Abstract
Cofilin is a regulator of actin filament dynamics. We studied whether during platelet activation Rho kinase stimulates LIM kinase (LIMK) leading to subsequent phosphorylation and inactivation of cofilin. Platelet shape change and aggregation/secretion were induced by low and high concentrations of thrombin, respectively. We found that during these platelet responses Rho kinase activation was responsible for mediating rapid Thr508 phosphorylation and activation of LIMK-1 and for the F-actin increase during shape change and, in part, during secretion. Surprisingly, during shape change cofilin phosphorylation was unaltered, and during aggregation/secretion cofilin was first rapidly dephosphorylated by an okadaic acid–insensitive phosphatase and then slowly rephosphorylated by LIMK-1. LIMK-1 phosphorylation and cofilin dephosphorylation and rephosphorylation during aggregation were independent of integrin αIIbβ3 engagement. Cofilin phosphorylation did not regulate cofilin association with F-actin and was unrelated to the F-actin increase in thrombin-activated platelets. Our study identifies LIMK-1 as being activated by Rho kinase in thrombin-stimulated platelets. Two counteracting pathways, a cofilin phosphatase and LIMK-1, are activated during platelet aggregation/secretion regulating cofilin phosphorylation sequentially and independently of integrin αIIbβ3 engagement. Rho kinase–mediated F-actin increase during platelet shape change and secretion involves a mechanism other than LIMK-1–mediated cofilin phosphorylation, raising the possibility of another LIMK substrate regulating platelet actin assembly.
Introduction
Dynamic remodeling of actin structures underlies the different morphologic and functional platelet responses such as shape change, spreading, secretion, and aggregation.1,2 The remodeling of actin is mediated by factors that regulate actin polymerization and depolymerization, disassembly of existing filaments, formation of new filaments, crosslinking of filaments to networks, and bundling of actin filaments.3-5 They include signaling proteins that regulate actin dynamics as well as proteins that bind directly to actin and modulate the diverse actin structures. A key protein regulating actin remodeling is cofilin,6,7 an essential, ubiquitously expressed and highly conserved actin-binding protein. Binding of cofilin to actin filaments stabilizes a twisted form of F-actin, thereby weakening lateral subunit interactions and promoting filament severing and depolymerization.8,9 However, filament severing by cofilin also results in the generation of free barbed ends, which in turn is crucial for efficient enhancement of actin polymerization.10-12 Cofilin is therefore an actin dynamizing protein, which favors depolymerization or polymerization of actin, depending on the cellular content of actin filaments relative to actin monomers and free barbed ends.13 In unstimulated cells cofilin is present both in a phosphorylated and a nonphosphorylated form.14,15 The depolymerizing activity of cofilin and cofilin binding to actin are inhibited by its phosphorylation on Ser316 mediated by LIM kinases (LIMKs).17,18 Two forms of LIM kinase have been described, LIMK-1 and LIMK-2.19
Rho-like small GTPases such as Rho, Rac, and Cdc42 play a major role in regulating actin dynamics in stimulated cells. The kinase downstream of Rho-GTPase, the Rho kinase (p160ROCK), first isolated from platelets,20 plays a major role in platelet shape change.21,22 Rho kinase directly phosphorylates the 130-kDa myosin phosphatase targeting subunit (MYPT) of myosin phosphatase, thereby inhibiting the catalytic subunit of the enzyme leading to an increase of myosin light chain phosphorylation. Phosphorylated myosin interacts with actin filaments and develops actin-activated adenosine triphosphatase (ATPase) activity. These properties of myosin are important for the contraction of the actin cytoskeleton during shape change and facilitate platelet secretion.1,4 On the basis of in vitro and in vivo studies of transfected cells, it has been proposed that Rho kinase also phosphorylates and activates LIMKs,23 leading to cofilin phosphorylation and stabilization of actin cytoskeletal structures.17 LIMKs can also be phosphorylated by Rac-activated PAKs (p21-activated kinases).24 Both Rac25,26 and PAK27,28 are stimulated during platelet activation.
So far it is not known whether the LIMK/cofilin phosphorylation pathway is stimulated during physiologic activation of platelets. Also, it is ambiguous whether Rho-mediated Rho kinase activation or Rac-mediated PAK activation causes LIMK phosphorylation in physiologically activated platelets. We studied which forms of LIMK are expressed in platelets, whether LIMKs are activated on platelet activation, and whether Rho kinase is the kinase responsible for LIMK activation. We also analyzed whether LIMK activation leads to cofilin phosphorylation and whether a Rho kinase/LIM kinase/cofilin pathway might regulate the increase of F-actin underlying platelet shape change and secretion/aggregation stimulated by thrombin.
Our study identifies LIMK-1 as being rapidly activated by Rho kinase during shape change and aggregation. However, cofilin phosphorylation did not change during shape change, and unexpectedly cofilin was rapidly and reversibly dephosphorylated during aggregation. Two counteracting pathways, a cofilin phosphatase and LIMK-1, regulate cofilin phosphorylation differently during shape change and secretion.
Materials and methods
Reagents
Human thrombin (T-7009) was from Sigma (St Louis, MO). Y-27632, H-1152, okadaic acid, and Na3VO4 were from Calbiochem, Merck Biosciences GmbH (Darmstadt, Germany). RGDS peptide was from Bachem Biochemica (Heidelberg, Germany). Chrono-Lumi luciferase luciferin reagent was from Chrono-Log (Havertown, PA). Alexa Fluor-546 phalloidin was from Molecular Probes (Eugene, OR). Anti–phospho-MYPT (Thr696) and anti–phospho-MYPT (Thr853) were from Upstate Biotechnology (Lake Placid, NY). Anti-cofilin antibody (ACFL02) was from Cytoskeleton (Denver, CO). Anti–LIMK-1, anti–LIMK-2, and anti–phospho-LIMK-1/LIMK-2 (Thr508/505) antibodies were from Cell Signaling Technology (Beverly, MA). In the initial experiments, the anti–phospho-cofilin (Ser3) antibody used was produced and characterized by Meberg et al.15 During progress of the work a commercial antibody (from Cell Signaling Technology) was used. The unphosphorylated peptide (CKNDRKKRYTVVGN) and phosphorylated peptide (CKNDRKKRYTPVVGN) of the LIM kinase domain, which are identical in LIMK-1 and LIMK-2, were synthesized by Dr G. Arnold (Genzentrum, Munich, Germany). Protein A–Sepharose (P-3391) was from Sigma. λ Protein phosphatase (P0753) was from New England Biolabs (Beverly, MA). All other materials were obtained as reported previously.29
Isolation of human platelets and measurement of platelet shape change, aggregation and ATP secretion
Approval was obtained from the Ethic Commission of the Medical Faculty of the University of Munich. Informed consent was provided according to the Declaration of Helsinki. Platelets from acetylsalicylic acid–treated human blood were isolated by centrifugation in the presence of apyrase and resuspended in a buffer (pH 7.4) containing 20 mM HEPES, 138 mM NaCl, 2.9 mM KCl, 1 mM MgCl2, and 0.6 ADPase U/mL apyrase (4 × 108 cells/mL) as described previously.30 Platelet shape change induced by thrombin (0.075 U/mL) or aggregation and secretion induced by thrombin (0.5 U/mL) were measured by recording the light transmission in an aggregometer as described.29,30 Y-27632 (20 μM) and H-1152 (20 μM) were added 30 minutes and RGDS (0.5 mM) was added 2 minutes before platelet stimulation.
Isolation of platelet F-actin
F-actin was isolated as described by Kovacsovics and Hartwig31 with slight modifications. Unstimulated and stimulated platelet suspensions (0.1 mL) were lysed with equal volume of buffer containing 2% Triton X-100, 100 mM Tris-HCl, 10 mM EGTA, 10 mM NaF, 2 mM Na3VO4, phosphatase cocktail inhibitor-I (1:100 dilution), and one tablet of complete-mini protease inhibitor per 10 mL buffer, pH 7.4. After 5 minutes of lysis, the Triton X-100–insoluble fraction was separated by centrifugation at 100 000g for 30 minutes at 4°C using a tabletop ultracentrifuge (Optima TLX; Beckman Instruments, Palo Alto, CA). Pellets were washed twice with ice-cold phosphate-buffered saline and dissolved in SDS-PAGE sample buffer (62.5 mM Tris, 2% SDS, 5% glycerol, 5% 2-mercaptoethanol, 1 mM EDTA, pH 6.8). Proteins were separated on 12% polyacrylamide gels. Actin was stained with Coomassie brilliant blue, and cofilin in the F-actin fraction was immunoblotted. Actin and cofilin were quantified by densitometry.
Expression, purification, and antibody production against the kinase domain of LIMK-2
The cDNA encoding LIMK-2 was kindly provided by Prof Kensaku Mizuno (Tohoku University, Sendai, Japan). The plasmid coding for GST and the kinase domain (315-638 amino acids) of LIMK-2 were constructed and subcloned into EcoRI and XhoI sites of the pGEX5X-2 vector. GST kinase was expressed in Escherichia coli. The cells were grown at 16°C and induced with 0.1 M IPTG for 12 hours. GST kinase was purified on glutathione-Sepharose (Amersham Bioscience, Buckinghamshire, England) according to instructions of the manufacturer. Antiserum against purified GST kinase was raised in rabbits (Pab Production, Herbertshausen, Germany).
Expression of EGFP–LIMK-2 in endothelial cells
The cDNA encoding LIMK-2 was subcloned into EcoRI and SalI sites of pEGFP-C1 vector (Clontech, Franklin Lakes, NJ) to obtain LIMK-2 fused with EGFP. Human umbilical venous endothelial cells were cultured and transfected with EGFP–LIMK-2 plasmid as described.32,33 The cells were lysed in SDS sample buffer and immunoblotted with anti–LIMK-1 and anti–LIMK-2 antibodies to test their specificity.
Immunoblotting and measurement of protein phosphorylation
Aliquots (100 μL) of unstimulated and stimulated platelet suspensions were transferred from the aggregometer cuvettes to an equal volume of 2 × SDS-PAGE sample buffer. In case of aggregation, the reactions were quenched at given time points by adding an equal volume of 2 × SDS-PAGE sample buffer directly to the platelet suspensions. Proteins were separated on 12% SDS-PAGE (cofilin and phospho-cofilin) or 10% SDS-PAGE (phospho-MYPT, LIMK-1, LIMK-2, and phospho-LIMK). Equal amounts of samples were loaded on 2 gels in parallel, for detecting the unphosphorylated and phosphorylated form of the proteins. Proteins were blotted to nitrocellulose membranes using the Mini Trans-Blot electrophoresis cell (Bio-Rad, Hercules, CA). Membranes were blocked with 5% (wt/vol) nonfat milk in TBS (Tris-buffered saline) and incubated with the respective primary and secondary antibodies. The dilutions of the primary antibodies were anti–GST kinase antibody (1:1000); anti-cofilin (1:12 000); both anti–phospho-MYPT antibodies (1:3000); and anti–phospho-cofilin, anti–LIMK-1, anti–LIMK-2, and anti–phospho-LIMK (1:1000). The dilution of horseradish peroxidase–linked secondary antirabbit and antimouse antibodies was 1:5000. The membranes were developed with Super Signal West Pico Chemiluminescent (Pierce, Rockford, IL) and exposed to Hyperfilm (Amersham). The films were scanned into TIF format using ScanJet 5300C (Hewlett-Packard, Palo Alto, CA). Calibration of the scanner to an optical density scale (Kodak step tablet st34; Eastman Kodak, Rochester, NY) and densitometric analysis of the proteins were done using the public domain of National Institutes of Health (NIH, Bethesda, MD) ImageJ (1.34s) software. The densitometric values of phosphorylated proteins were divided by the corresponding values of unphosphorylated proteins, respectively. Absorption of proteins in unstimulated control samples was set to 100%. Data are presented as mean ± SD of individual experiments from different blood donors.
Cofilin and phospho-cofilin in resting platelets were separated by isoelectric focusing electrophoresis. The isoelectric focusing gels consisted of 4% pH 7-9 ampholyte, 1% 3-10 ampholyte (Serva, Heidelberg, Germany), 5% glycerol, 6% acrylamide, 6 M urea, polymerized with 0.015% ammonium persulfate, 0.0005% riboflavin 5-phosphate, and 0.03% TEMED (0.4-mm thick). Gels were run for 30 minutes at 100 V, 30 minutes at 200 V, and 2 hours at 450 V. Proteins were detected by anti-cofilin immunoblot.34
Immunoprecipitation of LIMK-1 from platelets
The experiments were carried out in the presence of RGDS to avoid platelet aggregation. Platelet suspensions (4 × 108/mL, 0.4 mL) were stimulated with thrombin in the presence or absence of the inhibitor Y-27632. Samples were lysed in an equal volume of 2 × immunoprecipitation lysis buffer (2% NP40, 300 mM NaCl, 20 mM Tris [pH 7.5], 2 mM EGTA, 2 mM EDTA, 5 mM Na3VO4, complete mini protease inhibitor 1 tablet/5 mL, phosphatase cocktail 1:100, and 0.1% SDS) for 45 minutes on ice. The lysates were clarified by centrifugation at 16 000g for 15 minutes, and then 40 μL 50% protein A–Sepharose slurry was added to the supernatants and incubated for 1 hour at 4°C to preclear the supernatant. Protein A–Sepharose was prepared by incubating the beads in swelling buffer (20 mM NaH2PO4, 0.15 M NaCl, and 0.1% NaN3) containing 2% BSA to block unspecific binding. Precleared supernatants were incubated overnight with anti–LIMK-1 antibody (1:50 dilution) followed by addition of 80 μL 50% protein A–Sepharose slurry and incubation at 4°C for 1 hour. The immunoprecipitates were collected by centrifugation at 16 000g for 25 seconds, washed 3 times with 1 mL ice-cold 1 × immunoprecipitation lysis buffer, and processed for immunoblot analysis or for the kinase assay. All steps were performed at 4°C.
LIMK-1 kinase assay
The protein concentration of the immunoprecipitated samples was measured using dotMETRIC protein assay kit (Geno Technology, St Louis, MO). For the kinase assay, equal amount of protein was added to 100 μL kinase buffer containing 50 mM HEPES (pH 7.5), 5 mM MgCl2, 10 mM NaF2, and 1 mM Na3VO4. The reaction was started by the addition of ATP (10 mM) and λ-phosphatase–treated His-tagged cofilin (16 μg) and incubated for 1 hour at 37°C. Reactions were quenched by adding 100 μL 2 × SDS-PAGE sample buffer. The samples were then immunoblotted with anti–phospho-cofilin and anti-cofilin antibodies. The activity of LIMK-1 was measured by cofilin phosphorylation.
Immunofluorescence
Resting and activated platelet suspensions (0.1 mL) were fixed with an equal volume of buffer containing 7.4% formaldehyde for 10 minutes at room temperature and spun onto polylysine-coated coverslips at 800g for 5 minutes. Platelets were permeabilized with PBS containing 0.2% Triton X-100 for 5 minutes, washed thrice with PBS, and incubated with Alexa Fluor 546 phalloidin (60 nM) for 15 minutes to stain F-actin. After having been washed 3 times with PBS, coverslips were mounted on glass slides with mounting medium (Gel/Mount; Biomedia, Foster City, CA). Platelet fluorescence was observed using a Zeiss LSM510 confocal laser-scanning microscope (Carl Zeiss, Jena, Germany) and a Plan-Apochromat 63×/1.4 numeric aperture oil differential interference contrast objective. Stacks of images were analyzed using Zeiss LSM510 Meta software.
Results
F-actin increase during thrombin-induced platelet shape change is mediated by Rho kinase activation
Washed platelets were stimulated with low concentrations of thrombin (0.075 U/mL) to induce selectively platelet shape change, without platelet aggregation (Figure 1A) and secretion (data not shown). As observed previously,22 shape change after this low concentration of thrombin was inhibited by Y-27632, indicating that shape change was mediated by Rho kinase activation. Thrombin induced in the same samples an increase in F-actin from 43% ± 2% of total in resting platelets to 55% ± 3% (mean ± SD, n = 4) 30 seconds after thrombin stimulation which was inhibited by Y-27632 (Figure 1B). Interestingly, Y-27632 increased significantly the F-actin content in unstimulated platelets.
To analyze Rho kinase activation, phosphorylation of one of its substrates, the MYPT was measured. Thrombin induced a rapid irreversible increase of MYPT phosphorylation at Thr853 (about 2-fold) during shape change (Figure 1C). The established Rho kinase inhibitor Y-2763235 abolished the thrombin-induced increase of MYPT phosphorylation (Figure 1C). These results indicate that Rho kinase is activated during shape change and mediates the F-actin increase underlying shape change.
The Rho kinase inhibitor Y-27632 inhibits shape change, F-actin increase, and MYPT phosphorylation induced by thrombin. Platelets were incubated with Y-27632 (20 μM) or solvent (H2O) for 30 minutes and then stimulated by thrombin (0.075 U/mL). (A) Inhibition of shape change by Y-27632. Shape change was measured by the decrease in light transmission, (left) and by confocal fluorescence microscopy of platelets stained for F-actin with Alexa Fluor 546 phalloidin (right). Bar, 10 μm. (B) Effect of Y-27632 on F-actin content during shape change. Values are mean + SD of 4 independent experiments. *Statistically significant; P < .05 with respect to time (0 seconds). †Significance between control (□) and Y-27632 (▪)–treated unstimulated platelets. (C, left) Platelet lysates were immunoblotted with anti–phospho-Thr853-MYPT antibody. Graphic representation of the result of MYPT phosphorylation in thrombin (0.075 U/mL)–stimulated platelets, evaluated by densitometry. Values are mean + SD of 3 experiments with platelets from different donors. (Right) Representative Western blot of MYPT phosphorylation in the absence and presence of Y-27632.
The Rho kinase inhibitor Y-27632 inhibits shape change, F-actin increase, and MYPT phosphorylation induced by thrombin. Platelets were incubated with Y-27632 (20 μM) or solvent (H2O) for 30 minutes and then stimulated by thrombin (0.075 U/mL). (A) Inhibition of shape change by Y-27632. Shape change was measured by the decrease in light transmission, (left) and by confocal fluorescence microscopy of platelets stained for F-actin with Alexa Fluor 546 phalloidin (right). Bar, 10 μm. (B) Effect of Y-27632 on F-actin content during shape change. Values are mean + SD of 4 independent experiments. *Statistically significant; P < .05 with respect to time (0 seconds). †Significance between control (□) and Y-27632 (▪)–treated unstimulated platelets. (C, left) Platelet lysates were immunoblotted with anti–phospho-Thr853-MYPT antibody. Graphic representation of the result of MYPT phosphorylation in thrombin (0.075 U/mL)–stimulated platelets, evaluated by densitometry. Values are mean + SD of 3 experiments with platelets from different donors. (Right) Representative Western blot of MYPT phosphorylation in the absence and presence of Y-27632.
Rho kinase stimulates LIMK-1 during thrombin-induced shape change
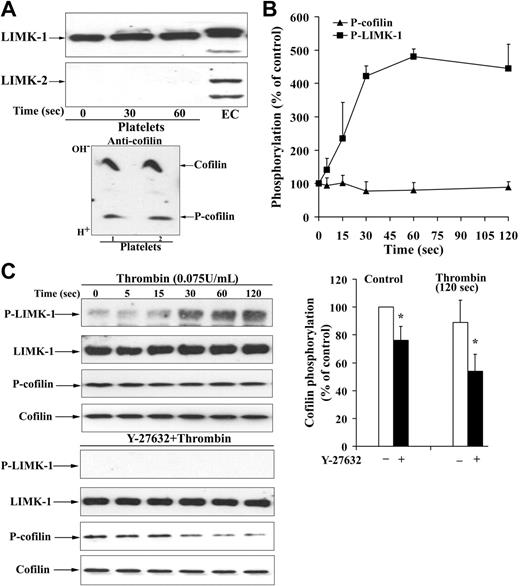
To analyze how Rho kinase activation regulates the increase of F-actin, the activation of LIM kinases known to phosphorylate and inactivate cofilin was analyzed. We investigated first whether platelets contain LIM kinases, and which type of LIM kinase is expressed in platelets. The polyclonal antibody we had produced against the recombinant kinase domain of LIMK-2 recognized a specific band at 72 kDa in platelets (data not shown). Because this antibody might not distinguish between LIMK-1 and LIMK-2, specific peptide antibodies against these 2 forms of LIMKs were used. The specificity of the anti–LIMK-1 and anti–LIMK-2 was probed by immunoblotting endothelial cells transfected with different GFP–LIMK-2 constructs. The anti–LIMK-2 antibody identified the different GFP–LIMK-2 constructs at their respective positions in the immunoblot, whereas the anti–LIMK-1 antibody did not (data not shown). LIMK-1 but not LIMK-2 is expressed in platelets (Figure 2A). In contrast, endothelial cells contain both LIMK-1 and LIMK-2.
To explore whether LIMK-1 phosphorylation was increased during shape change, a specific anti–phospho-LIMK antibody was used, which recognizes the phosphorylated Thr508 in the kinase domain. LIMK-1–Thr508 phosphorylation increased during shape change, and LIMK-1 phosphorylation was rapid and irreversible (Figure 2B). LIMK-1 phosphorylation was slightly slower than MYPT phosphorylation (compare with Figure 1C). The specificity of the anti–phospho-LIMK antibody was probed using the respective unphosphorylated and phosphorylated peptide (amino acids 500-512) of LIMK-1. Only the phosphorylated peptide blocked the signal of the phosphorylated LIMK-1 on immunoblots of activated platelets (data not shown). LIMK-1 phosphorylation was completely Rho kinase dependent, because it was abolished after platelet preincubation with Y-27632 (Figure 2C).
Regulation of cofilin phosphorylation and cofilin binding to F-actin in resting platelets and during shape change
In resting platelets, one third of total cofilin is present in its phosphorylated form as measured after separation of these 2 forms by isoelectric focusing and subsequent cofilin immunoblot (Figure 2A). Preincubation of platelets with Y-27632 completely inhibited basal LIMK-1 phosphorylation and decreased cofilin phosphorylation (Figure 2C). We investigated how cofilin phosphorylation was related to the amount of F-actin and the amount of cofilin associated with F-actin in resting platelets. Inhibition of LIMK-1 by Y-27632 reduced cofilin phosphorylation by 20% (Figure 2C), increased F-actin, and increased cofilin association with F-actin in resting platelets (Figures 1B, 3A). Therefore, LIMK-1–mediated cofilin phosphorylation might reduce F-actin and cofilin association with F-actin in resting platelets.
Unexpectedly, despite the rapid and pronounced LIMK-1 phosphorylation, we could not observe a concomitant increase in cofilin phosphorylation during shape change (Figure 2B). After pretreatment with Y-27632, thrombin induced a decrease of cofilin phosphorylation (from 76% ± 10% to 54% ± 12% of control, P < .05; Figure 2C). These results suggest that cofilin dephosphorylation by a cofilin phosphatase might mask the concomitant stimulation of cofilin phosphorylation by LIMK-1 during shape change.
Confirming previous studies,36 we found that cofilin only in its unphosphorylated form bound with F-actin in platelets, whereas the phosphorylated cofilin did not (data not shown). Because there was no net increase in the phospho-cofilin pool during the shape change, where an increase in F-actin was observed, we wondered whether the association of active unphosphorylated cofilin with F-actin was changed and analyzed the cofilin association with F-actin at different time intervals during shape change. We found a significant small increase of cofilin (about 5%) with platelet F-actin (Figure 3B). However, cofilin relative to F-actin did not increase as measured by the ratio of F-actin–associated cofilin with F-actin (Figure 3C). Furthermore, pretreatment of platelets to Y-27632 increased cofilin association with F-actin in resting platelets and inhibited the increase during shape change. Y-27632, which resulted in cofilin dephosphorylation of 40% 2 minutes after thrombin stimulation (Figure 2C), rather decreased than increased the association of cofilin with F-actin at this time point (Figure 3B-C). We conclude that the F-actin increase and the association of the small pool of cofilin with F-actin (5%) during shape change are regulated by a mechanism other than cofilin phosphorylation. No association of LIMK-1 and phospho–LIMK-1 with the actin cytoskeleton could be detected in resting or activated platelets (data not shown).
Rho kinase–dependent activation of LIMK-1 without stimulation of cofilin phosphorylation during shape change. (A, top) LIMK-1 but not LIMK-2 is expressed in platelets. Resting platelets, platelets stimulated for 30 and 60 seconds with thrombin, and endothelial-cell (EC) lysates were immunoblotted with specific anti–LIMK-1 and anti–LIMK-2 antibodies. (Bottom) Identification of cofilin in its unphosphorylated and phosphorylated states in resting platelets. Resting platelet lysates were subjected to isoelectric focusing (IEF) electrophoresis and subsequently immunoblotted with anti-cofilin antibody. Top band is the more basic, dephosphorylated form of cofilin and bottom band is the more acidic, phosphorylated form of cofilin. (B) LIMK-1 and cofilin phosphorylation during platelet shape change induced by thrombin (0.075 U/mL). Graphic representation of the result for LIMK-1 and cofilin phosphorylation. Values are the mean + SD for 3 independent experiments. (C) Effect of Y-27632 (20 μM) on LIMK-1 phosphorylation and cofilin phosphorylation in resting platelets and during thrombin (0.075 U/mL)–induced shape change. (Left) Representative immunoblots of platelets blotted with anti–phospho-LIMK-1/LIMK-2 (Thr508/505), anti–LIMK-1, anti–P-cofilin (Ser3) and anti-cofilin antibodies. (Right) Bar diagram showing cofilin phosphorylation of nontreated (□) and Y-27632-treated (▪) platelets in control and after thrombin stimulation (120 seconds). Values for cofilin phosphorylation in resting platelets and activated platelets are mean + SD of 8 and 4 independent experiments, respectively. *Statistically significant; P < .05 with respect to nontreated control.
Rho kinase–dependent activation of LIMK-1 without stimulation of cofilin phosphorylation during shape change. (A, top) LIMK-1 but not LIMK-2 is expressed in platelets. Resting platelets, platelets stimulated for 30 and 60 seconds with thrombin, and endothelial-cell (EC) lysates were immunoblotted with specific anti–LIMK-1 and anti–LIMK-2 antibodies. (Bottom) Identification of cofilin in its unphosphorylated and phosphorylated states in resting platelets. Resting platelet lysates were subjected to isoelectric focusing (IEF) electrophoresis and subsequently immunoblotted with anti-cofilin antibody. Top band is the more basic, dephosphorylated form of cofilin and bottom band is the more acidic, phosphorylated form of cofilin. (B) LIMK-1 and cofilin phosphorylation during platelet shape change induced by thrombin (0.075 U/mL). Graphic representation of the result for LIMK-1 and cofilin phosphorylation. Values are the mean + SD for 3 independent experiments. (C) Effect of Y-27632 (20 μM) on LIMK-1 phosphorylation and cofilin phosphorylation in resting platelets and during thrombin (0.075 U/mL)–induced shape change. (Left) Representative immunoblots of platelets blotted with anti–phospho-LIMK-1/LIMK-2 (Thr508/505), anti–LIMK-1, anti–P-cofilin (Ser3) and anti-cofilin antibodies. (Right) Bar diagram showing cofilin phosphorylation of nontreated (□) and Y-27632-treated (▪) platelets in control and after thrombin stimulation (120 seconds). Values for cofilin phosphorylation in resting platelets and activated platelets are mean + SD of 8 and 4 independent experiments, respectively. *Statistically significant; P < .05 with respect to nontreated control.
Analysis of the Rho kinase/LIMK-1 pathway, cofilin phosphorylation, F-actin increase, and cofilin/F-actin association during thrombin-induced platelet aggregation and/or secretion; LIMK-1 phosphorylation and regulation of cofilin phosphorylation are independent of integrin αIIbβ3
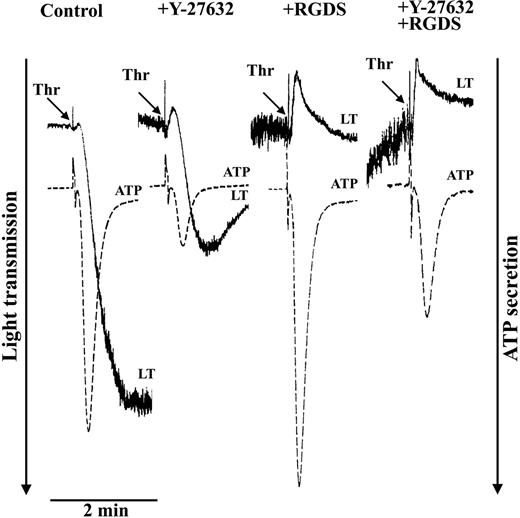
Platelets were stimulated with thrombin (0.5 U/mL) to induce platelet aggregation and secretion (Figure 4). Because Rho kinase phosphorylates MYPT not only at Thr85337 but also at Thr696,38 both phosphorylation sites were measured after thrombin stimulation. Thrombin induced a rapid and reversible increase of MYPT phosphorylation at both Thr696 (Figure 5A) and Thr853 (not shown) which was not only inhibited by Y-27362 (Figure 6A) but also by the new specific Rho kinase inhibitor H-115239 20 μM; data not shown). Y-27632 inhibited thrombin-induced aggregation and secretion (Figure 4). The Y-27632–mediated inhibition of secretion was also observed in the presence of RGDS, indicating that inhibition of aggregation by Y-27632 is due to inhibition of secretion. These results suggest a role of a Rho kinase–dependent pathway in platelet secretion.
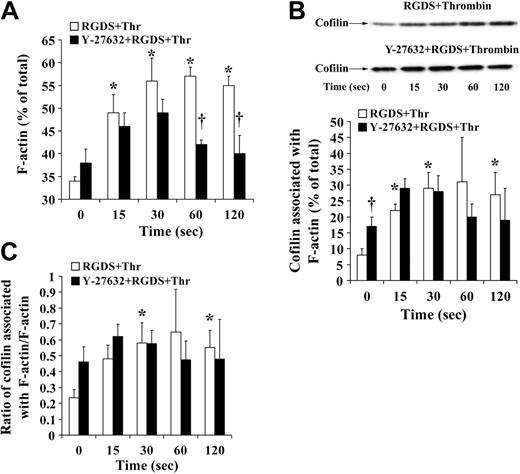
Cofilin association with F-actin during platelet shape change. (A, left, top) Representative gel and immunoblot of actin and cofilin, respectively. Total indicates whole platelets; F-actin, F-actin fraction of the same number of platelets. (Left, bottom) Immunoblot of cofilin associated with F-actin during thrombin-induced shape change. Effect of Y-27632. (Right) Graphic representation of the results. Values are the mean + SD for 4 independent experiments. *Statistically significant; P < .05 with respect to time (0 seconds) in nontreated samples. (B) Bar diagram showing the ratio of cofilin associated with F-actin to F-actin in nontreated platelets (□) and platelets treated with Y-27632 (▪) during thrombin-induced shape change. Values are the mean + SD for 4 independent experiments.
Cofilin association with F-actin during platelet shape change. (A, left, top) Representative gel and immunoblot of actin and cofilin, respectively. Total indicates whole platelets; F-actin, F-actin fraction of the same number of platelets. (Left, bottom) Immunoblot of cofilin associated with F-actin during thrombin-induced shape change. Effect of Y-27632. (Right) Graphic representation of the results. Values are the mean + SD for 4 independent experiments. *Statistically significant; P < .05 with respect to time (0 seconds) in nontreated samples. (B) Bar diagram showing the ratio of cofilin associated with F-actin to F-actin in nontreated platelets (□) and platelets treated with Y-27632 (▪) during thrombin-induced shape change. Values are the mean + SD for 4 independent experiments.
Inhibition of thrombin-induced aggregation and secretion by the Rho kinase inhibitor Y-27632. Platelets were stimulated with thrombin (0.5 U/mL) in the absence or presence of the integrin αIIbβ3 blocker RGDS (0.5 mM) and the Rho kinase inhibitor Y-27632 (20 μM). Representative tracings for change in light transmission (LT) and ATP secretion are shown.
Inhibition of thrombin-induced aggregation and secretion by the Rho kinase inhibitor Y-27632. Platelets were stimulated with thrombin (0.5 U/mL) in the absence or presence of the integrin αIIbβ3 blocker RGDS (0.5 mM) and the Rho kinase inhibitor Y-27632 (20 μM). Representative tracings for change in light transmission (LT) and ATP secretion are shown.
The reversible MYPT phosphorylation pattern during platelet aggregation was different from the irreversible time course of MYPT phosphorylation during shape change. MYPT phosphorylation even decreased under control levels in aggregated platelets (Figure 5A). These differences might be explained by an aggregation-dependent stimulation of dephosphorylation of MYPT at Thr696 and Thr853. Indeed, the tetrapeptide RGDS, which blocks the integrin αIIbβ3 and platelet aggregation, or the absence of stirring during platelet incubation with thrombin inhibited the dephosphorylation of MYPT at Thr696 and Thr853 completely (Figure 5A; data not shown). Therefore, in thrombin-aggregated platelets, the increase of MYPT phosphorylation is due to activation of Rho kinase, and the rapid and pronounced decrease of MYPT phosphorylation is due to activation of a phosphatase acting on MYPT Thr696 and Thr853 phosphorylation sites that is activated by engagement of integrin αIIbβ3.
The increase of LIMK-1 phosphorylation was higher during aggregation (13-fold) than during shape change (5-fold) (compare Figure 5B with Figure 2B). The phosphorylation of LIMK-1 in thrombin-aggregated platelets was again Rho kinase dependent, because it was completely inhibited by Y-27632 and H-1152 (Figure 6A; data not shown). Despite LIMK-1 activation, cofilin phosphorylation rapidly decreased during platelet aggregation/secretion. The decrease of cofilin phosphorylation was about 60% 30 seconds after thrombin addition and was followed by slow rephosphorylation (Figure 5C). LIMK-1 phosphorylation during aggregation was independent of integrin αIIbβ3 engagement, because the pattern of LIMK-1 phosphorylation was unchanged in the presence of RGDS or by the absence of platelet stirring (Figure 5B). Also the rapid dephosphorylation and subsequent slow rephosphorylation of cofilin were independent of outside-in signaling of integrin αIIbβ3 (Figure 5C).
In platelets preincubated with Y-27632 and stimulated with thrombin, no change in the initial phase of cofilin dephosphorylation was observed, but the subsequent rephosphorylation of cofilin was completely blocked (Figure 6B). Similar results were obtained with the novel and specific Rho kinase inhibitor H-1152 (data not shown). To measure LIMK-1 activity, LIMK-1 was immunoprecipitated from thrombin-stimulated and unstimulated platelets, and LIMK-P immunoblots and kinase assays using the specific LIMK substrate cofilin were performed. Thrombin stimulated both LIMK-1 phosphorylation and LIMK-1 activity in platelets, both of which were inhibited by Y-27632 (Figure 6C). Hence, these results strongly suggest that in thrombin-stimulated platelets cofilin rephosphorylation is mediated by Rho kinase activation of LIMK-1.
Thrombin-induced reversible phosphorylation of MYPT, irreversible phosphorylation of LIMK-1, but reversible dephosphorylation of cofilin during platelet aggregation and secretion: LIMK-1 phosphorylation and changes of cofilin phosphorylation are independent of integrin αIIbβ3engagement. Platelet suspensions were not treated (stirring [▪] and nonstirring [▵] conditions) or treated with RGDS (0.5 mM; □) for 2 minutes before stimulation with thrombin (0.5 U/mL). (A) Thrombin-induced MYPT phosphorylation. Platelet lysates were immunoblotted with anti–phospho-Thr696-MYPT antibody. (Left) Graphic representation of results. (Right) Representative immunoblot of MYPT phosphorylation. (B) Thrombin-induced LIMK-1 phosphorylation. Platelet lysates were immunoblotted with anti–phospho-LIMK-1/LIMK-2 (Thr508/505) antibody. (Left) Graphic representation. (Right) Representative immunoblots. (C) Cofilin dephosphorylation and rephosphorylation during thrombin-induced secretion/aggregation. Platelet lysates were immunoblotted with anti–phospho-cofilin antibody. (Left) Graphic representation of results. (Right) Representative immunoblots for cofilin phosphorylation. Values are mean + SD or mean – SD of 3 independent experiments.
Thrombin-induced reversible phosphorylation of MYPT, irreversible phosphorylation of LIMK-1, but reversible dephosphorylation of cofilin during platelet aggregation and secretion: LIMK-1 phosphorylation and changes of cofilin phosphorylation are independent of integrin αIIbβ3engagement. Platelet suspensions were not treated (stirring [▪] and nonstirring [▵] conditions) or treated with RGDS (0.5 mM; □) for 2 minutes before stimulation with thrombin (0.5 U/mL). (A) Thrombin-induced MYPT phosphorylation. Platelet lysates were immunoblotted with anti–phospho-Thr696-MYPT antibody. (Left) Graphic representation of results. (Right) Representative immunoblot of MYPT phosphorylation. (B) Thrombin-induced LIMK-1 phosphorylation. Platelet lysates were immunoblotted with anti–phospho-LIMK-1/LIMK-2 (Thr508/505) antibody. (Left) Graphic representation. (Right) Representative immunoblots. (C) Cofilin dephosphorylation and rephosphorylation during thrombin-induced secretion/aggregation. Platelet lysates were immunoblotted with anti–phospho-cofilin antibody. (Left) Graphic representation of results. (Right) Representative immunoblots for cofilin phosphorylation. Values are mean + SD or mean – SD of 3 independent experiments.
To explore which type of phosphatase might be involved in the rapid dephosphorylation of cofilin after platelet stimulation with thrombin, platelets were incubated with okadaic acid, a PP1/PP2A type phosphatase inhibitor. Okadaic acid did not inhibit cofilin dephosphorylation, indicating that cofilin is not dephosphorylated by a PP1/PP2A type phosphatase (data not shown).
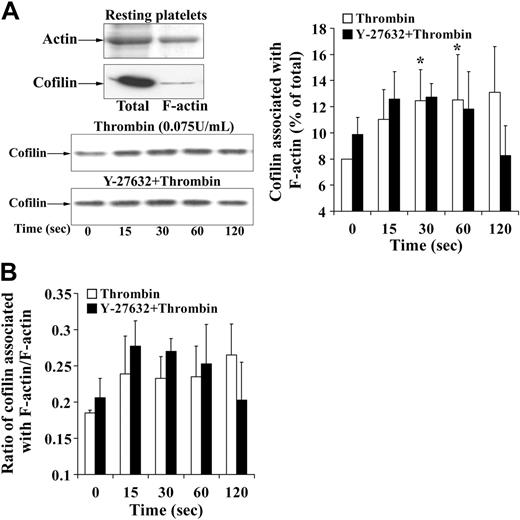
Platelet aggregation and secretion is associated with a different actin reorganization in platelets as compared with shape change.1 Because cofilin was rapidly dephosphorylated during platelet secretion independently of whether platelets aggregated or not (Figures 4 and 5C), we wondered whether the association of cofilin with F-actin was increased. Experiments were performed in the presence of RGDS to avoid possible artifacts during isolation of platelet F-actin associated with platelet aggregation (such as unspecific trapping of cofilin in aggregated platelets and inefficient lysis of platelet aggregates with Triton X-100). The presence of RGDS during stirring decreased F-actin in resting platelets as compared with control but did not change cofilin phosphorylation and the association of cofilin with F-actin (8%) in resting platelets (Figures 1B, 3B, Figure 7A-B, and 5C).
Confirming previous observations, we found that the increase in F-actin was higher during platelet secretion as compared with shape change: F-actin increased from 34% ± 1% in resting platelets to 56% ± 5% of total 30 seconds after addition of thrombin (Figure 7A). Cofilin association with F-actin increased from 8% ± 2% in resting platelets to 29% ± 5%. This increase was a net increase, because the ratio of F-actin–associated cofilin relative to F-actin increased 3-fold from 0.23 in resting platelets to 0.64 30 seconds after addition of thrombin (Figure 7C). These results suggest that the rapid cofilin dephosphorylation (maximum at 30 seconds) generating more active cofilin enhances its association with F-actin and increases F-actin during platelet secretion. Cofilin remained associated with F-actin 60 and 120 seconds after thrombin stimulation (Figure 7B-C), indicating that LIMK-1–mediated cofilin rephosphorylation did not cause cofilin dissociation from F-actin. Inhibition of Rho kinase/LIMK-1 led to a reduced F-actin increase at 30 seconds, did not reduce the rapid cofilin dephosphorylation and cofilin association with F-actin during the first 30 seconds (Figures 6A and 7A-B), and reversed the increase of F-actin and the cofilin association with F-actin (Figures 6A and 7A-B). We conclude that Rho kinase regulates the F-actin increase during platelet secretion through a mechanism other than cofilin phosphorylation and association with F-actin.
Discussion
Our study identifies LIMK-1 as being activated by Rho kinase in thrombin-stimulated platelets. Rho kinase mediated LIMK-1 phosphorylation during shape change and aggregation/secretion. Although LIMK-1 was activated, stimulation of cofilin phosphorylation was not observed. Either cofilin phosphorylation was found to be unchanged (as seen during shape change), or cofilin was reversibly dephosphorylated (as found during aggregation/secretion). Both LIMK-1 activation and changes of cofilin phosphorylation were independent of integrin αIIbβ3 engagement.
Activation of Rho kinase in activated platelets was measured using a new approach, that is, by quantifying the phosphorylation of the substrate MYPT on Thr69638 or Thr853.37 Rho kinase activation preceded the increase of F-actin during shape change, and Y-27632 inhibited the increase of F-actin during shape change and reduced and reversed the F-actin increase during secretion, indicating that Rho kinase activation mediates the increase of F-actin during shape change and, in part, secretion. We found that of the 2 isoforms of LIM-kinase only LIMK-1 is expressed in platelets. LIMK-1 was rapidly phosphorylated during shape change and secretion/aggregation. The kinetics of LIMK-1 phosphorylation during shape change and secretion were similar to the kinetics of Rho kinase activation as measured by MYPT phosphorylation, suggesting that Rho kinase phosphorylates LIMK-1 in intact platelets. Indeed, LIMK-1 phosphorylation was completely inhibited by 2 different Rho kinase inhibitors, Y-27632 and H-1152. Our study is to our knowledge the first to provide evidence that Rho kinase activates LIMK-1 during physiologic cell activation. Y-27632, which does not affect the activity of PAK even at high concentrations (100 μM)35 inhibited completely LIMK-1 phosphorylation during thrombin-induced platelet shape change, secretion, and aggregation and blocked the increase of LIMK-1 activity in thrombin-stimulated platelets. Moreover, the novel specific Rho kinase inhibitor, H-1152, provided similar results. Other groups using transfected cells have reported that LIMK-1 can be phosphorylated and activated either by p21-activated kinase (PAK)24 or Rho kinase.10 PAK has been shown to be activated during thrombininduced shape change and aggregation,27,28 raising the possibility that PAK might phosphorylate LIM kinase. Our study clearly shows that LIMK-1 phosphorylation and activation was entirely dependent on Rho kinase.
Inhibition of MYPT phosphorylation, LIMK-1 phosphorylation, LIMK-1 activity, and cofilin rephosphorylation by the Rho kinase inhibitor Y-27632 during thrombin-stimulated aggregation/secretion. Platelet samples stimulated by thrombin (0.5 U/mL) in the absence or presence of the Rho kinase inhibitor Y-27632 (20 μM) were immunoblotted with anti–phospho-MYPT, anti–phospho-LIMK-1/LIMK-2 (Thr508/505), and anti–phospho-cofilin antibodies. (A) Representative immunoblots showing concomitant inhibition of MYPT and LIMK-1 phosphorylation by Y-27632. (B) Immunoblot and graphic representation of the results of cofilin phosphorylation after thrombin stimulation of platelets in the absence (□) or presence of Y-27632 (▪). Values are mean + SD for 3 independent experiments. (C) LIMK-1 phosphorylation parallels LIMK-1 activity in thrombin-stimulated platelets. Inhibition by Y-27632. Platelets were preincubated with Y-27632 (20 μM) or solvent for 30 minutes and stimulated with thrombin in the presence of RGDS. LIMK-1 from resting and activated platelets (120 seconds) was immunoprecipitated. (Top) LIMK-1 immunoprecipitates (LIMK-1 IP) were blotted with anti–LIMK-1 antibody and anti–phospho-LIMK-1/LIMK-2 (Thr508/505) antibody. (Bottom) Immunoprecipitates were assayed for LIMK-1 activity using His-cofilin as substrate. Cofilin phosphorylation was measured by blotting the samples with anti–phospho-cofilin antibodies.
Inhibition of MYPT phosphorylation, LIMK-1 phosphorylation, LIMK-1 activity, and cofilin rephosphorylation by the Rho kinase inhibitor Y-27632 during thrombin-stimulated aggregation/secretion. Platelet samples stimulated by thrombin (0.5 U/mL) in the absence or presence of the Rho kinase inhibitor Y-27632 (20 μM) were immunoblotted with anti–phospho-MYPT, anti–phospho-LIMK-1/LIMK-2 (Thr508/505), and anti–phospho-cofilin antibodies. (A) Representative immunoblots showing concomitant inhibition of MYPT and LIMK-1 phosphorylation by Y-27632. (B) Immunoblot and graphic representation of the results of cofilin phosphorylation after thrombin stimulation of platelets in the absence (□) or presence of Y-27632 (▪). Values are mean + SD for 3 independent experiments. (C) LIMK-1 phosphorylation parallels LIMK-1 activity in thrombin-stimulated platelets. Inhibition by Y-27632. Platelets were preincubated with Y-27632 (20 μM) or solvent for 30 minutes and stimulated with thrombin in the presence of RGDS. LIMK-1 from resting and activated platelets (120 seconds) was immunoprecipitated. (Top) LIMK-1 immunoprecipitates (LIMK-1 IP) were blotted with anti–LIMK-1 antibody and anti–phospho-LIMK-1/LIMK-2 (Thr508/505) antibody. (Bottom) Immunoprecipitates were assayed for LIMK-1 activity using His-cofilin as substrate. Cofilin phosphorylation was measured by blotting the samples with anti–phospho-cofilin antibodies.
F-actin increase and association of cofilin with F-actin during thrombin-induced secretion: effect of Y-27632. Platelets treated with H2O (□) or Y-27632 (▪) were stimulated with thrombin (0.5 U/mL) in the presence of the integrin αIIbβ3 blocker RGDS (0.5 mM) and lysed with 1% Triton X-100 for isolation of F-actin. F-actin and cofilin associated with F-actin (percentage of total) were measured. (A) F-actin content, bar diagram. (B) Cofilin association with F-actin. (Top) Representative immunoblots. (Bottom) bar diagram (C) Bar diagram showing the ratio of F-actin–associated cofilin to F-actin. Values are the mean + SD for 4 independent experiments. *Statistically significant; P < .05 with respect to nonstimulated samples at 0 seconds. †Significance between control (□) and Y-27632 (▪)–treated platelets.
F-actin increase and association of cofilin with F-actin during thrombin-induced secretion: effect of Y-27632. Platelets treated with H2O (□) or Y-27632 (▪) were stimulated with thrombin (0.5 U/mL) in the presence of the integrin αIIbβ3 blocker RGDS (0.5 mM) and lysed with 1% Triton X-100 for isolation of F-actin. F-actin and cofilin associated with F-actin (percentage of total) were measured. (A) F-actin content, bar diagram. (B) Cofilin association with F-actin. (Top) Representative immunoblots. (Bottom) bar diagram (C) Bar diagram showing the ratio of F-actin–associated cofilin to F-actin. Values are the mean + SD for 4 independent experiments. *Statistically significant; P < .05 with respect to nonstimulated samples at 0 seconds. †Significance between control (□) and Y-27632 (▪)–treated platelets.
In agreement with observations made previously,14 we found that about one third of total cofilin is phosphorylated in resting platelets. We observed a significant decrease of cofilin phosphorylation in resting platelets after incubation with Y-27632, indicating that the Rho kinase/LIMK-1 pathway is needed to maintain this basal level of cofilin phosphorylation. Surprisingly, activation of LIMK-1 failed to increase cofilin phosphorylation during shape change. The lack of increase of cofilin phosphorylation is explained either by inaccessibility of cofilin for LIMK-1 in intact platelets and/or by simultaneous activation of a cofilin phosphatase. In support of the latter, we found after inhibiting LIMK-1 a decrease of cofilin phosphorylation during shape change, indicating that a cofilin phosphatase is activated during shape change counteracting the effect of LIMK-1 on cofilin. Therefore, we suggest that phosphocycling of cofilin increases as a result of simultaneous activation of both LIMK-1 and a cofilin phosphatase. A similar observation was made previously during neurite extension stimulated with growth factors.15 A further possibility for the lack of cofilin phosphorylation by LIMK-1 during shape change is the presence of another LIMK-1 substrate in platelets.
Platelets were stimulated with higher concentrations of thrombin to induce platelet aggregation and secretion subsequent to shape change. In agreement with a previous study showing that Rho and Rho kinase activation in platelets are upstream of integrin αIIbβ3,26 we found that Rho kinase activation and subsequent LIMK-1 activation were independent of integrin αIIbβ3 engagement in thrombin-aggregated platelets. Also cofilin dephosphorylation/rephosphorylation occurred independently, whether platelets aggregated or not, indicating that the regulation of cofilin phosphorylation was independent of integrin αIIbβ3.
The results of rapid cofilin dephosphorylation in thrombin-stimulated platelets are similar to previous and recent findings.14,40 However, in TRAP-stimulated platelets no decrease of cofilin phosphorylation, only an increase 3 to 5 minutes after activation was found.41 In contrast to our results, the study of Falet et al40 showed that during thrombin-induced platelet aggregation, cofilin remained dephosphorylated, suggesting that integrin αIIbβ3 activation leads to cofilin dephosphorylation. Also, a recent study in epithelial cells showed that β3 overexpression reduced phospho-cofilin levels.42 A possible explanation for the discrepancies between the study by Falet et al40 and our study might be the different methods used to isolate washed platelets. We applied a commonly used platelet preparation protocol, whereas Falet et al40 isolated platelets from plasma using a metrizamide gradient.
LIMK-1 phosphorylation during secretion/aggregation was rapid, irreversible, and higher than during shape change. In contrast to shape change, cofilin phosphorylation during platelet secretion was rapidly decreased followed by LIMK-1–mediated rephosphorylation to the level of resting platelets within 2 minutes. The results of inhibition of the Rho kinase/LIMK-1–mediated cofilin rephosphorylation with Y-27632 indicate that this pathway regulates primarily secretion, not aggregation. Y-27632 did not inhibit cofilin dephosphorylation up to 30 seconds. These results suggest that during platelet secretion the 2 counteracting pathways regulating cofilin phosphorylation, that is, cofilin phosphatase and LIMK-1, sequentially act on cofilin. The rapid and large dephosphorylation of cofilin shows that a cofilin phosphatase is activated very rapidly and that this enzyme has better access to its substrate phospho-cofilin than LIMK-1 (which is also activated rapidly) to cofilin. Cofilin might be dephosphorylated by a specific cofilin phosphatase such as the recently identified “slingshot,” which is insensitive to inhibition by okadaic acid.43 Such an assumption is supported by our results, showing that the PP1/PP2A-type phosphatase inhibitor okadaic acid did not affect cofilin dephosphorylation. Because LIMK-1 is rapidly activated in thrombin-stimulated platelets, but cofilin is rapidly dephosphorylated, one has also to assume a compartmentalization to explain these results. LIMK-1 is known to bind to a number of different proteins, including slingshot,44 14-3-3,45 and some transmembrane receptors,46 so even in its activated form it may not have direct access to cofilin. In summary, we interpret the cofilin phosphorylation data in thrombin-stimulated platelets by rapid activation of a cofilin phosphatase generating active cofilin which is after 30 seconds rephosphorylated and inactivated by LIMK-1. However, we cannot exclude that cofilin is phosphorylated in intact platelets by other kinases than LIMK-1.
We investigated how cofilin phosphorylation was related to the amount of F-actin and to the amount of cofilin associated with F-actin. The results with Y-27632 in resting platelets suggested that LIMK-1–mediated cofilin phosphorylation might reduce F-actin content and cofilin association with F-actin. However, during shape change there was no relation of the unchanged phospho-cofilin levels with the increase of F-actin (11%) and cofilin association with F-actin (5%). During the initial phase of thrombin-induced secretion (up to 30 seconds), cofilin dephosphorylation (Figure 5C) was associated with a larger increase of F-actin (22%) and a higher amount of cofilin associated with F-actin (21%) as compared with shape change (Figure 7A-B). However, cofilin rephosphorylation after 30 seconds did not decrease F-actin and cofilin association with F-actin. Moreover, Y-27632 pretreatment reversed the increase of F-actin and cofilin association with F-actin which was associated with an inhibition of cofilin rephosphorylation. We conclude that LIMK-1–mediated cofilin phosphorylation does not regulate the F-actin increase and cofilin association with F-actin in thrombin-activated platelets. Confirming our results, Falet et al40 showed that cofilin, but not phospho-cofilin, bound to F-actin and that the initial cofilin dephosphorylation was accompanied with an increase in F-actin content and increased association of cofilin with F-actin. However, in contrast to our results, cofilin rephosphorylation 5 to 10 minutes after thrombin stimulation was paralleled by its dissociation from F-actin. This difference in the study by Falet et al40 and ours might be due to different lysis methods used for isolation of F-actin. We observed that phalloidin, which was not used in our method but in the study by Falet et al,40 displaced cofilin from F-actin and caused F-actin increase during lysis (data not shown). The incubation time for platelet lysis is also critical to avoid changes that occur after platelet lysis.47
In conclusion, our study indicates that Rho kinase mediates rapidly LIMK-1 activation and the increase of F-actin during thrombin-induced shape change and aggregation/secretion. The rapid LIMK-1 activation did, however, not lead to an increased cofilin phosphorylation during shape change and the early phase of secretion. This might be explained by 2 counteracting pathways regulating cofilin phosphorylation, cofilin phosphatase, and LIMK-1, and by compartmentalization of LIMK-1 with reduced accessibility to cofilin in intact platelets. LIMK-1 activation and the pathways regulating cofilin phosphorylation were independent of integrin αIIbβ3 activation. Rho kinase regulates the F-actin increase during platelet shape change and secretion through a mechanism other than LIMK-1–mediated cofilin phosphorylation, raising the possibility that another LIMK-1 substrate regulates F-actin assembly in platelets.
Prepublished online as Blood First Edition Paper, October 11, 2005; DOI 10.1182/blood-2004-11-4377.
Supported by the Deutsche Forschungsgemeinschaft (DFG)–Graduate Program GK 438 “Vascular Biology in Medicine” (D.P. and P.G.), by grants of the August-Lenz-Stiftung, and DFG (SFB 413 and Si 274/9) (W.S.), and by the National Institutes of Health (grants GM35126 and NS40371) (J.R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Nicole Wilke for technical assistance. The results are part of the PhD thesis of D.P. at the University of Munich.


![Figure 5. Thrombin-induced reversible phosphorylation of MYPT, irreversible phosphorylation of LIMK-1, but reversible dephosphorylation of cofilin during platelet aggregation and secretion: LIMK-1 phosphorylation and changes of cofilin phosphorylation are independent of integrin αIIbβ3 engagement. Platelet suspensions were not treated (stirring [▪] and nonstirring [▵] conditions) or treated with RGDS (0.5 mM; □) for 2 minutes before stimulation with thrombin (0.5 U/mL). (A) Thrombin-induced MYPT phosphorylation. Platelet lysates were immunoblotted with anti–phospho-Thr696-MYPT antibody. (Left) Graphic representation of results. (Right) Representative immunoblot of MYPT phosphorylation. (B) Thrombin-induced LIMK-1 phosphorylation. Platelet lysates were immunoblotted with anti–phospho-LIMK-1/LIMK-2 (Thr508/505) antibody. (Left) Graphic representation. (Right) Representative immunoblots. (C) Cofilin dephosphorylation and rephosphorylation during thrombin-induced secretion/aggregation. Platelet lysates were immunoblotted with anti–phospho-cofilin antibody. (Left) Graphic representation of results. (Right) Representative immunoblots for cofilin phosphorylation. Values are mean + SD or mean – SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2004-11-4377/4/m_zh80020689380005.jpeg?Expires=1766129993&Signature=AmlgJB8XGP018rwYCjz1XvisMk1bk89jS17tKf7ok7Y~-D~biliR2to5Y-FgC4oEECFl9aKJNlmCGYrGkUGJhAEWfdXVCojsWEH~XVjooIBOyCxWib5VkSlosrmPhez0yDJbyo7MqfQqziS0fUs9IDq4TIk1EsQupoT7EHQ~GujL0IUq8g1f-adEdH4LnPZe4maRJVT7KEZ9UyV-MUAgWLCtWcRP7TnXczIEiENyIZGx5V0kddeeqO39-BKBVof8jJt1gKYwdadP488v7uj6AEpuryWht2ubw1sIRWSaQ8VyV2jQ2sY1CmGfLrId-xPIlyFAx0hLbRfhl-ha56NE0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)