Abstract
Kaposi sarcoma herpesvirus (KSHV) infection is consistently associated with primary effusion lymphomas (PELs) that are non-Hodgkin lymphomas of B-cell origin. All PEL cells are latently infected with KSHV and express latent viral proteins such as the viral cyclin (v-cyclin), which has previously been implicated in down-regulation of cell-cycle inhibitor p27KIP1 levels via phosphorylation on Thr187. PEL cells retain high levels of p27KIP1 but yet proliferate actively, which has left the biologic significance of this p27KIP1 destabilization somewhat elusive. We have recently demonstrated that v-cyclin and p27KIP1 stably associate in PEL cells. Here we demonstrate that v-cyclin together with its kinase partner CDK6 phosphorylates the associated p27KIP1 in PEL cells, which represent a biologically relevant model system for KSHV pathobiology. During latent viral replication p27KIP1 was phosphorylated by v-cyclin-CDK6 predominantly on Ser10, which enhances its cytoplasmic localization. Interestingly, upon reactivation of KSHV lytic cycle, v-cyclin-CDK6 phosphorylated p27KIP1 on Thr187, which resulted in down-regulation of p27KIP1 protein levels. These findings indicate that v-cyclin modulates the cell-cycle inhibitory function of p27KIP1 by phosphorylation in PELs, and also suggest a novel role for v-cyclin in the lytic reactivation of KSHV. (Blood. 2006;107:725-732)
Introduction
Kaposi sarcoma herpesvirus (KSHV) infection is strongly involved in a rare, AIDS-related form of B-cell lymphoproliferative disorder called primary effusion lymphoma (PEL).1-3 PEL is a non-Hodgkin-type lymphoma derived from post-germinal-center B cells.4 KSHV genome encodes several homologs of cellular proteins, which deregulate checkpoints in the signaling pathways, govern cell proliferation, and modulate apoptosis.5-7 All KSHV-positive PEL cells express latent viral proteins,8 suggesting a role for these proteins in oncogenesis of this lymphoma. One of these is the viral cyclin (v-cyclin), which is closely related to cellular cyclin D2 and is expressed both during the latency and the lytic viral replication cycle.9-11 As its cellular counterparts, v-cyclin associates with cyclin dependent kinases (CDKs), having the highest specificity toward CDK6.12,13 v-cyclin-CDK6 promotes cell-cycle progression by phoshorylating specific target proteins such as pRb and Histone H1. However, compared with cellular cyclin D-CDK4/6, v-cyclin-CDK6 has a broader substrate range in vitro which includes Id-2, cdc25A, ORC-1, CDC6, and Bcl-2.14-17
Cell-cycle progression and activity of cyclin-CDK complexes is regulated by the CIP/KIP family of inhibitors, which function by forming ternary complexes with cellular cyclin-CDKs.18,19 Association of p27KIP1 with cyclin E-CDK2 inhibits CDK2 kinase activity and cell-cycle progression. The function of CDK inhibitors is often altered in human cancer, and the CIP/KIP inhibitors are regulated primarily at posttranslational level. Phosphorylation of p27KIP1 is a known regulatory event occurring at the G1-S transition of the cell cycle. Phosphorylation of p27KIP1 on Thr187 residue by cyclin E-CDK2 complex20,21 is necessary for binding of p27KIP1 to Skp2 and subsequent destabilization.22 There are 3 putative phosphorylation sites on p27KIP1 that have the minimal consensus for CDKs, namely Ser10, Ser178, and Thr187. Recent studies indicate that phosphorylation of Ser10 is involved in nuclear export and plays a physiologic role in stabilization of the p27KIP1 protein in G0 phase.23-26 Thus, functional inactivation of p27KIP1 occurs either through loss of expression or through phosphorylation-dependent cytoplasmic sequestration.
Previous studies in overexpression models have shown that p27KIP1 is a substrate for the v-cyclin-CDK6 complex in vitro, and that by phosphorylation of p27KIP1 on Thr187, v-cyclin can bypass the growth arrest imposed by this inhibitor.14,15 However, this mechanism does not seem to be effective in PEL cells, which appear to consistently overexpress p27KIP1, and yet proliferate actively.27 We have recently demonstrated that v-cyclin and p27KIP1 stably associate in PEL cells, suggesting a mechanism by which p27KIP1 is inactivated in PEL cells.28 We have further addressed the mechanism of p27KIP1 inactivation by analyzing v-cyclin complexes purified using gel filtration chromatography of PEL cells. Here we show that p27KIP1 is phosphorylated in vivo by v-cyclin-CDK6. Moreover, our results suggest a new role for v-cyclin in the reactivation of PEL cells to KSHV lytic replication.
Materials and methods
Cell culture and transfections
Primary effusion lymphoma cell line BC-129 is derived from a human immunodeficiency virus (HIV)-positive patient. The cell line is co-infected with KSHV and Epstein-Barr virus (EBV) and was obtained from American Type Culture Collection (ATCC; Manassas, VA). The BC-3 cell line30 is negative for HIV and infected with KSHV, but not EBV. BC-3 cells were kindly provided by Dr Ethel Cesarman (Cornell Medical College, New York, NY). JOK-1 is a hairy-cell leukemia line (a gift from Dr Leif Andersson, University of Helsinki, Finland), which served as a KSHV-negative control cell line in the experiments. PEL cell lines and JOK-1 were cultured in a humidified 5% CO2 atmosphere at 37°C in RPMI 1640 medium supplemented with 15% fetal calf serum (FCS; Invitrogen, Carlsbad, CA), 100 U/mL penicillin G, and 100 μg/mL streptomycin. To induce KSHV lytic replication, cells were first synchronized31 and then treated with 20 ng/mL 12-O-tetradecanoyl phorbol-13-acetate (TPA; Sigma, St Louis, MO) per milliliter for 24 to 48 hours.31 U2OS human osteosarcoma cells were routinely cultured in a humidified 5% CO2 atmosphere at 37°C in Dulbecco modified Eagle medium (DMEM) containing 10% (wt/vol) FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin. U2OS cells were transiently transfected using Fugene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions.
Expression vectors
Mammalian expression vectors were as follows: pcDNA3-Myc-p27KIP1, pcDNA3-Myc-S10Ap27KIP1, and pcDNA-v-cyclin (a gift from Dr Sibylle Mittnacht, The Institute of Cancer Research, London, United Kingdom). Plasmid constructs for expressing recombinant glutathione-S-transferase (GST) fusion protein of the wild-type p27KIP1 (GST-p27KIP1) and the phosphorylation site mutants GST-p27KIP1 (S10A), GST-p27KIP1 (T187A), and GST-p27KIP1 (S10AT187A) were kindly provided by Dr Nisar Malek (The Institute for Molecular Biology, Hannover Medical School, Hannover, Germany).
Production of anti-v-cyclin antibodies
In order to produce recombinant v-cyclin fusion protein (GST-v-cyclin), the pCool vector (a modified version of pGEX-2T [Amersham Pharmacia Biotech, Piscataway, NJ]; a gift from Dr N. Pavletich, Memorial Sloan-Kettering Cancer Center, New York, NY) was expressed in Escherichia coli.32 The fusion protein was then bound to the glutathione 4B sepharose beads. The beads were washed 2 times with cold phosphate-buffered saline (PBS) to remove protease inhibitors followed by incubation with thrombin buffer (1mM CaCl2; 50 mM Tris, pH 8.0; 5 U/mL thrombin; Sigma, Steinheim, Germany) for 18 hours at 14°C. Rabbit polyclonal immunoglobulin G (IgG) antibodies directed against the thrombin cleaved recombinant v-cyclin were produced by BIOTREND Chemikalien (Köln, Germany).
Antibodies and reagents
Rabbit polyclonal antibodies to p27KIP1 (C-19), p-p27(Ser 10), CDK6 (C-21), CDK2 (M2), and Sp1 (PEP-2), and mouse monoclonals to β-tubulin (D-10) and actin (C-2) were all purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal antibody to CDK4 (DCS-35) and CDK6 (Ab-3; Cocktail) were from NeoMarkers (Fremont, CA). Anti-human p27KIP1/Kip-1 mouse monoclonal antibody was from Upstate Biotechnology (Lake Placid, NY), and rabbit polyclonal antibody to p-p27 (Thr187) was from Zymed (PT187; San Francisco, CA). Mouse monoclonal antibody recognizing the Myc-epitope (9E10) was from Babco (Berkeley, CA). Anti-ORF59 mouse monoclonal antibody was a kind gift from Dr Bala Chandran (The University of Kansas Medical Center, Kansas City, MO). Alexa Fluor 488 goat antirabbit and Alexa Fluor 594 goat antimouse antibodies were from Molecular Probes (Eugene, OR). Bisbenzimide Hoechst 33342 and Protein A Sepharose were obtained from Sigma-Aldrich Chemical (St Louis, MO). Recombinant proteins were produced in E coli and purified using Glutathione Sepharose 4B (Amersham Pharmacia Biotech, Uppsala, Sweden).14
Gel filtration chromatography
Cell lysates prepared in ELB lysis buffer (150 mM NaCl; 50 mM HEPES, pH 7.4; 0.1% Igepal; 5 mM EDTA; 2 mM DTT; 1 mM phenylmethylsulfonyl fluoride [PMSF]; 2 μg/mL leupeptin; 2 μg/mL pepstatin; and 1.5 μg/mL aprotinin) were passed through a 0.22-μm pore size MILLEX-GS filter (Millipore, Billerica, MA) and fractionated on a Superdex 200 HR column with a fast protein liquid chromatography (FPLC) system (Pharmacia Biotech, Uppsala, Sweden). Samples were loaded onto the column and separated in gel filtration buffer (50 mM HEPES, pH 7.5; 150 mM NaCl) at a flow rate of 0.3 mL/min. The molecular mass standards (Sigma) used to calibrate the column were blue dextran (2000 kDa) thyroglobulin (669 kDa), apoferritin (443 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), bovine serum albumine (BSA; 66 kDa), and carbonic anhydrase (29 kDa). For each fractionation 30 fractions of 0.5 mL were collected. Fifty microliters of each fraction was used for immunoblotting and 450 μL of each fraction for immunoprecipitation experiments.
Immunoprecipitation assay and immunoblotting
Cells were lysed in ELB lysis buffer, and lysates were then clarified by centrifugation at 20 800 g for 15 minutes at 4°C. For immunoprecipitation, whole-cell lysates (300 μg) or lysates fractionated by gel filtration chromatography were precleared by incubation with protein A-Sepharose beads for 2 hours at 4°C. Samples were then immunoprecipitated as described previously.28 For blocking treatments, 1 μg v-cyclin antibody was pretreated with 10 μg GST-v-cyclin (competing antigen) or BSA (nonspecific protein for the control) at room temperature (RT) for 2 hours prior to addition to the lysate. Proteins were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) according to standard protocols.
In vitro kinase assays
To determine phosphorylation of endogenous coprecipitated proteins, whole-cell extracts from PEL cells or fractions from gel filtration chromatography were incubated for 2 hours at 4°C with the rabbit polyclonal anti-v-cyclin antibody. Immunocomplexes were coupled to protein A Sepharose beads for an additional 2 hours at 4°C and washed 3 times with the lysis buffer followed by 2 washes with the kinase buffer (20 mM Tris, pH 7.5; 50 mM KCl; 7.5 mM MgCl2; 10mM MnCl2 1 mM DTT; 25 mM β-glycerophosphate; 2 μg/mL leupeptin; 2 μg/mL pepstatin; and 1.5 μg/mL aprotinin). Kinase reactions were performed by incubating the immunocomplexes with 10 μL kinase buffer containing 2 μCi (0.074 MBq) [32P] adenosine triphosphate (ATP) for 20 minutes at 30°C. For measuring the in vitro kinase activity toward Histone H1 (Roche Diagnostics), GST-pRb,33 or GST-p27KIP1, 2 μg Histone H1 and GST-pRb or 0.5 μg GST-p27KIP1 were added to the kinase reactions as substrates. Reactions were terminated by the addition of 5 × SDS-PAGE sample buffer, and resolved in SDS-PAGE and autoradiography. The extent of [32P] incorporation into the substrates was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer (Amersham Biosciences, Piscataway, NJ). The level of [32P] incorporation obtained from autoradiographs using the v-cyclin immunoprecipitates from JOK-1 (a KSHV-negative control cell line) represented the background level, which was subtracted from the values obtained with v-cyclin immunoprecipitates from PEL cell lines. Immunodepletion experiments were performed as described previously.28 Quantitative analysis of incorporated radioactivity was performed with the Typhoon 9400 Image Quant analyzer.
Cytocentrifugation and indirect immunofluorescence
PEL cells (1 × 105 cells/0.2 mL) were applied onto glass slides by cytocentrifugation (Cytospin; Shandon, Pittsburgh, PA) at 110 g for 3 minutes. Slides were air dried for 10 minutes, fixed with 3.5% (wt/vol) paraformaldehyde (PFA), and permeabilized with 0.5% NP-40 for 5 minutes. Transfected cells on coverslips were fixed with 3.5% (wt/vol) paraformaldehyde (PFA) and permeabilized with 0.1% Triton X-100 for 5 minutes. Immunofluorescence labeling was performed as before.28 The fluorochromes were visualized with a Zeiss Axioplan 2 fluorescent microscope (Carl Zeiss, Jena, Germany) equipped with Zeiss PLAN-NEOFLUOR × 40/0.50 numeric aperture objective lenses. Images were acquired with a Zeiss Axiocam H RC, using Zeiss AxioVision and Adobe Photoshop 7.0 (Adobe, San Jose, CA) software.
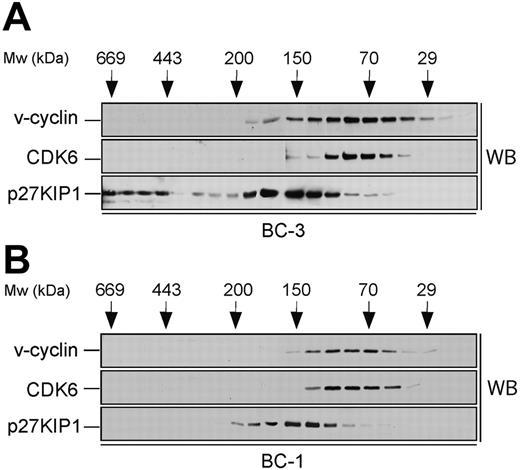
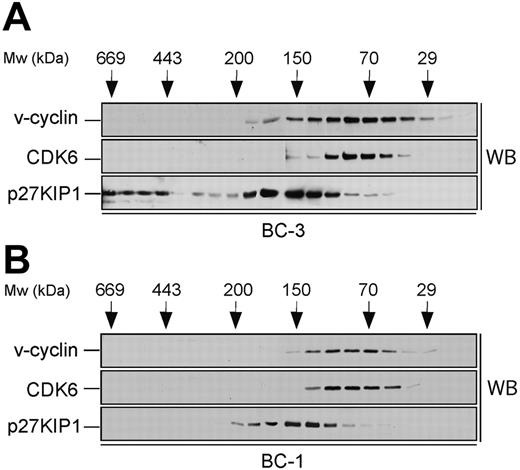
Size fractionation of PEL cell lysates. Lysates from BC-3 (A) and BC-1 (B) cell lines were separated using gel filtration chromatography on a Superdex 200HR column. Fractions were resolved by SDS-PAGE (12%) and analyzed by Western blotting (WB) with antibodies against v-cyclin, CDK6, and p27KIP1. The elution profile of the molecular weight (Mw) standards is indicated on the top.
Size fractionation of PEL cell lysates. Lysates from BC-3 (A) and BC-1 (B) cell lines were separated using gel filtration chromatography on a Superdex 200HR column. Fractions were resolved by SDS-PAGE (12%) and analyzed by Western blotting (WB) with antibodies against v-cyclin, CDK6, and p27KIP1. The elution profile of the molecular weight (Mw) standards is indicated on the top.
Subcellular fractionation
Cells at a concentration of 5 × 107 cells/mL were resuspended in hypotonic lysis buffer (20 mM Tris, pH 7.5; 10 mM NaCl; 1.5 mM MgCl2; 2 mM EDTA; 0.1% Triton X-100; MnCl2; 20% glycerol; 1 mM DTT; 25 mM β-glycerophosphate; 2 μg/mL leupeptin; 2 μg/mL pepstatin; and 1.5 μg/mL aprotinin). Samples were incubated on ice for 5 minutes with gentle mixing. Nuclei were pelleted by spinning at 180 g for 5 minutes at 4°C. The supernatant (cytoplasm) was then carefully removed from the pellet, which was washed with PBS. Cytoplasmic extract was further clarified by centrifugation at 20 800 g for 15 minutes at 4°C. The nuclear pellet was resuspended in hypotonic lysis buffer containing 0.5M NaCl and vortexed 2 × 10 sec. Nuclei were spun at 20 800 g for 15 minutes at 4°C. Sp1 (specificity protein 1)34 was used as a nuclear protein marker to follow the efficiency of the fractionation procedure. β-tubulin was used as a marker for the cytoplasm.
Results
v-cyclin forms a binary complex with CDK6 and associates with p27KIP1 in conjunction with CDK6 or CDK2
To identify the v-cyclin-containing complexes in PEL cells, a biochemical analysis was performed. To this end, lysates from 2 KSHV-infected PEL cell lines, BC-3 and BC-1, were subjected to gel filtration chromatography using a Superdex 200HR column, and fractions were analyzed by immunoblotting. v-cyclin eluted in complexes between 30 to 170 kDa in both cell lines (Figure 1, top panels). v-cyclin elution profile in BC-3 was somewhat broader than in BC-1, which may be due to the fact that BC-3 cells express approximately 5 times more v-cyclin than BC-1.27,28 We next analyzed the elution profiles of CDK6 and p27KIP1 to determine whether they coeluted with v-cyclin. CDK6 eluted with the peak of v-cyclin in both cell lines (Figure 1, middle panels) while p27KIP1 elution profile (80-220 kDa) partially overlapped with that of v-cyclin and CDK6 (Figure 1, bottom panels). Additionally, in BC-3 cells there was a pool of p27KIP1 present at approximately 500 to 600 kDa (Figure 1A, bottom panel). Since v-cyclin did not coelute with these high-molecular-weight complexes of p27KIP1, we did not analyze them further. Previous studies in transfected cells as well as PEL cells suggest that v-cyclin also associates with CDK2.15,35,36 Therefore, we determined the elution profile of CDK2 by immunoblotting the gel filtration fractions with anti-CDK2 antibodies. CDK2 was eluted at 30 to 50 kDa and 150 to 180 kDa in both BC-3 and BC-1 cells (data not shown).
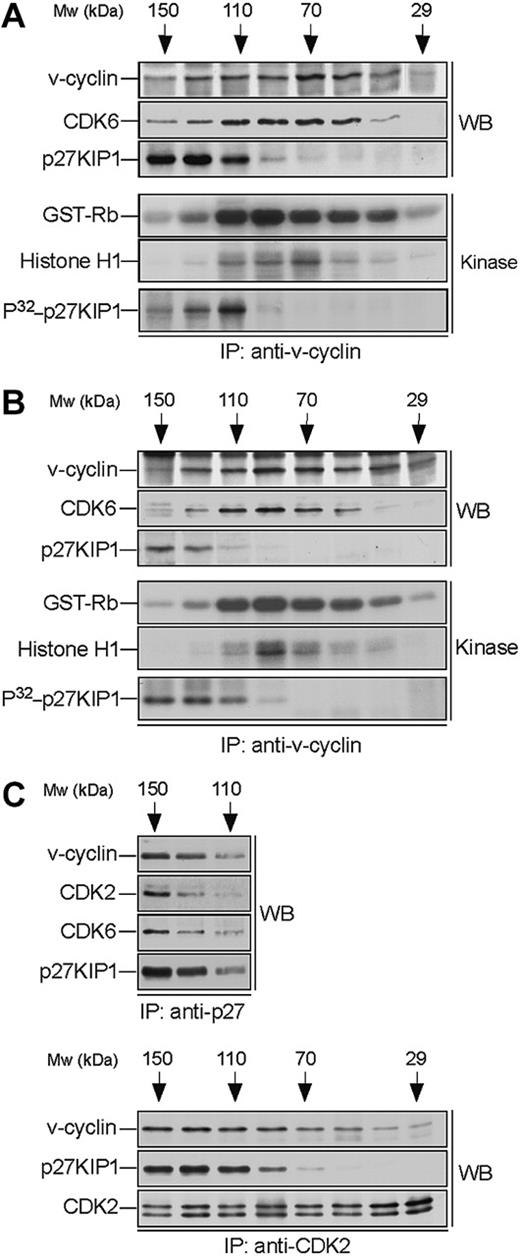
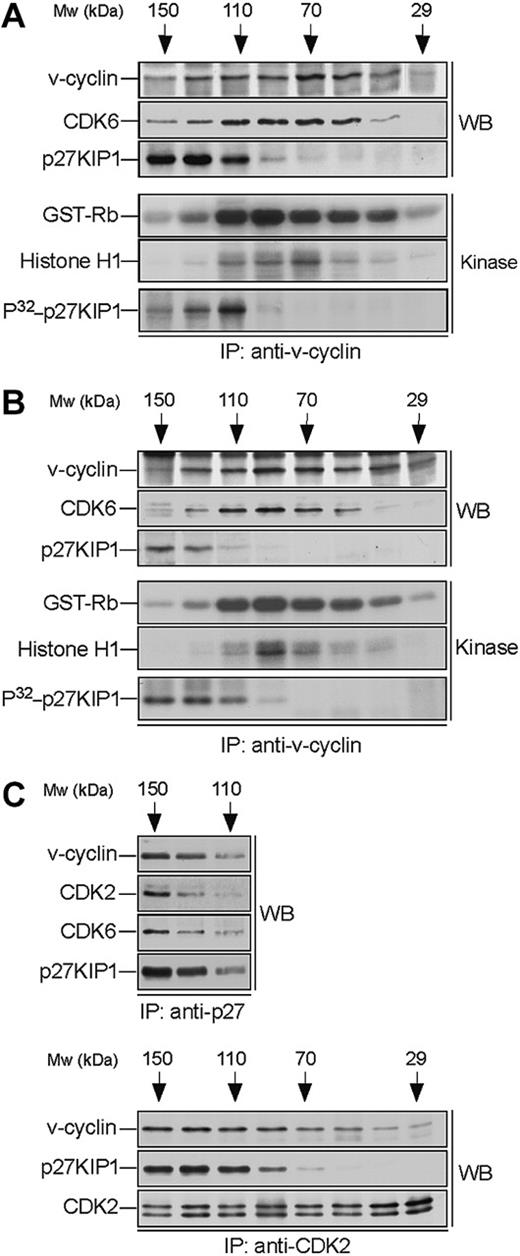
v-cyclin forms a binary complex with CDK6 and associates with p27KIP1. Lysates from BC-3 (A) and BC-1 (B) cell lines were separated by gel filtration chromatography and fractions were immunoprecipitated with anti-v-cyclin antibody. Immunocomplexes were then immunoblotted with antibodies against v-cyclin, CDK6, and p27KIP1 (WB panels). The interactions between v-cyclin and p27KIP1 were detectable in a short exposure from BC-3 cells, whereas a longer exposure was required for the BC-1 cell line. The immunoprecipitates were subjected to an in vitro kinase assay using recombinant GST-Rb and Histone H1 or coprecipitated endogenous proteins as substrates (Kinase panels). Kinase activity was determined by SDS-PAGE (12%) and autoradiography. (C) BC-3 whole-cell extract was separated as in panel A and the fractions were immunoprecipitated with anti-p27KIP1 (3 top panels) or anti-CDK2 (3 bottom panels) antibodies. Associated proteins were analyzed in SDS-PAGE by immunoblotting (WB) with anti-v-cyclin, anti-p27KIP1, anti-CDK6, and anti-CDK2 antibodies as indicated.
v-cyclin forms a binary complex with CDK6 and associates with p27KIP1. Lysates from BC-3 (A) and BC-1 (B) cell lines were separated by gel filtration chromatography and fractions were immunoprecipitated with anti-v-cyclin antibody. Immunocomplexes were then immunoblotted with antibodies against v-cyclin, CDK6, and p27KIP1 (WB panels). The interactions between v-cyclin and p27KIP1 were detectable in a short exposure from BC-3 cells, whereas a longer exposure was required for the BC-1 cell line. The immunoprecipitates were subjected to an in vitro kinase assay using recombinant GST-Rb and Histone H1 or coprecipitated endogenous proteins as substrates (Kinase panels). Kinase activity was determined by SDS-PAGE (12%) and autoradiography. (C) BC-3 whole-cell extract was separated as in panel A and the fractions were immunoprecipitated with anti-p27KIP1 (3 top panels) or anti-CDK2 (3 bottom panels) antibodies. Associated proteins were analyzed in SDS-PAGE by immunoblotting (WB) with anti-v-cyclin, anti-p27KIP1, anti-CDK6, and anti-CDK2 antibodies as indicated.
To analyze complex formation we subjected the v-cyclin-containing fractions to immunoprecipitation with anti-v-cyclin antibodies. The immune complexes were then separated by SDS-PAGE and analyzed by immunoblotting with anti-CDK6 and anti-p27KIP1 antibodies. CDK6 was detected in the v-cyclin immunoprecipitates (peaking at 70-110 kDa; Figure 2A-B), thus confirming that they form a complex. In both cell lines p27KIP1 was detected in the v-cyclin immunoprecipitates in fractions ranging from 110 to 150 kDa. In BC-3 cells, p27KIP1 and CDK6 were coprecipitated with v-cyclin at approximately 110 kDa, indicating formation of a ternary complex (Figure 2). This was further supported by the reciprocal immunoprecipitations using anti-p27KIP1 antibodies where both v-cyclin and CDK6 were coprecipitated with p27KIP1 from the same fractions (Figure 2C, top panels). Interestingly, we also detected evident association between v-cyclin, CDK2, and p27KIP1 when the same fractions were subjected to immunoprecipitation using anti-CDK2 antibodies (Figure 2C, bottom panels). These results suggest that v-cyclin forms binary complexes with CDK6 and higher-order molecular complexes that consist of at least CDK6-p27KIP1 and CDK2-p27KIP1.
The binary v-cyclin-CDK6 complex is responsible for the pRb- and histone H1-kinase activity
Previous studies from transfected cells,12,13 as well as from PEL cells,28 indicate that v-cyclin forms active complexes with CDK6. Next we wanted to determine in which of the above-identified complexes the v-cyclin-associated kinase activity resides. To this end, v-cyclin immunoprecipitates from gel filtration fractions were subjected to an in vitro kinase assay using GST-pRb and Histone H1 as substrates. The highest GST-Rb- and Histone H1 kinase activity resided in the fractions where binary v-cyclin-CDK6 complexes eluted in both cell lines (Figure 2A-B). These findings indicate that the majority of v-cyclin-associated pRb and histone H1 kinase activity in PEL cells resides in the binary v-cyclin-CDK6 complex.
p27KIP1 is phosphorylated by v-cyclin-CDK6 in vivo
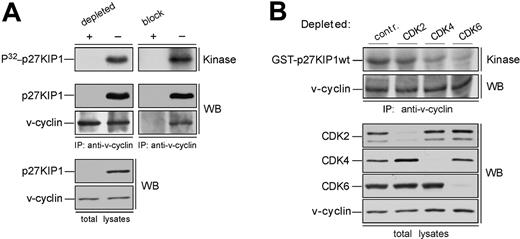
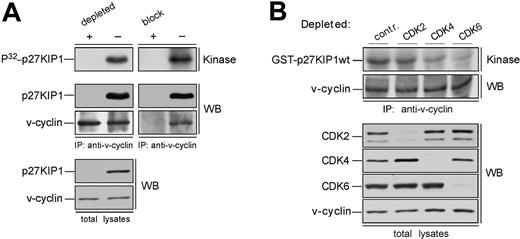
In overexpression models v-cyclin-CDK6 has been implicated in down-regulation of the p27KIP1 levels via phosphorylation.14,15 To address whether p27KIP1 is a substrate for v-cyclin-associated kinase also in PEL cells we subjected the anti-v-cyclin immunoprecipitates from gel filtration fractions to a kinase assay toward coprecipitated endogenous proteins. The kinase reactions were analyzed by SDS-PAGE and autoradiography. Among the phosphorylated proteins we detected a band migrating approximately at 25 to 30 kDa in SDS-PAGE (Figure 2A-B, bottom panels), which comigrated with p27KIP1 in the following immunoblotting of the kinase filter. This phosphorylation occurred in fractions at approximately 110 to 120 kDa in both cell lines, therefore suggesting that the ternary complex of v-cyclin-CDK6-p27KIP1 is responsible for this phosphorylation (Figure 2A-B, bottom panels). Repeating the experiment using anti-v-cyclin antibody pretreated with the antigen (recombinant full-length v-cyclin) resulted in complete abolishment of the phosphorylation on p27KIP1 (Figure 3A, right panels), thus demonstrating the specificity of the experiment. These results confirm that p27KIP1 is a substrate for v-cyclin-associated kinase in PEL cells in vivo.
Because several other proteins were phosphorylated in this assay we wanted to confirm that this substrate was indeed p27KIP1. To that end, fractions were subjected to immunodepletion with either anti-p27KIP1 antibody or normal rabbit IgG as a control. Three rounds of immunodepletion were sufficient to reduce all detectable p27KIP1 (Figure 3A, left panels). These immunodepleted fractions were then immunoprecipitated with anti-v-cyclin antibody and subjected to the kinase assay without exogenous substrates as described. Depletion of p27KIP1 completely abolished phosphorylation of the 27-kDa protein, whereas parallel mock depletion using purified rabbit IgG had no effect.
Finally, we examined whether other CDKs than CDK6 would be involved in v-cyclin-mediated p27KIP1 phosphorylation in PEL cells. To this end, CDK2, CDK4, or CDK6 were removed individually by immunodepletion from BC-3 cell lysate. Three consecutive rounds of immunoprecipitation efficiently removed individual CDKs from the total lysate (Figure 3B). The CDK-depleted lysates were immunoprecipitated with anti-v-cyclin antibody and the kinase activity of each immune complex was assessed toward recombinant GST-p27KIP1. Depletion of CDK6 and CDK4 resulted in a significant decrease of v-cyclin kinase activity toward GST-p27KIP1 (Figure 3B, top panel). The level of phosphorylation was 3-fold and 8-fold less in CDK4 and CDK6 depleted lysates, respectively, compared with the rabbit IgG-depleted control. In contrast, CDK2 depletion did not affect phosphorylation of GST-p27KIP1. v-cyclin can weakly associate with CDK4 both in overexpression systems12,13 and PELs,35 but this leads to only residual activation of CDK4 kinase. In the gel filtration chromatography CDK4 eluted in complexes of approximately 150 to 170 kDa in size together with cyclin D2 and p27KIP1 (data not shown), while no significant overlap with the v-cyclin-containing fractions was observed. Moreover, immunoblotting of the v-cyclin immunoprecipitates from fractions between 110 to 150 kDa with anti-CDK6 antibodies reveals colocalization of CDK6 with the peak of phosphorylated p27KIP1 (at approximately 110 kDa; Figure 2A, P32-p27KIP1 and CDK6 panels), indicating that CDK6 is the favored kinase partner of v-cyclin for the p27KIP1 phosphorylation in BC-3 cells.
p27KIP1 is phosphorylated by v-cyclin-CDK6 in vivo. (A) Lysates from BC-3 cells were separated by gel filtration chromatography. In the left panels the peak fraction for p27KIP1 phosphorylation (the 110-kDa fraction from Figure 2A) was immunodepleted of p27KIP1 with 3 consecutive rounds of immunoprecipitation using anti-p27KIP1 antibody (depleted +) or with rabbit immunoglobulin G for control (-). The p27KIP1-and control-depleted extracts were immunoprecipitated with anti-v-cyclin antibody and subjected to an in vitro kinase assay (top left panel). A parallel sample of the same fraction was also immunoprecipitated with anti-v-cyclin antibody neutralized by pretreatment with GST-v-cyclin (block +) or with anti-v-cyclin antibody treated with a nonspecific control protein BSA (-), and assayed for kinase activity toward coprecipitated endogenous p27KIP1 (top right panel). Kinase activity was determined by autoradiography after 12% SDS-PAGE (P32-p27KIP1; Kinase). Immunoprecipitated proteins were detected by immunoblotting with anti-v-cyclin and anti-p27KIP1 antibodies (WB). The p27KIP1 depleted total lysate was immunoblotted with antibodies against p27KIP1 and v-cyclin (left, 2 bottom panels). (B) Total extracts from BC-3 cell line were immunodepleted for CDK2, CDK4, and CDK6 by 3 consecutive rounds of immunoprecipitation with anti-CDK2, anti-CDK4, or anti-CDK6 antibodies, respectively. In the control (contr.), lysates were subjected to immunoprecipitation with rabbit IgG. The depleted extracts were subjected to immunoprecipitation by anti-v-cyclin antibody and in vitro kinase assay toward GST-p27KIP1. Kinase activity was determined as in panel A (top panel). Successful depletion of individual proteins from the total lysates was confirmed by immunoblotting with antibodies against CDK2, CDK4, and CDK6 (WB).
p27KIP1 is phosphorylated by v-cyclin-CDK6 in vivo. (A) Lysates from BC-3 cells were separated by gel filtration chromatography. In the left panels the peak fraction for p27KIP1 phosphorylation (the 110-kDa fraction from Figure 2A) was immunodepleted of p27KIP1 with 3 consecutive rounds of immunoprecipitation using anti-p27KIP1 antibody (depleted +) or with rabbit immunoglobulin G for control (-). The p27KIP1-and control-depleted extracts were immunoprecipitated with anti-v-cyclin antibody and subjected to an in vitro kinase assay (top left panel). A parallel sample of the same fraction was also immunoprecipitated with anti-v-cyclin antibody neutralized by pretreatment with GST-v-cyclin (block +) or with anti-v-cyclin antibody treated with a nonspecific control protein BSA (-), and assayed for kinase activity toward coprecipitated endogenous p27KIP1 (top right panel). Kinase activity was determined by autoradiography after 12% SDS-PAGE (P32-p27KIP1; Kinase). Immunoprecipitated proteins were detected by immunoblotting with anti-v-cyclin and anti-p27KIP1 antibodies (WB). The p27KIP1 depleted total lysate was immunoblotted with antibodies against p27KIP1 and v-cyclin (left, 2 bottom panels). (B) Total extracts from BC-3 cell line were immunodepleted for CDK2, CDK4, and CDK6 by 3 consecutive rounds of immunoprecipitation with anti-CDK2, anti-CDK4, or anti-CDK6 antibodies, respectively. In the control (contr.), lysates were subjected to immunoprecipitation with rabbit IgG. The depleted extracts were subjected to immunoprecipitation by anti-v-cyclin antibody and in vitro kinase assay toward GST-p27KIP1. Kinase activity was determined as in panel A (top panel). Successful depletion of individual proteins from the total lysates was confirmed by immunoblotting with antibodies against CDK2, CDK4, and CDK6 (WB).
v-cyclin-CDK6 phosphorylates p27KIP1 predominantly on Ser10
Previous reports have focused on the v-cyclin-mediated Thr187 phosphorylation and subsequent down-regulation of p27KIP1.14,15 Ellis et al14 have reported that v-cyclin-CDK6 also phosphorylates the Ser10 residue on p27KIP1 in vitro. However, the role of Ser10 phosphorylation by v-cyclin-CDK6 has remained elusive. To identify which sites are phosphorylated on p27KIP1 by v-cyclin-CDK6 in PEL cells, anti-v-cyclin immunoprecipitates from BC-3 and BC-1 lysates were subjected to an in vitro kinase assay using wild-type and phosphosite mutants of GST-p27KIP1 as substrates. These mutants included GST-p27KIP1 S10A and GST-p27KIP1 T187A in which the residues Ser10 or Thr187, respectively, were individually replaced with Ala and a double-mutant GST-p27KIP1S10AT187A with both sites replaced with Ala. The extent of phosphorylation on these recombinant p27KIP1 proteins was analyzed by autoradiography and quantified using the phosphoimager (Figure 4). Substitution of Ser10 to Ala markedly reduced the level of phosphorylation by v-cyclin-CDK6. The amount of [32P] incorporated into the S10A mutant was 16% in BC-3 and 18% in BC-1 of that incorporated into the wild-type GST-p27KIP1. The corresponding numbers for the T187A mutant were 63% and 72% in BC-3 and BC-1, respectively (Figure 4C-D). This suggests that Ser10 is the major site on p27KIP1 phosphorylated by v-cyclin-CDK6 in PEL cells. When both Ser10 and Thr187 were replaced by Ala (the GST-p27KIP1S10AT187A double mutant), the incorporation of radioactivity was reduced to 11% in BC-3 and 13% in BC-1 when compared with the wild type. This may indicate phosphorylation of other remaining phosphosites, although in the previous phosphopeptide analysis, these were the only identified residues for v-cyclin-associated kinase in vitro.14 Similar results were obtained with 2 additional PEL cell lines, BCBL-1 and JSC-1, while no phosphorylation on p27KIP1 was observed in the in vitro kinase assays from a KSHV-negative control cell line JOK-1 (data not shown).
v-cyclin-CDK6 phosphorylates p27KIP1 predominantly on Ser10. BC-3 (A) and BC-1 (B) whole-cell extracts were immunoprecipitated with anti-v-cyclin antibody and assayed for kinase activity toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A). The kinase reactions were resolved by SDS-PAGE (12%) followed by autoradiography (P32-p27KIP1; Kinase). Immunoprecipitation of equal levels of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody (WB). The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present the average of 3 independent experiments.
v-cyclin-CDK6 phosphorylates p27KIP1 predominantly on Ser10. BC-3 (A) and BC-1 (B) whole-cell extracts were immunoprecipitated with anti-v-cyclin antibody and assayed for kinase activity toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A). The kinase reactions were resolved by SDS-PAGE (12%) followed by autoradiography (P32-p27KIP1; Kinase). Immunoprecipitation of equal levels of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody (WB). The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present the average of 3 independent experiments.
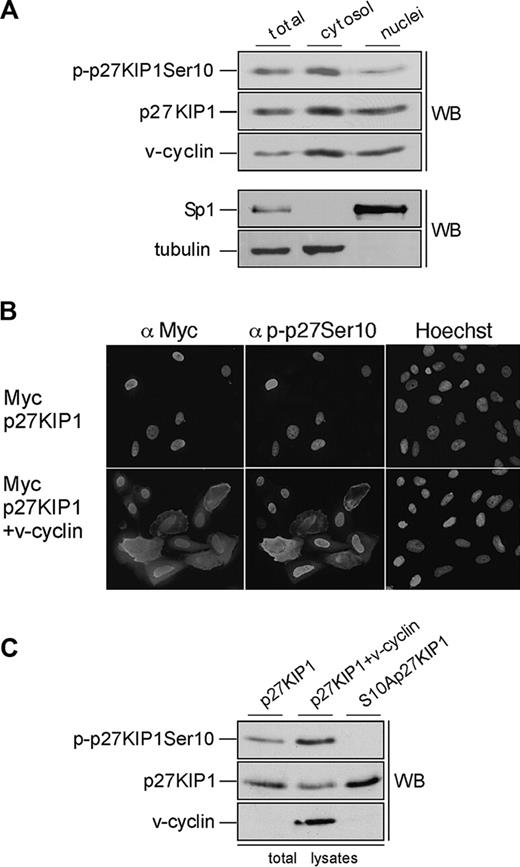
Ser10-phosphorylated p27KIP1 is predominantly cytoplasmic in PEL cells
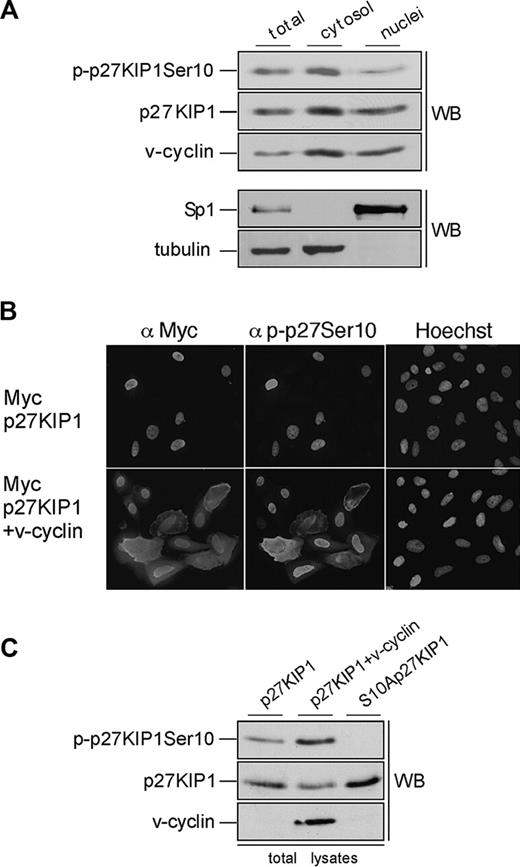
Phosphorylation of p27KIP1 on Ser10 has been implicated as the nuclear export signal that allows cell-cycle progression in response to mitogens.24,25 To address the role of Ser10 phosphorylation in BC-3 cells we examined the subcellular localization of p27KIP1 and v-cyclin from nuclear and cytoplasmic extracts by immunoblotting (Figure 5A). Consistent with recent results by Van Dross et al,36 p27KIP1 and v-cyclin were detected in both cellular compartments, and slightly more abundant in the cytoplasmic extracts than in the nuclear extracts. Both cytoplasmic and nuclear v-cyclin complexes had kinase activity and phosphorylated GST-pRb and Histone H1 in vitro (data not shown). Interestingly, when the immunoblotting was performed using antibody against Ser10-phosphorylated p27KIP1 (p-p27 [Ser10]), the Ser10-phosphorylated form was 2-fold more abundant in the cytoplasmic fractions than in the nucleus (Figure 5A, top panel). These results suggest that v-cyclin-mediated Ser10 phosphorylation may promote the pronounced cytoplasmic localization of p27KIP1 in PEL cells, and thereby inhibit its antiproliferative function.
Ser10-phosphorylated p27KIP1 is predominantly cytoplasmic in PEL cells. (A) BC-3 cells were separated into cytoplasmic and nuclear extracts as described in “Materials and methods.” The resulting fractions were resolved on SDS-PAGE (12%) and analyzed by immunoblotting with anti-p-p27 (Ser10), anti-p27KIP1, and anti-v-cyclin antibodies (top 3 panels). The distribution of the nuclear marker Sp1 and the cytoplasmic marker β-tubulin confirmed the purity of the fractions (bottom 2 panels). (B) U2OS cells were transfected with expression vector for Myc-p27KIP1 alone or together with v-cyclin. Cells were analyzed 48 hours after transfection by indirect immunofluorescence. The cells were labeled by anti-Myc and anti-p-p27KIP1 Ser10 antibodies as indicated and their nuclear morphology was visualized by Hoechst staining. (C) U2OS cells were transfected with expression vector for Myc-p27KIP1 alone or together with v-cyclin or with S10A-p27KIP1 alone. Total-cell lysates of transfected cells were resolved by SDS-PAGE (12%) and immunoblotted with anti-p-p27 (Ser10), anti-p27KIP1, and anti-v-cyclin antibodies as indicated (WB).
Ser10-phosphorylated p27KIP1 is predominantly cytoplasmic in PEL cells. (A) BC-3 cells were separated into cytoplasmic and nuclear extracts as described in “Materials and methods.” The resulting fractions were resolved on SDS-PAGE (12%) and analyzed by immunoblotting with anti-p-p27 (Ser10), anti-p27KIP1, and anti-v-cyclin antibodies (top 3 panels). The distribution of the nuclear marker Sp1 and the cytoplasmic marker β-tubulin confirmed the purity of the fractions (bottom 2 panels). (B) U2OS cells were transfected with expression vector for Myc-p27KIP1 alone or together with v-cyclin. Cells were analyzed 48 hours after transfection by indirect immunofluorescence. The cells were labeled by anti-Myc and anti-p-p27KIP1 Ser10 antibodies as indicated and their nuclear morphology was visualized by Hoechst staining. (C) U2OS cells were transfected with expression vector for Myc-p27KIP1 alone or together with v-cyclin or with S10A-p27KIP1 alone. Total-cell lysates of transfected cells were resolved by SDS-PAGE (12%) and immunoblotted with anti-p-p27 (Ser10), anti-p27KIP1, and anti-v-cyclin antibodies as indicated (WB).
To address if v-cyclin can induce cytoplasmic mislocalization of p27KIP1, U2OS cells were transfected with expression vectors for Myc-p27KIP1 or Myc-S10Ap27KIP1 with and without v-cyclin. To assess the requirement of v-cyclin-CDK6 kinase activity for this, cotransfection of v-cyclin, p27KIP1, and kinase-deficient CDK6 (CDK6DN) was also included. Transfected cells were analyzed by indirect immunofluorescence for p27KIP1 localization using anti-Myc, anti-p-p27 (Ser10), and anti-v-cyclin antibodies. In about 75% to 80% of the cells p27KIP1 showed a predominantly nuclear localization when expressed alone (Figure 5B, top panel). Coexpression of v-cyclin with the wild-type p27KIP1 reduced the number of cells with exclusively nuclear p27KIP1 to 50%, which correlated with increased Ser10 phosphorylation on p27KIP1 in both immunofluorescence and immunoblotting analyses (Figure 5B, bottom panel; and Figure 5C, top panel). Coexpression of CDK6DN with v-cyclin also resulted in more efficient nuclear retention of p27KIP1, with 77% of the cells having nuclear p27KIP1. Moreover, Myc-S10Ap27KIP1 coexpressed with v-cyclin was more efficiently retained in the nucleus (in 70% of the cells) than wt p27KIP1. These results support the finding from PEL cells that v-cyclin-CDK6-induced phosphorylation on Ser10 residue of p27KIP1 enhances its cytoplasmic mislocalization.
Lytic cycle triggers Thr-187 phosphorylation. BC-3 (A) and BC-1 (B) cells were treated with 20 ng/mL TPA for 24 hours and cell lysates were immunoprecipitated with anti-v-cyclin antibody. In vitro kinase assay toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A) was determined by autoradiography after 12% SDS-PAGE (P32-p27KIP1; Kinase). Immunoprecipitation of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody. The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present an average of 3 independent experiments.
Lytic cycle triggers Thr-187 phosphorylation. BC-3 (A) and BC-1 (B) cells were treated with 20 ng/mL TPA for 24 hours and cell lysates were immunoprecipitated with anti-v-cyclin antibody. In vitro kinase assay toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A) was determined by autoradiography after 12% SDS-PAGE (P32-p27KIP1; Kinase). Immunoprecipitation of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody. The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present an average of 3 independent experiments.
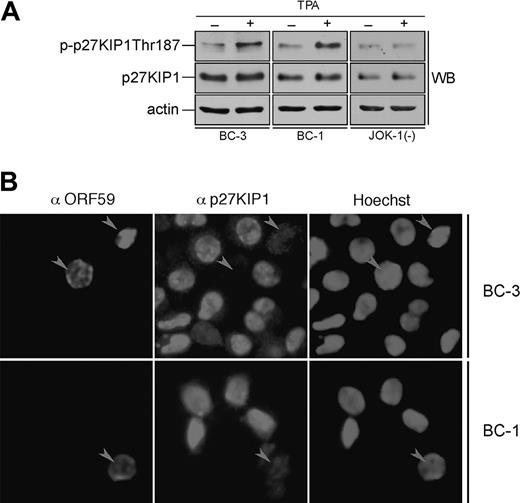
Lytic reactivation of KSHV leads to down-regulation of p27KIP1 in PEL cells
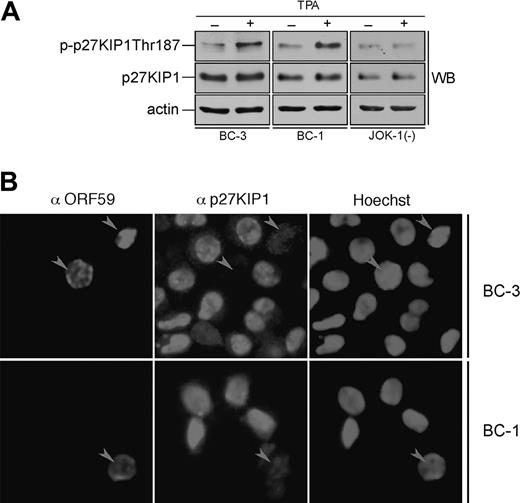
Sequential reactivation of KSHV lytic gene expression can be achieved upon treatment of latently infected PEL cells with TPA. Next we addressed the effect of lytic reactivation on phosphorylation of p27KIP1 by v-cyclin. BC-3 and BC-1 cells were induced by TPA treatment for 24 and 48 hours. The efficiency of lytic induction was analyzed by indirect immunofluorescence using antibodies against a KSHV early lytic marker, anti-ORF59. Cell lysates from TPA-induced cells after 24 hours were immunoprecipitated with anti-v-cyclin antibody and subjected to an in vitro kinase assay using GST-p27KIP1 wild-type and phosphosite mutants (S10A, T187A, and S10AT187A) as substrates. The extent of phosphorylation on the substrates was analyzed by autoradiography and quantified using the phosphoimager. Interestingly, substitution of Ser10 with Ala did not reduce phosphorylation of p27KIP1 (Figure 6A-D) as it did in the kinase assay from latently infected PEL cells (Figure 4). Surprisingly, the GST-T187Ap27KIP1 mutant had this time incorporated significantly less radioactivity than wild-type p27KIP1 (25% in BC-3 and only 7% in BC-1 of the amount incorporated to the wild type; Figure 6A-D), suggesting that Thr187 is the predominant site phosphorylated by v-cyclin-associated kinase in PEL cells induced to lytic replication cycle. Intriguingly, the v-cyclin-associated endogenous p27KIP1 also was phosphorylated more efficiently than in latently infected cells (data not shown). When whole-cell extracts from untreated and TPA-treated BC-3, BC-1, and the KSHV-negative control cell line JOK-1 were analyzed by immunoblotting with p-p27 (Thr187) antibodies, Thr187 phosphorylation on p27KIP1 was increased by 2.3-fold and 2-fold in the BC-3 and BC-1 cells, respectively, but remained unaltered in JOK-1 (Figure 7A). This indicates that Thr187 phosphorylation was indeed occurring in PEL cells reactivated to KSHV lytic cycle.
Lytic reactivation of KSHV leads to down-regulation of p27KIP1 in PEL cells. (A) BC-3, BC-1, and JOK-1 (a KSHV-negative control cell line) cells were either treated with TPA or with DMSO (noninduced control) for 24 hours. Whole-cell extracts were resolved by 12% SDS-PAGE and analyzed by immunoblotting with antibodies against p-p27 (Thr187), p27KIP1, and actin (loading control). (B) BC-3 and BC-1 cells were induced to viral replication with TPA for 48 hours. The p27KIP1 down-regulation in anti-ORF59-positive cells was determined by double-label immunofluorescence experiment after cytocentrifugation. Arrowheads point to ORF59-positive cells with down-regulated p27KIP1.
Lytic reactivation of KSHV leads to down-regulation of p27KIP1 in PEL cells. (A) BC-3, BC-1, and JOK-1 (a KSHV-negative control cell line) cells were either treated with TPA or with DMSO (noninduced control) for 24 hours. Whole-cell extracts were resolved by 12% SDS-PAGE and analyzed by immunoblotting with antibodies against p-p27 (Thr187), p27KIP1, and actin (loading control). (B) BC-3 and BC-1 cells were induced to viral replication with TPA for 48 hours. The p27KIP1 down-regulation in anti-ORF59-positive cells was determined by double-label immunofluorescence experiment after cytocentrifugation. Arrowheads point to ORF59-positive cells with down-regulated p27KIP1.
Phosphorylation of p27KIP1 on Thr187 is essential for its degradation via the proteasome dependent pathway. Hence, we wanted to evaluate the p27KIP1 levels in TPA-induced PEL cells. BC-1 and BC-3 cells were treated with TPA for 48 hours and analyzed for p27KIP1 and ORF59 expression by indirect immunofluorescence (Figure 7B). Intriguingly, 65% and 59% of ORF59-positive cells in BC-1 and BC-3, respectively, had diminished levels of p27KIP1 when compared with ORF59-negative cells (12% and 10%, respectively). Decrease in p27KIP1 levels was not due to an adverse TPA effect, since also the ORF59-positive, spontaneously reactivated cells in non-TPA-treated samples showed the same effect (data not shown). v-cyclin-mediated Thr187 phosphorylation has previously been implicated in down-regulation of p27KIP1 levels in overexpression models.14,15 Our results here suggest that this may indeed be a functional mechanism for p27KIP1 inactivation also in PEL cells reactivated to KSHV lytic cycle.
Discussion
Our recent results have demonstrated that KSHV viral cyclin stably associates with p27KIP1 in PEL cells,28 suggesting that v-cyclin helps PEL-derived cell lines to tolerate high levels of p27KIP1. In this study we demonstrate that p27KIP1 is a substrate for v-cyclin-CDK6 in vivo, and that phosphorylation in latently infected PEL cells occurs predominantly on Ser10. This contributes to the inactivation of the antiproliferative function of p27KIP1 by enhancing its relocalization to the cytoplasm. Interestingly, reactivation of KSHV lytic cycle in PEL cells triggered a change in the preferred phosphorylation site by v-cyclin-CDK6 to Thr187 on p27KIP1, which resulted in down-regulation of p27KIP1 protein levels.
The biochemical analysis of v-cyclin complexes performed in this study identified CDK6 as the favored CDK partner for v-cyclin in vivo and confirms that the majority of v-cyclin-associated kinase activity in PELs resides in the binary v-cyclin-CDK6 complexes. In accordance with previous findings on cyclin D-CDK4 complexes,37,38 p27KIP1 was not required for the formation of active v-cyclin-CDK6 complex. Interaction of increasing amounts of p27KIP1 with cyclin D-CDK4 has been shown to maximize the accumulation but at the same time inhibit the activity of these complexes. Fractions where p27KIP1 was associated with v-cyclin and CDK6 had less kinase activity toward GST-Rb and Histone H1 (Figure 2A-B) implying that the ternary complexes either were less kinase active or had an altered substrate specificity. In favor of the latter alternative, the associated p27KIP1 was phosphorylated by v-cyclin-CDK6 in these fractions. This also confirms that p27KIP1 is not only phosphorylated by v-cyclin-associated kinase in overexpression models,14,15 but is also a substrate for v-cyclin in vivo in PELs.
v-cyclin and p27KIP1 were also detected in association with CDK2 in fractions between 150 and 110 kDa. While several biochemical and transfection approaches12,13,15,35,36 have suggested that v-cyclin associates with CDK2, this does not seem to result in a significant activation of the CDK2 kinase activity. What might be the functional implication of this observed interaction? By associating with CDK2, v-cyclin may help to sequester p27KIP1 from binding and inhibiting the active cyclin E/A-CDK2 complexes thereby promoting progression of the cell cycle. On the other hand, the molecular mass of the complex containing v-cyclin, CDK2, and p27KIP1 suggests that it may contain also additional proteins. One putative binding partner could be the proliferating nuclear antigen (PCNA). Quaternary complexes of cyclin-CDK-p21CIP1/p27KIP1-PCNA have been detected in cells but their physiologic significance remains unsettled.39
In human tumors the antiproliferative activity of p27KIP1 is often abrogated by cytoplasmic relocalization or increased degradation.40 Ser10 phosphorylation by human kinase interacting stathmin (hKIS) is necessary for nuclear export of p27KIP1 and progression of the cell cycle.25,41 The identification of Ser10 as the major p27KIP1 phosphorylation site for v-cyclin-CDK6 as well as the pronounced cytoplasmic localization of p27KIP1 in latently infected PEL cells suggest a novel mechanism by which v-cyclin may override the antiproliferative function of p27KIP1.
In normal cells, degradation of p27KIP1 is regulated by cyclin E-CDK2-mediated phosphorylation on Thr187, which serves as a signal for the ubiquitination machinery.42 Previous studies in transfected cells have provided evidence that v-cyclin can also bypass the p27KIP1-induced cell-cycle arrest by destabilizing the p27KIP1 protein via Thr187 phosphorylation.14,15,43 However, since the KSHV-infected PELs retain high levels of p27KIP1, the biologic significance of the v-cyclin-CDK6-mediated degradation of p27KIP1 has remained elusive. Here we show that upon lytic reactivation the levels of p27KIP1 are down-regulated, and this correlates with the increased v-cyclin-CDK6 kinase activity toward Thr187 of p27KIP1. Why is p27KIP1 down-regulated upon reactivation of KSHV lytic cycle? Induction of KSHV lytic cycle replication in PEL cells induces a G1 cell-cycle arrest, presumably to facilitate viral gene expression and DNA replication on the expense of host DNA synthesis.44,45 Several lines of evidence support the relevance of cyclin E-cdk2 activity not only for cellular but also for viral DNA synthesis.46-48 Moreover, in a recent report, cyclin E-CDK2 loading onto the HIV-1 genome in vivo was required for viral replication in T cells.49 Accordingly, the presence of elevated levels of p27KIP1 in PEL cells might prevent cyclin E-CDK2 from activating the KSHV DNA synthesis upon induction of the lytic replication cycle. By inducing destabilization of p27KIP1, v-cyclin would ensure that cyclin E-CDK2 promotes the replication and accumulation of viral DNA.
In summary, our results demonstrate that v-cyclin-CDK6 abrogates p27KIP1 function by phosphorylation in PEL cells. In latently infected cells, v-cyclin-CDK6 promotes S-phase progression through Ser10 phosphorylation and relocalization of a pool of p27KIP1 to the cytoplasm. Moreover, we postulate that the biologic relevance of v-cyclin-CDK6-induced destabilization of p27KIP1 is to ensure efficient viral DNA replication upon lytic reactivation of KSHV in PEL cells.
Prepublished online as Blood First Edition Paper, September 13, 2005; DOI 10.1182/blood-2005-06-2534.
Supported by the Sigrid Juselius Foundation (G.S., P.M.O.), Helsinki Graduate School in Biotechnology and Molecular Biology (A.J.), and grants from the Academy of Finland and Finnish Cancer Foundations (P.M.O.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Nisar Malek, Sibylle Mittnacht, Bala Chandran, Ethel Cesarman, Leif Andersson, and Marikki Laiho for reagents, and Susanna Räsänen for excellent technical assistance. Tomi Mäkelä and Johanna Furuhjelm are acknowledged for comments and critical review of this manuscript. We are also grateful to the members of Mäkela, Klefström, and Ojala laboratories for helpful discussions and constructive criticism.

![Figure 4. v-cyclin-CDK6 phosphorylates p27KIP1 predominantly on Ser10. BC-3 (A) and BC-1 (B) whole-cell extracts were immunoprecipitated with anti-v-cyclin antibody and assayed for kinase activity toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A). The kinase reactions were resolved by SDS-PAGE (12%) followed by autoradiography (P32-p27KIP1; Kinase). Immunoprecipitation of equal levels of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody (WB). The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present the average of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-06-2534/4/m_zh80020689350004.jpeg?Expires=1765922339&Signature=EHz2TGzKxman-Wq0Z4924D9F5wws6hAp1pu0QAeiaICHasb8AVZOQxCrNTo7aepYVqiIbp2pekafw0USPRdI9jjxlZRsXMiBgZHtC7EQyEa3bWBa4KlpRwdCWPkm38mzN-JBBlQLxVhsCkrYHTMXXS2oi~Hz2indaplNX5Xr0mZb1MPqHflNl1xdp~bZtLtnpU5KnpPo8ZTciCCHNKWlOUxiAYxntILr9oIy5CLJZKVQqzMqYfM-dqS~myGB0oOWXEZXWjl8frs2cR47IoreVJpFPnkAAEk6vo1sJ9SjboUlJh6Q1aTEqj7CSIuRQJIuiXq3e1l9L-Qr1Ojg72SqwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Lytic cycle triggers Thr-187 phosphorylation. BC-3 (A) and BC-1 (B) cells were treated with 20 ng/mL TPA for 24 hours and cell lysates were immunoprecipitated with anti-v-cyclin antibody. In vitro kinase assay toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A) was determined by autoradiography after 12% SDS-PAGE (P32-p27KIP1; Kinase). Immunoprecipitation of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody. The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present an average of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-06-2534/4/m_zh80020689350006.jpeg?Expires=1765922339&Signature=DtJB9AVlimhDHrWeVH5xbdUH1Ztny8Z9XD22mtOv1XCm6zoqPB4o~P8eBT1cq~akSuJc0-~AxJYERxKlWJf7GcjqWsOu0eukFdKTdR7YjTrKwu-QA7-vTL~RFQSlQUMW8M1jy0qlCikA~ZZOCsP8dN~Gvir~aLyZshfLRbBvVL6Pj7b4KFBZ3hLFrpCxMkVcJKuTqGWzcGyP7jSk9NzTu2evxmv5Yln5dPVQZDCNTOUYJ2l5C8xoMAZOikXA3JmNcCd-VthygaJtxCVp6gXUA8UoiX4F0wztB~RcYCjEtY9DMhb3TXtrNf61F2PnnZmE3XJLSOOXZYO7ojEc90vIHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. v-cyclin-CDK6 phosphorylates p27KIP1 predominantly on Ser10. BC-3 (A) and BC-1 (B) whole-cell extracts were immunoprecipitated with anti-v-cyclin antibody and assayed for kinase activity toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A). The kinase reactions were resolved by SDS-PAGE (12%) followed by autoradiography (P32-p27KIP1; Kinase). Immunoprecipitation of equal levels of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody (WB). The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present the average of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-06-2534/4/m_zh80020689350004.jpeg?Expires=1765922340&Signature=kQ2uNPZxq2PzFkePf8m1xCSQt5l9s5-BzM9JjwERSDRjXtKErePiSlkwBcd43mOKcNvrkFy212tiYjddT4V05n86gFpenFk0TvOdVYchnr5Nmt3GRWB9RPKadmR5uyfV0rDasK4O5Ez7SkzViKxy-KUdi9Ba5lsXZM7QoxozP1iMyVWNA8DsZFXWUCoE~pPayEsmSjX1ZNf5e8xGfsrPBpT7qVUyye6fJLSJ4xFZ314DKOgzbUDWG3~7GkjAx73fydskvfXm3I3Hb-15DNXvH7wyZoTxMBMlJ6oqMdxIt-Othf-zhHKzh3EAGHMlStN4Dh6oLwTG1YNO1FoWy5admQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Lytic cycle triggers Thr-187 phosphorylation. BC-3 (A) and BC-1 (B) cells were treated with 20 ng/mL TPA for 24 hours and cell lysates were immunoprecipitated with anti-v-cyclin antibody. In vitro kinase assay toward GST-p27wt (WT), GST-p27S10A (S10A), GST-p27T187A (T187A), and GST-p27S10AT187A (S10AT187A) was determined by autoradiography after 12% SDS-PAGE (P32-p27KIP1; Kinase). Immunoprecipitation of v-cyclin was confirmed by immunoblotting with anti-v-cyclin antibody. The extent of [32P] incorporation into the wild-type and mutants of GST-p27KIP1 in BC-3 (C) and BC-1 (D) was quantified from the autoradiographs by Typhoon 9400 Image Quant analyzer as detailed in “Materials and methods.” The level of [32P] incorporation by wild-type GST-p27KIP1 was defined as 100%. Data present an average of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-06-2534/4/m_zh80020689350006.jpeg?Expires=1765922340&Signature=SZRc9a2w7sd521gZ8dgZhDeMycXpinBFvrZpCEmLT2jz-MEPFwqfGyxSE-TL2tr1kjHmEbMfXAx-~~xHnUvV07RKmoccZv6x3xX99IwLnebAtV8Gd9oE6WA783-FPEjXdNC8vyUZK6KvsyrnFb95hlrOgGeC3uWC0YuG3O~avndgFVd1wd3Wg2nlec34VOrwxwHBpD0fAebDXi~tXgCQyqeesdk1pajrA6ZjRGBMyjjkt7QeJWXoCUfx0fjqHHKRNSMP95YObiKcK-bpOha6pTKr-7kksIwsQRVAsJQ4NKDApIZGaYZNoV6vhYXodQzgfygoDqqNZqZKAvTJob7pkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)