Abstract
Efficient clearance of apoptotic cells seems to be a prerequisite to prevent the development of autoimmunity. Here we identify that macrophage colony-stimulating factor (M-CSF)–driven macrophages (Mø2s) are potent phagocytes that have the unique capacity to preferentially bind and ingest early apoptotic cells. This macrophage subset has intrinsic anti-inflammatory properties, characterized by high interleukin-10 (IL-10) production in the absence of proinflammatory cytokines, such as IL-6 and tumor necrosis factor-α (TNF-α). Importantly, whereas the IL-6 and TNF-α production by granulocyte-macrophage (GM)–CSF–driven macrophages (Mø1s) is inhibited upon uptake of apoptotic cells, the anti-inflammatory status of Mø2 is retained during phagocytosis. Mø2s were shown to use CD14 to tether apoptotic cells, whereas recognition of phosphatidylserine (PS) contributed to uptake of early apoptotic cells. Mø2s showed more potent macropinocytosis compared with dendritic cells (DCs) and Mø1s, and uptake of apoptotic cells was inhibited by a macropinocytosis inhibitor. Our studies suggest that, under steady-state conditions, IL-10–producing Mø2s are prominently involved in the clearance of early apoptotic cells.
Introduction
During normal homeostasis and tissue turnover large numbers of cells undergoing apoptosis are promptly removed and replaced. The removal of apoptotic material plays an important role in the suppression of inflammation and the regulation of immune responses.1-3 Apoptotic cells are a rich source of autoantigens,4 which are involved in the physiologic maintenance of self-tolerance. The uptake and processing of apoptotic cells has been proposed to be a silent process, meaning that release of pro-inflammatory cytokines by phagocytes is prevented.5-8 Impaired clearance of apoptotic cells, resulting in an accumulation of late apoptotic and secondary necrotic cells, might provide a danger signal to antigen-presenting cells (APCs), thus activating autoreactive T cells, and finally leading to the breakdown of peripheral tolerance.3 Accumulating evidence has been provided that defective clearance of apoptotic cells can lead to exacerbation of inflammation and predisposes to the development of autoimmunity, such as in systemic lupus erythematosus (SLE).9
The apoptotic cell clearance machinery includes professional phagocytes (ie, immature dendritic cells [DCs] and macrophages [Møs]), and nonprofessional phagocytes, including epithelial cells, fibroblasts, and mesangial cells.10 It has become clear that there are various subsets in both DCs and Møs.11-13 Recent in vitro data show that Møs can be polarized into proinflammatory (Mø1) and anti-inflammatory (Mø2) cells by granulocyte-macrophage colony-stimulating factors (GM-CSFs) and M-CSFs (also called CSF-1), respectively.14,15 Classically, GM-CSF and M-CSF are thought to be the primary growth factors for the differentiation of macrophages.16 Mice lacking M-CSF develop a general Mø deficiency,17 whereas GM-CSF knockout mice showed no major deficiency of Mø.18,19 In humans, M-CSF, but not GM-CSF, is an ubiquitous cytokine circulating in the human body.14,20 Thus, M-CSF could be the default cytokine to drive Mø differentiation under steady-state conditions.
The removal of apoptotic cells is an ongoing and constitutive process. Apoptotic cells provide “eat me” signals to phagocytes to promptly and efficiently engulf apoptotic cells during the very early stage of cell death, preventing them from releasing noxious intracellular contents.21 Although the silent removal of early apoptotic cells is well appreciated, the immunologic mechanisms involved are incompletely defined. There are several checkpoints conceivable which together determine the immunologic response toward the clearance of apoptotic cells. First, the process and the stage of cell death and/or the specific death pathways triggered are closely related to the consequence of apoptotic cell clearance.22 It has been established that late apoptotic or necrotic cells induce phagocyte activation, whereas early apoptotic cells do not.23,24 This is most likely due to differential expression of markers of cell death, such as heat shock proteins,25 HMGB1,26 and uric acid.27 Second, opsonization of apoptotic cells by components of the innate immune system such as complement factors facilitates and modulates the clearance of apoptotic cells (reviewed in Roos et al28 ). Third, the nature of phagocytes that take up apoptotic cells might provide a defined immunologic response.
Due to the large heterogeneity of phagocytes, the contribution of different phagocyte subsets in the clearance of apoptotic cells in terms of cytokine signature and the polarization of immune regulation remains largely unknown. In this respect, we hypothesize that under steady-state conditions, the scavenging of apoptotic cells is largely confined to a specialized phagocyte subset with anti-inflammatory properties. To test this hypothesis, we compared 3 types of phagocytes, DCs, Mø1s, and Mø2s, with respect to the phagocytosis of apoptotic cells at various stages of cell death. Our results strongly suggest that IL-10–producing Mø2s are prominently involved in the recognition and clearance of early apoptotic cells.
Materials and methods
Generation of monocyte-derived DCs, Mø1s, and Mø2s
Human mononuclear cells were isolated from buffy coats obtained from healthy donors using Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO), followed by anti-CD14 microbeads magnetic cell sorting, according to the manufacturer's instruction (Miltenyi Biotec/CLB, Amsterdam, The Netherlands). DCs were generated in 6-well culture plates (Costar, Cambridge, MA) in RPMI culture medium (RPMI 1640 containing 10% heat-inactivated fetal calf serum [FCS], 90 U/mL penicillin, and 90 μg/mL streptomycin; all from Gibco/Life technologies, Breda, The Netherlands) supplemented with 5 ng/mL GM-CSF (Leucomax; Novartis Pharma BV, Arnhem, The Netherlands) and 10 ng/mL IL-4 (PeproTech, Rocky Hill, NJ) for at least 6 days, as previously described.29 Two types of macrophages, namely Mø1 and Mø2, were generated in the same RPMI culture medium as DCs but supplemented with 5 ng/mL GM-CSF and 5 ng/mL M-CSF (R&D systems/ITK Diagnostics, Uithoorn, The Netherlands), respectively. In some experiments, Mø2s were generated in the presence of M-CSF and 10 μg/mL neutralizing anti–IL-10 receptor monoclonal antibody (mAb).30 In all experiments comparing these 3 types of phagocytes, they were generated in parallel from monocytes of the same donor. Separate unrelated donors were used for each independent experiment.
Analysis of cell-surface molecules by flow cytometry
Cells were harvested and washed in buffer containing 1% bovine serum albumin (BSA), 1% heat-inactivated normal human serum, and 0.02% NaN3. The following mAbs were used for flow cytometry analysis to analyze the surface molecules of phagocytes: anti-CD1a (Leu-6), anti-CD14 (Leu-M3), anti-CD11b/Mac-1 (all from BD Biosciences, San Jose, CA), anti–DC-SIGN/CD209 (AZN-D1; a gift from Dr Y. van Kooyk, Vrije Universiteit Medical Center, Amsterdam, The Netherlands), and anti–mannose receptor (MR)/CD206 (D547.3; a gift from F. Koning, LUMC, Leiden, The Netherlands). Staining was visualized by PE-conjugated goat anti–mouse immunoglobulin (Ig) (Dako, Glostrup, Denmark) using appropriate isotype controls. Cells were analyzed using FACSCalibur and CellQuest software (BD Biosciences). Dead cells, identified by propidium iodide (PI) uptake, were excluded from analysis.
Induction of apoptosis and necrosis
Jurkat T cells were cultured in RPMI culture medium. Apoptosis of Jurkat T cells was induced by culture with 50 μM etoposide (Vepesid; Bristol-Myers Squibb, New York, NY) for 18 hours in serum-free RPMI culture medium (RPMI 1640 containing 90 U/mL penicillin and 90 μg/mL streptomycin). Alternatively, Jurkat cells were washed with phosphate-buffered saline (PBS) and exposed to ultraviolet (UV)–C light (TUV lamp, predominantly 254 nm; Philips Electronic Instruments, Eindhoven, The Netherlands) at a dose of 50 J/m2. After UV irradiation, cells were cultured for 4 and 30 hours in serum-free RPMI medium to harvest the early and late apoptotic cells, respectively. Necrosis was induced by incubating Jurkat cells at 56°C in a water bath for 1 hour. Both apoptosis and necrosis were confirmed by double-staining with fluorescein isothiocyanate (FITC)–labeled annexin V and PI (VPS Diagnostics, Hoeven, The Netherlands) according to established methods.31
Next to apoptotic and necrotic cells, apoptotic blebs were used. Blebs were isolated from Jurkat cells treated with 50 μM etoposide for 48 hours, as described before.32 Blebs from 2.5 × 105 cells were used for the phagocytosis assay (see next section) per 1 × 105 phagocytes.
Phagocytosis assay
Prior to the induction of apoptosis, Jurkat cells were fluorescently labeled with carboxyfluorescein diacetate succinamidyl ester (CFSE; Molecular Probes, Leiden, The Netherlands), according to a previously described method.33 In brief, Jurkat cells were suspended in PBS at 2 × 107 cells/mL and incubated for 30 minutes at 37°C with 5 μM CFSE. Cells were washed and resuspended at 2 × 106 cells/mL in serum-free RPMI culture medium, used for apoptosis induction, as described in the previous section. For the phagocytosis assay, labeled apoptotic cells (1 × 105) were cocultured with DCs, Mø1s, or Mø2s at a 1:1 ratio for different time periods at 37°C or 4°C in 250 μL RPMI culture medium in round-bottom glass tubes. DCs, Mø1s, or Mø2s were stained with a PE-conjugated mAb against CD11b or APC-conjugated mAb against CD14 (BD Biosciences) and uptake was analyzed by 2-color flow cytometry. The percentage of CD11b-positive cells that stained positive for CFSE was used as a measure for the percentage of DCs, Mø1s, and Mø2s that ingested (37°C) and/or bound (4°C) apoptotic cells.
Phagocytosis of apoptotic cells by Mø1 and Mø2 was further investigated by confocal laser scanning microscopy with a Bio-Rad MRC1024 ES krypton–argon ion laser scanning imaging system (Hercules, CA), using appropriate filter settings. Images were visualized using a 40 ×/0.75 numeric aperture (NA) oil objective, were acquired using Laser Sharp 2000 software (Bio-Rad), and were processed using ImageJ software version 1.33 (NIH Image, Bethesda, MD). For this purpose, CFSE-labeled apoptotic cells (1 × 105) and phagocytes were cocultured at a 1:1 ratio on the Lab-TEK chamber slides (NUNC/Sanbio, Uden, The Netherlands) for 2 hours at 37°C, followed by washing to remove the noningested apoptotic cells, and staining with PE-conjugated mAb against CD11b. Cells were fixed with 4% paraformaldehyde before analysis. More than 600 single cells of Mø1 or Mø2 were randomly scored. Phagocytosis was presented as phagocytic index (percentage of phagocytosing Mø × average number of apoptotic cells per Mø).34
To measure a general phagocytic capacity of Mø1 and Mø2, sheep red blood cells (SRBCs, erythrocytes) were obtained and opsonized with rabbit anti–sheep red blood cell IgG (EIgG). Mø1s and Mø2s were precultured on Lab-TEK chamber slides at 37°C, followed by the addition of EIgG or nonopsonized erythrocytes at a 1:50 ratio for 0.5 hours. Unbound erythrocytes were washed away with PBS, and uningested erythrocytes were lysed by lysis buffer. Cells were fixed and stained with May-Grünwald/Giemsa. More than 300 single cells of Mø1 or Mø2 were scored by light microscopy and phagocytosis was presented as phagocytic index. Images were visualized using a Leica DC300F microscope (Leica, Rijswijk, The Netherlands) and a 40 × /1.30 NA oil objective. Images were captured using Leica IM50 software version 1.10 (Leica Microsystems, Heerbrugg, Switzerland).
Cytokine detection
DCs, Mø1s, and Mø2s were stimulated with 200 ng/mL lipopolysaccharide (LPS; Salmonella typhosa; Sigma-Aldrich) for 24 hours, and supernatants were harvested. Cytokines were detected in the supernatants using enzyme-linked immunosorbent assay (ELISA). The measurements of IL-6 and TNF-α were performed as described.35 The analysis of IL-10 was performed according to the manufacturer's instructions (Sanquin Research, Amsterdam, The Netherlands).
Coculture was performed by incubating DCs, Mø1s, and Mø2s with etoposide-induced apoptotic cells at a 1:1 ratio in RPMI culture medium. After 24 hours, supernatants were harvested and tested for IL-6, IL-10, and TNF-α production.
Endocytosis and macropinocytosis assays
Lectin-mediated endocytosis was examined following coincubation of phagocytes (1 × 105) with 100 μg/mL DextranFITC (Molecular Probes) for 30 or 60 minutes at 37°C in RPMI culture medium. Control experiments were done at 4°C. Blocking experiments were performed by preincubation of phagocytes for 20 minutes with 100 μg/mL mannan, or 50 mM d-mannose (both from Sigma-Aldrich). Incubation with 50 mM l-mannose (Sigma-Aldrich) or culture medium only was used as a negative control. Macropinocytosis was measured as the cellular uptake of 100 μg/mL lucifer yellow (LY) dipotassium salt (Molecular Probes) or 0.2 μg/ml BSAFITC (Sigma-Aldrich). Cells were washed extensively with cold PBS containing 1% FCS and 0.02% NaN3. Before analysis with flow cytometry, the cell-surface fluorescence was quenched with trypan blue (Sigma-Aldrich). To quantify the uptake, mean fluorescence intensity (MFI) values obtained at 4°C were subtracted from MFI values obtained at 37°C.
Inhibition of uptake of apoptotic cells
To assess the role of phosphatidylserine (PS) in the uptake of early apoptotic cells by Mø2, early apoptotic cells were preincubated with recombinant annexin V (up to 50 μg/mL; Sigma-Aldrich) at 4°C for 20 minutes to mask PS, before incubation with Mø2.
To assess the role of CD14 in the apoptotic cell clearance, Mø1s and Mø2s were treated with an anti-CD14–blocking mAb (61D3; a kind gift of Dr C. Gregory, University of Edinburgh, United Kingdom). Møs were preincubated with 61D3 (20 μg/mL) for 20 minutes at 4°C before addition of UV-induced early apoptotic cells or etoposide-induced apoptotic cells.
To investigate the role of macropinocytosis in the uptake of apoptotic cells by Mø1s and Mø2s, 5-(N,N-dimethyl)amiloride hydrochloride (DMA; Sigma-Aldrich) was used to inhibit macropinocytosis. Mø1s and Mø2s were preincubated with DMA (up to 1 mM) at 37°C for 20 minutes before early apoptotic cells or apoptotic blebs were added.
Statistical analysis
Statistical analysis was performed by 2-way analysis of variance (ANOVA), chi-square, or 1 sample t test using GraphPad Prism (GraphPad Software, San Diego, CA). Differences were considered statistically significant when P values were less than .05.
Results
Characterization of DCs, Mø1s, and Mø2s
Three types of phagocytes were generated from peripheral blood monocytes by different cytokines (ie, IL-4 and GM-CSF for DCs, GM-CSF for Mø1s, and M-CSF for Mø2s, respectively). Mø2s were less-adherent cells, with irregular shapes compared with Mø1s (Figure 1A). In contrast to DCs, Mø1s and Mø2s shared the typical macrophage phenotype, being positive for CD14, but negative for CD1a and DC-SIGN (Figure 1B). Notably, Mø2s expressed higher levels of CD14, but much lower level of mannose receptors (MR) than Mø1s. All 3 cell types showed expression of CD11b.
Uptake of apoptotic cells by DCs, Mø1s, and Mø2s
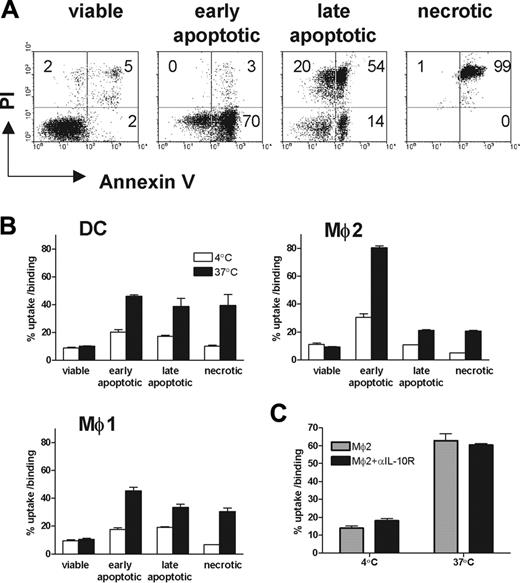
To investigate the phagocytosis of these 3 phagocyte subsets, apoptosis was induced in Jurkat cells using etoposide for 18 hours at 37°C in serum-free RPMI culture medium, as described.32 According to annexin V and PI staining, early apoptotic cells (Annexin+PI– populations) were routinely 27.52% ± 6.08%, and late apoptotic cells (PI+ populations) were 48.61% ± 10.76% (n = 8). Apoptosis was confirmed by nuclear fragmentation using Hoechst staining (data not shown). Exposure of apoptotic cells to DCs, Mø1s, and Mø2s resulted in a time- and temperature-dependent binding and ingestion, as shown by the appearance of CFSE- and CD11b double-positive populations (Figure 2A). Notably, from the 3 types of cells, Mø2s demonstrated the highest capacity for binding of apoptotic cells at 4°C, and for uptake of apoptotic cells at 37°C (Figure 2A). Mø2s have a 1.5- to 2-fold higher capacity for both binding and uptake of apoptotic cells compared with DCs and Mø1s (Figure 2B; ANOVA, P < .001). Similar results were obtained when different ratios of phagocytes to apoptotic cells (4:1, 16:1, and 64:1) were applied during coculture (data not shown). The uptake was significantly increased in time at 37°C (ANOVA, P < .05 for all 3 type of phagocytes), but the binding at 4°C was stable (Figure 2B).
Characterization of DCs, Mø1s, and Mø2s. (A) DCs, Mø1s, and Mø2s were generated in parallel from the same donor cultured for 6 or 7 days. Pictures show the morphology of these 3 different types of cells. Images were obtained using an Axiovert 25 inverted microscope (Carl Zeiss, Sliedrecht, The Netherlands) with a 20 × /0.3 NA objective and Zeiss Axiovision software version 3.1. Magnification, × 200. (B) Surface expression of CD1a, CD14, DC-SIGN, mannose receptor (MR), and CD11b on DCs, Mø1s, and Mø2s was determined by flow cytometry after 6 days of culture (closed histograms). Open histograms represent matched isotype controls. Data are representative of 3 to 8 independent experiments. Separate unrelated donors are used for each independent experiment.
Characterization of DCs, Mø1s, and Mø2s. (A) DCs, Mø1s, and Mø2s were generated in parallel from the same donor cultured for 6 or 7 days. Pictures show the morphology of these 3 different types of cells. Images were obtained using an Axiovert 25 inverted microscope (Carl Zeiss, Sliedrecht, The Netherlands) with a 20 × /0.3 NA objective and Zeiss Axiovision software version 3.1. Magnification, × 200. (B) Surface expression of CD1a, CD14, DC-SIGN, mannose receptor (MR), and CD11b on DCs, Mø1s, and Mø2s was determined by flow cytometry after 6 days of culture (closed histograms). Open histograms represent matched isotype controls. Data are representative of 3 to 8 independent experiments. Separate unrelated donors are used for each independent experiment.
To confirm and quantify the uptake of apoptotic cells by Mø1s and Mø2s, confocal microscopy was used. This analysis clearly showed the presence of CFSE-labeled apoptotic material inside the cells (Figure 2C). Quantification of phagocytic index confirmed our fluorescence-activated cell-sorting (FACS) data that Mø2s were more potent in the uptake of apoptotic cells than Mø1s (Figure 2D). The higher phagocytic index in Mø2s was due to a significantly higher percentage of Mø2s contributing to the uptake compared with Mø1s (chi-square test, P < .001), whereas the number of apoptotic cells taken up per macrophage between Mø1s and Mø2s were similar (2.33 vs 2.55, respectively). There seems to be a quantitative difference in the measurements by FACS and confocal microscopy. However, these experiments were not performed side by side and are therefore dependent on variation between donors. Moreover, for FACS analysis, cells were in solution, which might affect their phagocytic behavior. It is important to note that for differences between Mø1s and Mø2s, cells were in all cases generated in parallel from the same donor.
To determine whether the observed highly phagocytic capacity of Mø2s represents a generalized increase on phagocytosis, uptake of EIgG was investigated. We found that the phagocytic index of Mø2s is 6.84-fold higher than that of Mø1s (mean of 3 independent experiments; Figure 2E-F). This is especially explained by the fact the percentage of Mø2s contributing to the uptake was significantly higher than that of Mø1s (chi-square test, P < .001 for all 3 independent experiments), whereas the number of erythrocytes taken up per macrophage between Mø1s (1.83 ± 0.17) and Mø2s (2.23 ± 0.72) showed no difference (paired t test, P = .40). The nonopsonized erythrocytes were not ingested by Mø1s, whereas the uptake by Mø2s was of low efficiency (phagocytic index < 0.1; data not shown). Together, these data clearly show that Mø2s have a more generalized higher capacity of phagocytosis than Mø1s.
Uptake of apoptotic cells by DCs, Mø1s, and Mø2s. (A) Jurkat T cells were labeled with CFSE and induced into apoptosis by treating cells with etoposide for 18 hours. Apoptotic cells (0.1 × 106 cells) were coincubated with DCs, Mø1s, or Mø2s at a 1:1 ratio, for 0.5 hours and 2 hours at 37°C or 4°C. Prior to flow cytometry analysis, cells were stained with PE-conjugated mAb against CD11b. CFSE and CD11b double-positive populations represent the phagocytes that have bound and/or ingested apoptotic cells. Dot-plots represent the phagocytosis of apoptotic cells at 2 hours. (B) The percentages of uptake and binding (at 37°C) or binding (at 4°C) were calculated as 100% × [(CD11b+CFSE+)/CD11b+]. Data indicate the mean ± SD from 4 independent experiments performed in duplicate. Statistics were performed with 2-way ANOVA. *P < .01; **P < .001. (C) Confocal microscopy images show the uptake of apoptotic cells by Mø1s and Mø2s (see arrows). Red cells represent the CD11b-PE–positive Møs, and green cells are the CFSE-labeled apoptotic cells. Magnification, 400 ×. (D) Based on the confocal images, more than 600 cells of Mø1s and Mø2s were scored. Data are presented as phagocytic index (percentage of phagocytosing Møs × average number of apoptotic cells per Mø). A chi-square test was performed to evaluate the difference in the capacity of apoptotic cell uptake between Mø1s and Mø2s (P < .001). (E) Sheep erythrocytes were opsonized with rabbit anti–sheep red blood cell IgG (EIgG) and cocultured with Mø1s and Mø2s on Lab-TEK chamber slides at 37°C for 0.5 hours, followed by May-Grünwald/Giemsa staining. Pictures show that EIgG were ingested by Mø1s and Mø2s (arrows). Magnification, 400 ×. (F) More than 300 single cells of Mø1 or Mø2 were scored by light microscopy, and the phagocytosis of EIgG was presented as phagocytic index. Data are representative of 3 independent experiments using cells generated from 3 unrelated donors.
Uptake of apoptotic cells by DCs, Mø1s, and Mø2s. (A) Jurkat T cells were labeled with CFSE and induced into apoptosis by treating cells with etoposide for 18 hours. Apoptotic cells (0.1 × 106 cells) were coincubated with DCs, Mø1s, or Mø2s at a 1:1 ratio, for 0.5 hours and 2 hours at 37°C or 4°C. Prior to flow cytometry analysis, cells were stained with PE-conjugated mAb against CD11b. CFSE and CD11b double-positive populations represent the phagocytes that have bound and/or ingested apoptotic cells. Dot-plots represent the phagocytosis of apoptotic cells at 2 hours. (B) The percentages of uptake and binding (at 37°C) or binding (at 4°C) were calculated as 100% × [(CD11b+CFSE+)/CD11b+]. Data indicate the mean ± SD from 4 independent experiments performed in duplicate. Statistics were performed with 2-way ANOVA. *P < .01; **P < .001. (C) Confocal microscopy images show the uptake of apoptotic cells by Mø1s and Mø2s (see arrows). Red cells represent the CD11b-PE–positive Møs, and green cells are the CFSE-labeled apoptotic cells. Magnification, 400 ×. (D) Based on the confocal images, more than 600 cells of Mø1s and Mø2s were scored. Data are presented as phagocytic index (percentage of phagocytosing Møs × average number of apoptotic cells per Mø). A chi-square test was performed to evaluate the difference in the capacity of apoptotic cell uptake between Mø1s and Mø2s (P < .001). (E) Sheep erythrocytes were opsonized with rabbit anti–sheep red blood cell IgG (EIgG) and cocultured with Mø1s and Mø2s on Lab-TEK chamber slides at 37°C for 0.5 hours, followed by May-Grünwald/Giemsa staining. Pictures show that EIgG were ingested by Mø1s and Mø2s (arrows). Magnification, 400 ×. (F) More than 300 single cells of Mø1 or Mø2 were scored by light microscopy, and the phagocytosis of EIgG was presented as phagocytic index. Data are representative of 3 independent experiments using cells generated from 3 unrelated donors.
Mø2s retain their anti-inflammatory status after uptake of apoptotic cells
It has been proposed that clearance of apoptotic material is a noninflammatory process.1-3 Therefore, we investigated the functional consequences of uptake of apoptotic cells by Mø1s or Mø2s by measuring the cytokine release in supernatants. The capacity of cytokine production by the 3 phagocyte subsets was first investigated using LPS stimulation (Figure 3A-C). LPS-stimulated Mø2s produced large amounts of IL-10, but failed to secrete IL-6 and TNF-α. In contrast, Mø1s produced high amounts of IL-6 and TNF-α after LPS stimulation, whereas IL-10 was hardly detectable. DCs derived from the same donor were able to produce IL-10, IL-6, and TNF-α following LPS stimulation.
Mø2s showed intrinsic IL-10 production (Figure 3A,D), which was retained after uptake of apoptotic cells (Figure 3D), whereas production of IL-6 or TNF-α was not induced (Figure 3E-F). Importantly, uptake of apoptotic cells by Mø1s resulted in a down-regulation of IL-6 and TNF-α production, but no induction of IL-10 (Figure 3D-F). Upon exposure to apoptotic cells, DCs did not secrete IL-10, IL-6, or TNF-α.
Mø2s preferentially take up early apoptotic cells
To assess whether there are differences among these 3 types of phagocytes in the uptake of apoptotic cells in different stages of cell death, Jurkat cells were exposed to UV light. Four hours after UV irradiation, around 50% to 70% early apoptotic cells (annexin V+/PI–) were obtained (Figure 4A). Thirty hours after UV irradiation, around 70% to 95% of cells were late apoptotic (PI+). Necrotic cells generated by heat shock were all PI-positive. Viable cells were used as a negative control. All 3 types of phagocytes were unable to take up viable cells (Figure 4B). Importantly, Mø2s were the only phagocytes that were able to distinguish early apoptotic cells from late apoptotic and necrotic cells. Mø2s have a preferential uptake of early apoptotic cells, compared with late apoptotic or necrotic cells (Figure 4B). In contrast, DCs and Mø1s show an equal efficiency in the uptake of early apoptotic, late apoptotic, or necrotic cells. Furthermore, the efficiency of taking up early apoptotic cells by Mø2s is at least 2-fold higher than that by DCs and Mø1s (mean ± SD of phagocytosis of Mø2s: Mø1s from 5 independent experiments are 2.65 ± 0.62, P < .001, 2-way ANOVA).
It has been shown that IL-10–activated Møs have increased phagocytic capacity for the uptake of apoptotic cells.36 To investigate whether IL-10–producing Mø2s become highly phagocytic to early apoptotic cells as a consequence of the IL-10 environment created by themselves, we generated Mø2s in the presence of neutralizing anti–IL-10 receptor mAb. Although the anti–IL-10 receptor mAb inhibited the endogenous IL-10 production of Mø2s (data not shown), it did not inhibit phagocytosis of early apoptotic cells compared with Mø2s generated in the absence of anti–IL-10 receptor mAb (Figure 4C). We conclude that endogenous IL-10 produced by Mø2s is not involved in driving the development of high capacity for phagocytosis of early apoptotic cells.
Cytokine production by DCs, Mø1s, and Mø2s after LPS stimulation or uptake of apoptotic cells. (A-C) Day-6 DCs, Mø1s, and Mø2s were extensively washed, and subsequently stimulated with or without LPS for 24 hours. (D-F) DCs, Mø1s, or Mø2s (1 × 105 cells) were cocultured with etoposide-induced apoptotic cells at a 1:1 ratio for 24 hours in RPMI culture medium. Supernatants were harvested and measured by ELISA for IL-10, IL-6, and TNF-α. Data are presented as mean ± SD from duplicate cultures and are representative of at least 3 independent experiments where cells are generated from separate unrelated donors. Dashed lines represent the detection limits of ELISA.
Cytokine production by DCs, Mø1s, and Mø2s after LPS stimulation or uptake of apoptotic cells. (A-C) Day-6 DCs, Mø1s, and Mø2s were extensively washed, and subsequently stimulated with or without LPS for 24 hours. (D-F) DCs, Mø1s, or Mø2s (1 × 105 cells) were cocultured with etoposide-induced apoptotic cells at a 1:1 ratio for 24 hours in RPMI culture medium. Supernatants were harvested and measured by ELISA for IL-10, IL-6, and TNF-α. Data are presented as mean ± SD from duplicate cultures and are representative of at least 3 independent experiments where cells are generated from separate unrelated donors. Dashed lines represent the detection limits of ELISA.
Role of PS and CD14 in the uptake of apoptotic cells by Mø1s and Mø2s
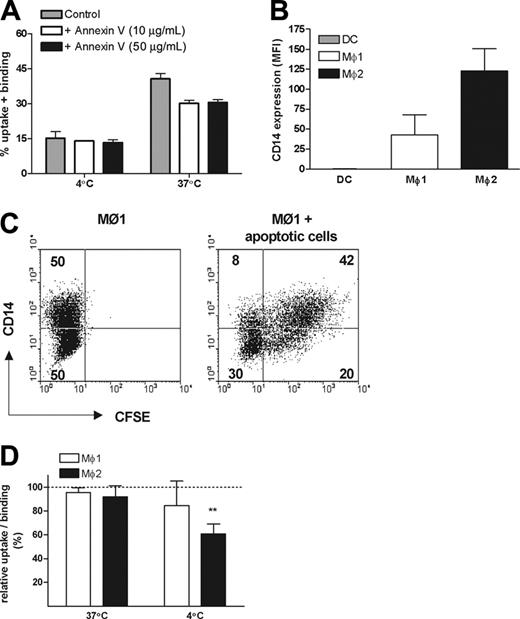
A major feature of early apoptosis is the redistribution of PS from the inner layer of the cell membrane to the outside. Therefore we next investigated whether PS is involved in the phagocytosis by Mø2s. Preincubation of early apoptotic cells with nonlabeled annexin V prevented binding of FITC-labeled annexin V, confirming an effective masking of PS (data not shown), as described.32,37 The uptake of early apoptotic cells by Mø2s was only partially inhibited after PS masking (inhibition was between 6.5% to 23.2% in 3 independent experiments), and this effect could not be improved by applying a higher concentration of annexin V (Figure 5A).
CD14 has been shown to be involved in the recognition of apoptotic cells by Møs.38 As a possible explanation for their efficient uptake of apoptotic cells, we noticed that Mø2s express a significantly higher level of CD14 compared with Mø1s (Figures 1B,5B). Surface expression of CD14 on Mø1s is heterogeneous (Figure 1B). Based on the FACS dot-plots, Mø1s were equally divided into 2 populations: CD14high and CD14low (Figure 5C). Phagocytosis experiments showed that CD14high cells were twice as efficient in binding and uptake of apoptotic cells compared with CD14low cells (Figure 5C). Thus, we further investigated the role of CD14 in the engulfment of early apoptotic cells by using a blocking antibody against CD14, mAb 61D3.38 The uptake of early apoptotic cells by both Mø1s and Mø2s was not significantly inhibited by this mAb. We did observe that the binding of early apoptotic cells by Mø2s at 4°C was inhibited (P < .001) (Figure 5D). Similar data were obtained when apoptosis was induced with either UV or etoposide. Together, these data indicate that CD14 is involved in tethering but not engulfing apoptotic cells.
Uptake of early apoptotic cells by Mø2s is mediated by macropinocytosis
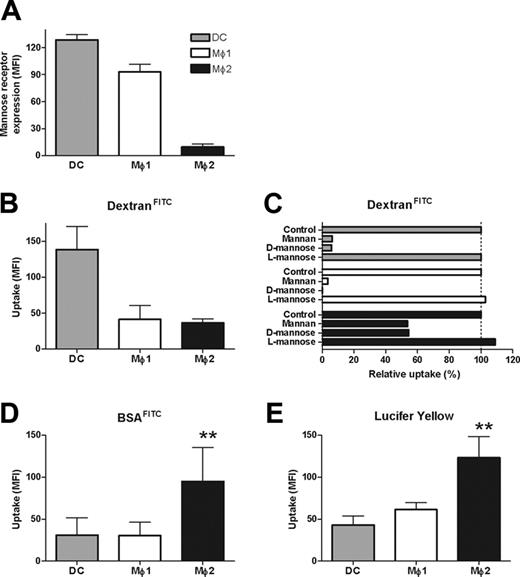
Phagocytes take up antigens via receptor-mediated endocytosis, mostly by C-type lectins, such as mannose receptors (MRs), or via macropinocytosis (fluid-phase endocytosis).39,40 Therefore, we investigated lectin-mediated endocytosis of soluble antigens using DextranFITC. Despite the fact that Mø2s showed a significantly lower expression of MRs (Figures 1B, 6A), the uptake of DextranFITC by Mø1s and Mø2s was comparable, whereas uptake by DCs was more efficient (Figure 6B). The uptake of DextranFITC by DCs and Mø1s was completely inhibited by mannan or D-mannose, whereas L-mannose, used as a negative control, did not inhibit the uptake (Figure 6C). In contrast, pretreatment of Mø2s by mannan or D-mannose inhibited the uptake of DextranFITC only by 50% (Figure 6C), suggesting the involvement of lectin-independent mechanisms such as macropinocytosis. Indeed, for the uptake of both BSAFITC and LY, Mø2s were shown to be 2- to 3-fold more efficient compared with Mø1s and DCs (ANOVA, P < .001) (Figure 6D-E).
To investigate a possible contribution of macropinocytosis in the uptake of early apoptotic cells by Mø2s, DMA, a Na+/H+ antiporter blocker, was used to inhibit the fluid phase uptake by macropinocytosis.39 DMA treatment of Mø2s not only inhibited the uptake of both BSAFITC and LY (Figure 7A), but also inhibited the uptake of early apoptotic cells in a dose-dependent fashion (Figure 7B), without interfering with the viability of the cells. Similarly, DMA also inhibited the uptake of early apoptotic cells by CD14high Mø1s (Figure 7C).
Uptake of viable, early apoptotic, late apoptotic, and necrotic cells by DCs, Mø1s, and Mø2s. (A) Early and late apoptotic cells were induced by UV-C light at a dose of 50 J/m2, then cultured for another 4 and 30 hours, respectively. Necrosis was induced by incubating Jurkat cells at 56°C for 1 hour. The untreated viable cells were used as control. Apoptosis was confirmed by double staining with FITC-labeled annexin V and PI. (B) These CFSE-labeled cells were cocultured with DCs, Mø1s, or Mø2s (1 × 105 cells) at a 1:1 ratio, in the same way as described in Figure 2. The percentages of uptake and binding (at 37°C) or binding (at 4°C) were quantified similarly as described in Figure 2. Data (mean ± SD) represent at least 3 independent experiments performed in duplicate. (C) Uptake of early apoptotic cells by Mø2s (mean ± SD) generated in the presence or absence of 10 μg/mL neutralizing anti–IL-10 receptor mAb.
Uptake of viable, early apoptotic, late apoptotic, and necrotic cells by DCs, Mø1s, and Mø2s. (A) Early and late apoptotic cells were induced by UV-C light at a dose of 50 J/m2, then cultured for another 4 and 30 hours, respectively. Necrosis was induced by incubating Jurkat cells at 56°C for 1 hour. The untreated viable cells were used as control. Apoptosis was confirmed by double staining with FITC-labeled annexin V and PI. (B) These CFSE-labeled cells were cocultured with DCs, Mø1s, or Mø2s (1 × 105 cells) at a 1:1 ratio, in the same way as described in Figure 2. The percentages of uptake and binding (at 37°C) or binding (at 4°C) were quantified similarly as described in Figure 2. Data (mean ± SD) represent at least 3 independent experiments performed in duplicate. (C) Uptake of early apoptotic cells by Mø2s (mean ± SD) generated in the presence or absence of 10 μg/mL neutralizing anti–IL-10 receptor mAb.
Role of PS and CD14 in the uptake of early apoptotic cells by Mø1s and Mø2s. (A) CFSE-labeled early apoptotic cells were preincubated with 10 μg/mL or 50 μg/mL unlabeled annexin V or medium as control at 4°C for 20 minutes, before the coincubation with Mø2s. The percentage of uptake and/or binding was calculated as described in Figure 2. Data shown are representative of 3 independent experiments performed in duplicate. (B) CD14 expression on DCs, Mø1s, and Mø2s generated in parallel from the same donor. Bars show the mean fluorescence intensity (MFI). Data shown are the mean ± SD from 4 independent experiments. (C) After coincubation of apoptotic cells with Mø1s, cells were stained with a APC-conjugated anti-CD14 mAb, instead of anti-CD11b. CD14 expression was divided equally into 2 populations: CD14high and CD14low. Dot-plots of the FACS showed the distinct uptake of apoptotic cells by CD14high and CD14low cells. (D) Mø1s and Mø2s were preincubated with or without a blocking anti-CD14 antibody (mAb 61D3) at 4°C for 20 minutes, before the coincubation with early apoptotic cells for 2 hours. Relative uptake/binding was calculated as 100% × (% in the presence of anti-CD14)/(% in the absence of anti-CD14). Data are shown as mean ± SD from 4 (Mø1s) to 6 (Mø2s) independent experiments performed in duplicate. Similar results were obtained when etoposide-induced apoptotic cells were applied. **P < .001, 1 sample t test. Separate unrelated donors are used for each independent experiment. Dashed line indicates 100% of relative uptake.
Role of PS and CD14 in the uptake of early apoptotic cells by Mø1s and Mø2s. (A) CFSE-labeled early apoptotic cells were preincubated with 10 μg/mL or 50 μg/mL unlabeled annexin V or medium as control at 4°C for 20 minutes, before the coincubation with Mø2s. The percentage of uptake and/or binding was calculated as described in Figure 2. Data shown are representative of 3 independent experiments performed in duplicate. (B) CD14 expression on DCs, Mø1s, and Mø2s generated in parallel from the same donor. Bars show the mean fluorescence intensity (MFI). Data shown are the mean ± SD from 4 independent experiments. (C) After coincubation of apoptotic cells with Mø1s, cells were stained with a APC-conjugated anti-CD14 mAb, instead of anti-CD11b. CD14 expression was divided equally into 2 populations: CD14high and CD14low. Dot-plots of the FACS showed the distinct uptake of apoptotic cells by CD14high and CD14low cells. (D) Mø1s and Mø2s were preincubated with or without a blocking anti-CD14 antibody (mAb 61D3) at 4°C for 20 minutes, before the coincubation with early apoptotic cells for 2 hours. Relative uptake/binding was calculated as 100% × (% in the presence of anti-CD14)/(% in the absence of anti-CD14). Data are shown as mean ± SD from 4 (Mø1s) to 6 (Mø2s) independent experiments performed in duplicate. Similar results were obtained when etoposide-induced apoptotic cells were applied. **P < .001, 1 sample t test. Separate unrelated donors are used for each independent experiment. Dashed line indicates 100% of relative uptake.
We next investigated the uptake of microparticles derived from apoptotic cells (apoptotic blebs). Mø2s were able to take up apoptotic blebs twice as efficiently as Mø1s (Figure 7D). DMA inhibited the uptake of apoptotic blebs by Mø2s dose-dependently (Figure 7E), and in concentrations that are lower than needed for the inhibition of uptake of early apoptotic cells.
Discussion
Over the last few years, it has become clear that both DCs and Møs show a large heterogeneity.11,13 We hypothesized that distinct phagocyte subsets contribute differentially to the clearance of apoptotic cells. Here we identify that the IL-10–producing Mø2s are potent phagocytes that have the unique capacity to preferentially bind and ingest early apoptotic cells. This macrophage subset has intrinsic anti-inflammatory properties, characterized by high IL-10 production in the absence of proinflammatory cytokines, such as IL-6 and TNF-α. IL-10 is an important anti-inflammatory cytokine, which is able to limit and ultimately terminate inflammatory responses and plays a role in differentiation of regulatory T cells.41
The generation of apoptotic cells is a continuous process. Although it is generally accepted that clearance of apoptotic cells is a noninflammatory process, this most likely happens only when apoptotic cells are removed during early stages of apoptosis progression. Late apoptotic or necrotic cells activate APCs, promoting them to present self-antigen to T cells, potentially resulting in autoimmunity.23,24 Thus, under normal conditions, apoptotic cells should be cleared before they progress into late stages. Our findings that Mø2s have the capacity to preferentially take up early apoptotic cells indicate that these IL-10–producing Mø subsets might be unique safeguards to promptly clear apoptotic cells in a silent way. Of note, increased capacity of phagocytosis by Mø2s is not unique for apoptotic cells, but also observed with IgG-opsonized erythrocytes. This suggests that Mø2s have a generalized higher capacity of phagocytosis compared with Mø1s. It remains to be established what the in vivo contribution is to apoptotic cell clearance by different myeloid subsets, and which mechanisms underlie the specific clearance of early apoptotic cells.
It has been shown that IL-10–activated Møs obtain enhanced capacity for the uptake of apoptotic cells.36 However, phagocytosis experiments using Mø2s generated in the presence of neutralizing anti–IL-10 receptor mAb suggested that endogenous IL-10 produced by Mø2s is not involved in driving the development of high capacity for phagocytosis of early apoptotic cells. However, inhibition of endogenous IL-10 production suggested that there is a positive feedback loop of IL-10 production. We should note that the endogenous production of IL-10 we observed was relatively low and was observed only at the end of the 6- to 7-day culture period. This might explain the suggested discrepancy with the study of Ogden et al,36 where higher amounts of IL-10 have been added from the start of the culture and clearly showed effects on the phagocytic capacity. In conclusion, we feel that in our experiments endogenous IL-10 production is not required for the development of cells with a high phagocytic activity.
Lectin-mediated endocytosis and macropinocytosis by DCs, Mø1s, and Mø2s. (A) Mannose receptor (MR)/CD206 expression on DCs, Mø1s, and Mø2s. Bars show the mean fluorescence intensity (MFI). Data shown are the mean (± SD) from 2 to 3 independent experiments. (B) The uptake of DextranFITC (100 μg/mL) by DCs, Mø1s, and Mø2s was measured at 1 hour. Data shown are the mean ± SD from 3 independent experiments. (C) The uptake of DextranFITC was measured by different phagocytes preincubated with 100 μg/mL mannan, or 50 mM d-mannose or l-mannose. The uptake by untreated phagocytes (control) is considered as 100%, and data show the relative uptake against the control. Data represent 3 independent experiments. (D) The uptake of BSAFITC (0.2 μg/mL) by DCs, Mø1s, and Mø2s at 1 hour. Data shown are the mean ± SD from 5 independent experiments. Dashed line indicates 100% of relative uptake. (E) The uptake of lucifer yellow (LY) (100 μg/mL) by DCs, Mø1s, and Mø2s at 1 hour. Data shown are the mean ± SD from 3 independent experiments (**P < .001, ANOVA).
Lectin-mediated endocytosis and macropinocytosis by DCs, Mø1s, and Mø2s. (A) Mannose receptor (MR)/CD206 expression on DCs, Mø1s, and Mø2s. Bars show the mean fluorescence intensity (MFI). Data shown are the mean (± SD) from 2 to 3 independent experiments. (B) The uptake of DextranFITC (100 μg/mL) by DCs, Mø1s, and Mø2s was measured at 1 hour. Data shown are the mean ± SD from 3 independent experiments. (C) The uptake of DextranFITC was measured by different phagocytes preincubated with 100 μg/mL mannan, or 50 mM d-mannose or l-mannose. The uptake by untreated phagocytes (control) is considered as 100%, and data show the relative uptake against the control. Data represent 3 independent experiments. (D) The uptake of BSAFITC (0.2 μg/mL) by DCs, Mø1s, and Mø2s at 1 hour. Data shown are the mean ± SD from 5 independent experiments. Dashed line indicates 100% of relative uptake. (E) The uptake of lucifer yellow (LY) (100 μg/mL) by DCs, Mø1s, and Mø2s at 1 hour. Data shown are the mean ± SD from 3 independent experiments (**P < .001, ANOVA).
Uptake of early apoptotic cells and apoptotic blebs by Mø2s is prevented by an inhibitor of macropinocytosis. Mø2s were preincubated with or without DMA at 37°C for 20 minutes before the coculture with BSAFITC (0.2 μg/mL), LY (100 μg/mL), or early apoptotic cells. (A) Data show the uptake of BSAFITC and LY at 1 hour by Mø2s in the presence (gray histograms) or absence (black histograms) of 500 μM DMA. The dashed lines represent the background mean fluorescence of Mø2s. (B) Data show the dose-dependent effect of DMA on uptake (37-4°C) of apoptotic cells and LY by Mø2s and represent the relative uptake of DMA-treated Mø2s against the controls (untreated cells). Data are shown as mean ± SD of duplicate cultures and represent 4 independent experiments. (C) Mø1s were treated with or without 500 μM DMA at 37°C for 20 minutes before the co-incubation with early apoptotic cells for 30 minutes. Data show the quantification of uptake (37-4°C) by CD14high and CD14low cells. Data (mean ± SD) represent 3 independent experiments. (D) Apoptotic blebs were isolated from CFSE-labeled Jurkat cells and were used for the phagocytosis assay with Mø1s and Mø2s. Data (mean ± SD) represent 4 independent experiments. (E) Mø2s were pretreated with or without DMA (up to 500 μM). Relative uptake is shown. Similar results were obtained from 2 independent experiments. Separate unrelated donors are used for each independent experiment.
Uptake of early apoptotic cells and apoptotic blebs by Mø2s is prevented by an inhibitor of macropinocytosis. Mø2s were preincubated with or without DMA at 37°C for 20 minutes before the coculture with BSAFITC (0.2 μg/mL), LY (100 μg/mL), or early apoptotic cells. (A) Data show the uptake of BSAFITC and LY at 1 hour by Mø2s in the presence (gray histograms) or absence (black histograms) of 500 μM DMA. The dashed lines represent the background mean fluorescence of Mø2s. (B) Data show the dose-dependent effect of DMA on uptake (37-4°C) of apoptotic cells and LY by Mø2s and represent the relative uptake of DMA-treated Mø2s against the controls (untreated cells). Data are shown as mean ± SD of duplicate cultures and represent 4 independent experiments. (C) Mø1s were treated with or without 500 μM DMA at 37°C for 20 minutes before the co-incubation with early apoptotic cells for 30 minutes. Data show the quantification of uptake (37-4°C) by CD14high and CD14low cells. Data (mean ± SD) represent 3 independent experiments. (D) Apoptotic blebs were isolated from CFSE-labeled Jurkat cells and were used for the phagocytosis assay with Mø1s and Mø2s. Data (mean ± SD) represent 4 independent experiments. (E) Mø2s were pretreated with or without DMA (up to 500 μM). Relative uptake is shown. Similar results were obtained from 2 independent experiments. Separate unrelated donors are used for each independent experiment.
Both GM-CSF and M-CSF are key growth factors, not only for Mø generation in vitro, but also for Mø differentiation in vivo. Op/op mice lacking M-CSF develop a profound macrophage deficiency, and this could be partially corrected by the implantation of diffusion chambers containing M-CSF-producing cells,17 confirming that M-CSF is crucial in Mø differentiation. GM-CSF knockout mice did not show major deficiency of Møs, although the Møs are smaller than normal.18,19 Under steady-state conditions, M-CSF is the only primary Mø growth factor detectable in peripheral blood.20 In contrast, GM-CSF is generally viewed as a proinflammatory cytokine involved in inflammation and is hardly detectable in circulation.42 Thus, under steady-state conditions, M-CSF could be the default cytokine driving Mø differentiation.
Cell-cell contact with apoptotic cells is sufficient to induce profound inhibition of IL-12 production by activated macrophages, thus preventing them to mount an immune response.8 We show here that, upon uptake of apoptotic cells, Mø2s do not produce proinflammatory cytokines such as IL-6 and TNF-α, while their IL-10 production was not inhibited. Importantly, and in line with previous results, uptake of apoptotic cells by Mø1s resulted in a down-regulation of IL-6 and TNF-α production. It has been shown that monocyte-derived Møs differentiated with M-CSF acquire the ability to selectively induce T-cell apoptosis in an activation-specific fashion,43 leading to the speculation that peripheral tolerance can be helped by selectively deleting autoreactive T cells that respond to self-antigen. Therefore, at least 2 mechanisms might be operational to prevent that uptake of apoptotic cells leads to immune activation.
Recently, CD14 has been reported to play an important role in the recognition of apoptotic cells,38 and was further suggested as a tethering molecule for Møs to recognize apoptotic cells.44 CD14–/– mice showed a persistence of apoptotic cells, supporting that CD14 plays a broad tethering role in the apoptotic cell clearance in vivo.45 In our study, CD14 expression is remarkably different between Mø1s (CD14+) and Mø2s (CD14+++). Notably, Mø2s have a strong interaction with apoptotic cells already at 4°C. Blocking studies using anti-CD14 antibody showed that CD14 is mainly involved in the surface binding, but hardly in the uptake of apoptotic cells, supporting the notion that CD14 is a tethering molecule rather than an engulfing molecule.38
Next to the tethering process, other mechanisms are involved in the engulfment of apoptotic cells. Uptake of antigens (Ag's) takes place via at least 2 different pathways: receptor-mediated endocytosis and macropinocytosis.39,40 Although macropinocytosis is thought to be a specific characteristic of DCs,39 it can be induced in other cells, including Møs stimulated with M-CSF.46,47 Here we show in a side-by-side comparison that Mø2s, generated in the presence of M-CSF but tested in the absence of M-CSF, exhibit more potent macropinocytosis compared with monocyte-derived DCs. The suggestion that macropinocytosis contributes to the uptake of apoptotic cells has already been put forward.48 Engagement of Mø surface receptors by apoptotic cells, either directly (via PS) or indirectly (via C1q or mannose binding lectin [MBL] binding to apoptotic cells) actively promotes macropinocytosis.34,49 Our studies using the inhibitor DMA suggest that the efficient uptake of early apoptotic cells or apoptotic blebs by Mø2s is mediated by Na+/H– antiporter-dependent macropinocytosis. Rac, Rho, and Cdc42 have been described as the major regulators of actin-driven macropinocytosis.50 Whether these regulators are involved in initiating macropinocytosis, particularly in the uptake of early apoptotic cells by Mø2s, remains to be established. Our findings are in agreement with the proposal that the uptake of apoptotic cells is a 2-step process:3 first, apoptotic cells are engaged with phagocytes by receptor-dependent tethering processes such as CD14 for Mø2s; second, formation of fluid-filled macropinosomes allows Mø2s to promptly and efficiently engulf early apoptotic cells or microparticles-derived from apoptotic cells.
In conclusion, different subsets of phagocytes, such as DCs, Mø1s, and Mø2s recognize and process early and late apoptotic cells in a differential manner. The cytokine environment and the nature of phagocytes at a certain location contribute to the decision whether apoptotic cells are cleared in a silent or inflammatory fashion. In this respect, we demonstrate that IL-10–producing Mø2s have the unique capability to preferentially bind and ingest early apoptotic cells. Our studies suggest that under steady-state conditions, M-CSF–driven Mø2s might be the default phagocytes to preferentially clear early apoptotic cells in a silent way, thereby contributing to the induction and maintenance of peripheral tolerance after encountering self-antigen.
Prepublished online as Blood First Edition Paper, February 23, 2006; DOI 10.1182/blood-2005-10-4144.
Supported by a grant (C02.2015) from the Dutch Kidney Foundation.
C.v.K., A.R., and M.R.D. designed the study; W.X. and N.S. performed research; A.M.W. provided vital new reagents and analytic tools; W.X., N.S., A.R., A.M.W., M.R.D., and C.v.K. analyzed data; W.X., A.R., and C.v.K. wrote the paper; and N.S., A.M.W., and M.R.D. reviewed and approved the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr C. Gregory (MRC center for Inflammation Research, University of Edinburgh, United Kingdom) for kindly providing anti-CD14 mAb. We thank A. R. Filon (Department of Toxicogenetics, Leiden University Medical Center [LUMC], The Netherlands) for help with UV irradiation. F. Prins (Department of Pathology, LUMC) and G. Lamers (Institute of Biology, Leiden University, The Netherlands) are acknowledged for their excellent technical assistance with confocal microscopy. We also thank J. H. Fransen (Nijmegen Center for Molecular Life Sciences, University Medical Center St Radboud, Nijmegen, The Netherlands) for valuable discussion.


![Figure 2. Uptake of apoptotic cells by DCs, Mø1s, and Mø2s. (A) Jurkat T cells were labeled with CFSE and induced into apoptosis by treating cells with etoposide for 18 hours. Apoptotic cells (0.1 × 106 cells) were coincubated with DCs, Mø1s, or Mø2s at a 1:1 ratio, for 0.5 hours and 2 hours at 37°C or 4°C. Prior to flow cytometry analysis, cells were stained with PE-conjugated mAb against CD11b. CFSE and CD11b double-positive populations represent the phagocytes that have bound and/or ingested apoptotic cells. Dot-plots represent the phagocytosis of apoptotic cells at 2 hours. (B) The percentages of uptake and binding (at 37°C) or binding (at 4°C) were calculated as 100% × [(CD11b+CFSE+)/CD11b+]. Data indicate the mean ± SD from 4 independent experiments performed in duplicate. Statistics were performed with 2-way ANOVA. *P < .01; **P < .001. (C) Confocal microscopy images show the uptake of apoptotic cells by Mø1s and Mø2s (see arrows). Red cells represent the CD11b-PE–positive Møs, and green cells are the CFSE-labeled apoptotic cells. Magnification, 400 ×. (D) Based on the confocal images, more than 600 cells of Mø1s and Mø2s were scored. Data are presented as phagocytic index (percentage of phagocytosing Møs × average number of apoptotic cells per Mø). A chi-square test was performed to evaluate the difference in the capacity of apoptotic cell uptake between Mø1s and Mø2s (P < .001). (E) Sheep erythrocytes were opsonized with rabbit anti–sheep red blood cell IgG (EIgG) and cocultured with Mø1s and Mø2s on Lab-TEK chamber slides at 37°C for 0.5 hours, followed by May-Grünwald/Giemsa staining. Pictures show that EIgG were ingested by Mø1s and Mø2s (arrows). Magnification, 400 ×. (F) More than 300 single cells of Mø1 or Mø2 were scored by light microscopy, and the phagocytosis of EIgG was presented as phagocytic index. Data are representative of 3 independent experiments using cells generated from 3 unrelated donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/12/10.1182_blood-2005-10-4144/4/m_zh80120696930002.jpeg?Expires=1765924028&Signature=XrqujzPCKop-931a8eLn0EkCH5OLL6~cL3d0dY-m90-kdxmP~iVnxy7N4TY~Jum-bfS~GqMdedvDP33bL7Fn8oZvPnF6AvCYTQjrZ~mejFJAOkTCietDFhs8n6pmzGaRz84SbexBVMXb9M998XaPWMNgL9wqiiMwCVReVh6hRNdqAGL1LeW96b8aR1oCuUjHxNYVT83L3mJvBVTeAhkfXeYg8MKd~FcZYk03bUICyyBHAtYAHum45XmaU0h2P1zZ5~X~Y~8R9SJjzHDJc266UyZhBPM9wvSM~ACUevUfcL3iOcknnuHtAVBoXK1M5lWdAqjdwiR5DZfbC2GBGmiBtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 2. Uptake of apoptotic cells by DCs, Mø1s, and Mø2s. (A) Jurkat T cells were labeled with CFSE and induced into apoptosis by treating cells with etoposide for 18 hours. Apoptotic cells (0.1 × 106 cells) were coincubated with DCs, Mø1s, or Mø2s at a 1:1 ratio, for 0.5 hours and 2 hours at 37°C or 4°C. Prior to flow cytometry analysis, cells were stained with PE-conjugated mAb against CD11b. CFSE and CD11b double-positive populations represent the phagocytes that have bound and/or ingested apoptotic cells. Dot-plots represent the phagocytosis of apoptotic cells at 2 hours. (B) The percentages of uptake and binding (at 37°C) or binding (at 4°C) were calculated as 100% × [(CD11b+CFSE+)/CD11b+]. Data indicate the mean ± SD from 4 independent experiments performed in duplicate. Statistics were performed with 2-way ANOVA. *P < .01; **P < .001. (C) Confocal microscopy images show the uptake of apoptotic cells by Mø1s and Mø2s (see arrows). Red cells represent the CD11b-PE–positive Møs, and green cells are the CFSE-labeled apoptotic cells. Magnification, 400 ×. (D) Based on the confocal images, more than 600 cells of Mø1s and Mø2s were scored. Data are presented as phagocytic index (percentage of phagocytosing Møs × average number of apoptotic cells per Mø). A chi-square test was performed to evaluate the difference in the capacity of apoptotic cell uptake between Mø1s and Mø2s (P < .001). (E) Sheep erythrocytes were opsonized with rabbit anti–sheep red blood cell IgG (EIgG) and cocultured with Mø1s and Mø2s on Lab-TEK chamber slides at 37°C for 0.5 hours, followed by May-Grünwald/Giemsa staining. Pictures show that EIgG were ingested by Mø1s and Mø2s (arrows). Magnification, 400 ×. (F) More than 300 single cells of Mø1 or Mø2 were scored by light microscopy, and the phagocytosis of EIgG was presented as phagocytic index. Data are representative of 3 independent experiments using cells generated from 3 unrelated donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/12/10.1182_blood-2005-10-4144/4/m_zh80120696930002.jpeg?Expires=1765924029&Signature=dQPh4C1leQ1zeYDVbkOLfCP-G4brzqDJ6ECW23yjSRITIMDx~v7-i~gt8ce276uliWfkn3SOhGZDxss5fvf8gM2HHy1-vnR2FlJQEzIe7TRg6kg7Ha2m-kIlMqVmQByE5P0~vTQoHIJjkYzyS67p4y-TUe8NlPS-4Q~YCXNWQBfMFJQU5f~rlEGDn82rga~49ZAbK1MALmiv0d-RKQ~3z3Vsm51VWrB8YRBljbSjBE9G1nKXRF6Lj6PNm8AwY3FLB1nLBU23iOa1~IrqhAbyoUWATo5a4w~l-g3haQx~3~bc9~xzZJ4hokldG~BlX9b-1mIGBAQxvulxjTCErS2OoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


