Abstract
Acute lymphoblastic leukemia (ALL) in adult patients is often resistant to current therapy, making the development of novel therapeutic agents paramount. We investigated whether mTOR inhibitors (MTIs), a class of signal transduction inhibitors, would be effective in primary human ALL. Lymphoblasts from adult patients with precursor B ALL were cultured on bone marrow stroma and were treated with CCI-779, a second generation MTI. Treated cells showed a dramatic decrease in cell proliferation and an increase in apoptotic cells, compared to untreated cells. We also assessed the effect of CCI-779 in a NOD/SCID xenograft model. We treated a total of 68 mice generated from the same patient samples with CCI-779 after establishment of disease. Animals treated with CCI-779 showed a decrease in peripheral-blood blasts and in splenomegaly. In dramatic contrast, untreated animals continued to show expansion of human ALL. We performed immunoblots to validate the inhibition of the mTOR signaling intermediate phospho-S6 in human ALL, finding down-regulation of this target in xenografted human ALL exposed to CCI-779. We conclude that MTIs can inhibit the growth of adult human ALL and deserve close examination as therapeutic agents against a disease that is often not curable with current therapy.
Introduction
While children with precursor B-cell acute lymphoblastic leukemia (ALL) are often cured, children with relapsed ALL and adults with ALL usually succumb to their disease with current therapy. Even with aggressive therapy, these patient groups have 5-year disease-free survival rates of only 28% to 39%.1 Thus, the development of novel therapeutic agents is crucial.
One potential class of novel therapeutics is mTOR inhibitors (MTIs). MTIs are a class of signal transduction inhibitors with anticancer activity that were initially developed as immunosuppressive agents.2-5 Rapamycin, a macrocyclic lactone produced by Streptomyces hydroscopicus,6 was the first MTI to be used in a clinical setting. Rapamycin is well tolerated in humans.7 MTIs also have been shown to be active against a wide variety of tumor types.8-10 We have previously shown that rapamycin induces apoptosis in precursor B ALL lines in vitro and has in vivo activity in transgenic mice with pre-B leukemia/lymphoma.11 Second generation MTIs, CCI-779 and RAD-001, are currently in phase 1 to phase 3 clinical trials in patients with various cancers,12-15 but preclinical studies have not previously been performed in primary human ALL.
Preclinical testing of chemotherapeutic agents often involves using transformed tumor lines and transgenic mouse models. While these are valuable tools, they may not be representative of human disease. More clinically relevant data may be obtained from systems using primary human ALL cells. Two of these systems, bone marrow stroma-supported culture16 and xenografting human ALL in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, have recently been developed.17 Both of these systems allow for direct testing of biologic and chemotherapeutic agents on human leukemic blasts in a preclinical setting.18,19 ALL xenografted into NOD/SCID mice has been shown to maintain its original phenotypic and genotypic characteristics even after serial passage into secondary and tertiary hosts.20 Phenotypic characteristics also are preserved in bone marrow stromal culture.21 The response of leukemic blasts to chemotherapeutic agents in the NOD/SCID xenograft model has been shown to directly correlate to human response to therapy.22
We studied the efficacy of a second generation MTI, CCI-779 (temsirolimus), in primary human ALL in both NOD/SCID xenografts and a bone marrow stromal culture system, demonstrating activity of this class of drugs against adult ALL.
Materials and methods
In vitro drug testing using bone marrow stromal culture
Lymphoblasts from patient samples were obtained from the Stem Cell and Leukemia Core of the University of Pennsylvania Cancer Center. Samples were collected from blood or bone marrow under approval from the institutional review board at the Children's Hospital of Philadelphia and the University of Pennsylvania School of Medicine after informed consent was given in accordance with the Declaration of Helsinki. Mononuclear cells were purified by Ficoll gradient, and cells were frozen as viable cells in dimethyl sulfoxide (DMSO).
Lymphoblasts were cultured on irradiated human primary stromal cells as previously described.23 Stromal cells were obtained from bone marrow cells and washed from 200- to 500-micron filters discarded from clinical bone marrow harvests. Stromal cells were isolated from the bone marrow cells by CD19 depletion and were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. The stromal cells were passaged at least 3 times to expand cell numbers and deplete nonadherent cells. The stromal cells were then trypsinized, irradiated to 2000 cGy, and placed into 24-well plates at 105 cells per well in RPMI 1640 with 10% FBS. The wells were washed with X-vivo 10 media (Cambrex, Walkersville, MD) after overnight incubation to allow for attachment of the stromal-cell layer.
Lymphoblasts and stromal cells were cocultured in X-vivo 10 media supplemented with 5% FBS and the growth factors stem cell factor (SCF) (20 mg/mL), IGF-1 (20 ng/mL), and FLT-3 (10 ng/mL) (R&D Systems, Minneapolis, MN). One half of the media and growth factors was replaced weekly. After aliquoting into wells containing the bone marrow stroma, 105 lymphoblasts were exposed to 0, 1, and 100 ng/mL CCI-779 (generously provided by Wyeth Research, Collegeville, PA) and allowed to grow in culture until confluence (4-5 weeks). New aliquots of drug and vehicle were added with weekly media changes. We assessed differences in proliferation as a measure of response to treatment by enumerating ALL cells after exposure to CCI-779 by multiple methods. We counted cells using light microscopy and verified cells were viable using trypan blue exclusion. We also verified cell counts by flow cytometry and distinguished live cells from dead cells using FSC/SSC and staining with 7-AAD-PE and stromal cells from lymphoblasts with CD-19-APC (BD Pharmingen, Franklin, NJ).
We used short-term cultures to assess for apoptosis. A total of 106 lymphoblasts were aliquoted into bone marrow stroma-containing wells. Lymphoblasts were exposed to 0 and 100 ng/mL CCI-779 for 12 and 24 hours. Cells were analyzed for apoptosis by flow cytometric assessment of annexin-V staining (BD Pharmingen).
Establishment of NOD/SCID xenografts
NOD/SCID mice were maintained in sterile micro-isolator cages and housed in ventilated racks on Institutional Animal Care and Use Committees (IACUC)-approved protocols. Mice received antibiotic prophylaxis with trimethoprim/sulfamethoxazole in drinking water. The protocol for engraftment is based on prior published work.19,20,22 Six- to ten-week-old NOD/SCID mice were irradiated in a single fraction of 275 cGy using a 135Cs γ irradiator. After irradiation, mice were injected with 107 lymphoblasts from patient samples. Starting at 3 to 5 weeks after injection, mice were screened for engraftment every 3 weeks by retro-orbital sampling of peripheral blood. Engraftment was detected by flow cytometric analysis using antibodies recognizing CD19 and CD45. In addition, mice were screened using anti-CD3 and anti-CD33 to ensure engraftment was of the lymphoblast population and not of a T-cell or myeloid lineage. All antibodies for these stains were obtained from Beckman Coulter (Brea, CA), except for anti-CD33, which was obtained from BD Pharmingen. All antibodies are non-cross-reactive to mouse proteins. After the engraftment of ALL, animals were humanely killed; bone marrow, spleen, and peripheral blood were harvested; and 107 of these primary engrafted lymphoblasts were injected into secondary hosts, which were used for experiments. This system could also be further extended to generate tertiary hosts and expand leukemia from a given patient into numerous animals.
In vivo activity of mTOR inhibitors in xenografted mice
To test MTIs in the NOD/SCID model, the xenografted mice were randomized to treatment with CCI-779 or vehicle after establishment of disease, defined as more than 5% blasts detected in peripheral blood. Mice were treated with 5 to 10 mg/kg/d CCI-779 6 days per week. CCI-779 was reconstituted in a mixture of 5% Tween, 5% PEG 400, and 4% EtOH in H2O (vehicle) according to the manufacturer's directions. Response to treatment was measured weekly by retro-orbital bleeds to determine white blood counts and percent blasts by flow cytometry (as described under “Establishment of NOD/SCID xenografts”). Ill-appearing mice were humanely killed, at which point spleens and bone marrow were harvested for flow cytometric analysis. CBC analysis was performed on a HemeVet 850FS hematology analyzer (CDC Technologies, Oxford, CT).
Immunoblotting
Immunoblots were performed to detect mTOR pathway targets, to assess inhibition of this pathway. For these experiments, we used a modification of the protocol of Huang et al.24 Mouse spleens were harvested and dissociated into a single-cell suspension after which erythrocytes were lysed using Tris/ammonium chloride. Ten to 20 × 106 splenocytes were lysed in ice-cold lysis buffer, derived from mixing 1 mL of 10× Lysis Buffer (Cell Signaling Technologies, Beverly, MA), 1 tablet of Complete Mini (Roche Diagnostics, Mannheim, Germany), and 100 μL 100 mM phenylmethylsulfonyl fluoride (PMFS) to a final volume of 10 mL with ddH2O. The samples were then frozen in liquid nitrogen and thawed in a 37°C water bath for a total of 3 cycles to lyse the cells. Nuclei were sedimented from the lysate at 15 000 g for 15 minutes at 4°C. Protein concentration in the supernatant was quantitated using the Bio-Rad Quick Start kit (Bio-Rad, Hercules, CA), based on the Bradford technique. A standard curve was generated using the Serum Albumin Standard Set (Bio-Rad). Equal quantities of protein (20-40 μg) from each lysate were resolved using NuPAGE Tris-bis 10% SDS gel electrophoresis (Cell Signaling Technologies) and transferred to polyvinylidenefluoride (PVDF) membranes (PerkinElmer Life Sciences, Boston, MA). The membranes were blocked using 3% immunoblot blocking reagent (Upstate Cell Signaling Solutions, Lake Placid, NY) in phosphate-buffered saline (PBS) with 0.01% Tween and rocked at room temperature for one hour, followed by labeling with antibodies to S6 and phospho-S6 (ser235/ser236) (Cell Signaling Technologies) for 18 hours at 4°C and detected using a horseradish-peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) and the Lumiglo chemiluminescence system (Cell Signaling Technologies). Bands were outlined and quantitated densitometrically using Quantity-One software (Bio-Rad).
Results
Efficacy of MTIs in bone marrow stroma-supported culture
We tested samples from 4 patients. These samples were chosen because they contained sufficient lymphoblasts for analysis in both the in vivo and in vitro systems. We hypothesized that signaling via the mTOR pathway is necessary for the survival of ALL cells. We initially tested this hypothesis in vitro by evaluating the impact of CCI-779 on ALL in long- and short-term stromal-supported culture systems. Choi and coworkers have shown that these stromal-supported cultures can support long-term ALL-cell survival and proliferation.25
We first used a long-term culture system to assess the effect of CCI-779. Bone marrow stromal cells were aliquoted into 24-well plates at 105 cells per well to establish a confluent layer of stromal cells. A total of 105 lymphoblasts from the 4 patient samples (characteristics are summarized in Table 1) were then aliquoted onto these bone marrow stromal layers in wells containing CCI-779 (1 ng/mL and 100 ng/mL) or vehicle alone as a control. Once lymphoblasts from control wells proliferated to reach confluence (4-5 weeks), we assessed the effect of CCI-779 on lymphoblast proliferation by enumeration with light microscopy using morphology and trypan blue exclusion. To ensure we were only counting lymphoblasts and not stromal cells, we also verified cell counts by counting total CD19+ cells by flow cytometry as well as distinguishing live cells from dead cells using FSC/SCC and the viability stain 7-AAD.
After starting with 105 cells, control wells reached 9 to 13 × 105 total lymphoblasts after 4 to 5 weeks in culture, demonstrating proliferation of the ALL cell in these culture conditions. Cell counts revealed a 15- to 32-fold decrease in cell number between control and treatment in the 4 patient samples. Blasts from all 4 patients responded to CCI-779 treatment to a similar degree as assessed by numbers of ALL cells remaining in the wells: a range of 0.4 to 0.6 × 105 blasts remained in the wells after treatment, with blasts from patient 200 at the high end of that range and blasts from patient 359 at the low end of the range. We demonstrated a 2- to 7-fold increased level of cell death as assessed by 7-AAD in treated cells compared to control in all patient samples. Results for 1 ng/mL and 100 ng/mL CCI-779 were essentially identical (not shown).
We then turned to a short-term culture system to look for induction of apoptosis as a result of CCI-779 treatment. Response to mTOR inhibition may differ by cell type, with some investigators reporting induction of apoptosis11,26 (as we have previously demonstrated) and others G1 arrest.27,28 Based on our prior work with murine ALL, we hypothesized that CCI-779-treated human ALL cells would undergo apoptosis. A total of 106 lymphoblasts from the 4 patient samples were cultured on bone marrow stroma for 24 hours. Cells were exposed to vehicle (control) or CCI-779 (100 ng/mL) for 12 or 24 hours. After 24 hours, CCI-779-treated cells demonstrated a 2- to 5-fold difference in apoptosis as detected by annexin-V as compared to control cells in all 4 patient samples (Figure 1). Taken together, the long-term and short-term in vitro culture results demonstrate that ALL cells require mTOR for survival, and ALL cells undergo apoptosis when the mTOR pathway is inhibited.
Establishment of multiple generations of NOD/SCID xenografts
To test the effect of CCI-779 on human ALL in an in vivo system, we used a xenograft model of primary human ALL. We attempted to establish NOD/SCID xenografts from frozen lymphoblasts from 9 adult patients with precursor B-cell ALL. We were able to successfully establish xenografts from 7 of the patient samples used (78%), a proportion equal to or better than other published work using similar models.17,20,22 One sample that did not engraft had a post-thaw viability of only 15%, suggesting that nonengraftment may have been related to the quality of the sample. Starting at 3 to 5 weeks after injection, mice were evaluated for establishment of disease by performing flow cytometric detection of human CD45+/CD19+ cells in the peripheral blood every 3 weeks. If no evidence of engraftment was found after 4 months, mice were killed for multicompartment analysis.
CCI-779 induces apoptosis in a short-term human ALL culture system. A total of 106 lymphoblasts from patients with adult human ALL were aliquoted onto a bone marrow stromal cell layer. All cells were maintained in culture for 24 hours and were either untreated (control) or treated with CCI-779 (100 ng/mL) for 12 or 24 hours. The x-axis depicts patient sample identification, and the y-axis depicts the percentage of apoptotic (annexin V-positive)/CD19-positive cells.
CCI-779 induces apoptosis in a short-term human ALL culture system. A total of 106 lymphoblasts from patients with adult human ALL were aliquoted onto a bone marrow stromal cell layer. All cells were maintained in culture for 24 hours and were either untreated (control) or treated with CCI-779 (100 ng/mL) for 12 or 24 hours. The x-axis depicts patient sample identification, and the y-axis depicts the percentage of apoptotic (annexin V-positive)/CD19-positive cells.
In 3 of the patient samples (96, 200, and 359) peripheral blasts were detected as early as 8 weeks after injection. The majority of xenografts generated from these samples had more than 90% replacement of bone marrow, and more than 60% replacement of spleen with human ALL. Xenografts from 3 other patient samples (240, 195, and 302) eventually developed peripheral blasts but not until 12 weeks after injection, and only one of these (240) developed more than 5% blasts. The majority of xenografts from these patients also had more than 90% replacement of bone marrow and 60% replacement of spleen with human ALL. Xenografts generated from one patient sample (176) did not demonstrate leukemia in spleen or peripheral blood but did show engraftment of less than 10% lymphoblasts in bone marrow. Figure 2 depicts a typical example of multicompartment analysis of one xenografted animal from patient 200. Each animal that developed xenografted ALL showed very significant disease burden in bone marrow and spleen. Low-level engraftment was seen only in mice that had been injected with sample 176.
To ensure the xenografted cells were not T lymphocytes or myeloid cells, which would have suggested engraftment of nonmalignant cells, mice were also screened for human CD3+ or CD33+ cells. No mouse demonstrated human CD33+ cells. Mice xenografted from one patient sample (200) intermittently displayed less than 5% human CD3+ cells, found in peripheral blood but not in spleen or bone marrow. However, these CD3+ cells only appeared in mice with advanced disease, and these CD3+ cells were also CD45+/CD19+, suggesting a mixed lineage phenotype of these cells.
To demonstrate leukemic stem-cell activity, we injected splenocytes and bone marrow lymphoblasts into secondary and tertiary hosts. Secondary hosts were successfully generated from each of the 6 patient samples attempted (Table 2). In 4 of these samples (96, 200, 359, and 240) secondary hosts developed more rapid engraftment. After 4 to 8 weeks, every mouse from those 4 patients who engrafted had between 5.5 and 41.5 × 107 lymphoblasts in femoral bone marrow and spleen, representing a minimum 5- to 42-fold increase in ALL in every mouse. Table 2 lists average time to primary engraftment, secondary engraftment, and magnitude of leukemic expansion for individual patient samples.
Establishment of xenografts. NOD/SCID mice were irradiated with 275 cGY and were injected with 107 lymphoblasts obtained from patients with pre-B ALL. Xenografts were established from 78% of patient samples used as assessed by flow cytometry for ALL. Top panel shows flow cytometric analysis of CD45 and CD19 staining of peripheral blood from a control mouse (A, 0.1%) and a xenografted mouse (D, 32%). Splenocytes from this mouse were 65% human ALL (B), and bone marrow was 94% human ALL (C). The majority of xenografted animals had more than 90% replacement of bone marrow and more than 60% replacement of spleen with human ALL. Also pictured are photomicrographs of Wright-Giemsa-stained peripheral blood (E) with human lymphoblast (arrow) and murine granulocyte (shown) and a cytospin prepared from bone marrow nucleated cells showing a monomorphic population of lymphoblasts (F). Images were captured using a Zeiss Axiovert 40C light microscope (Carl Zeiss, Thornwood, NJ) equipped with an apochromatic 40 ×/0.60 NA objective lens and a Nikon 995 camera (Nikon, Melville, NY).
Establishment of xenografts. NOD/SCID mice were irradiated with 275 cGY and were injected with 107 lymphoblasts obtained from patients with pre-B ALL. Xenografts were established from 78% of patient samples used as assessed by flow cytometry for ALL. Top panel shows flow cytometric analysis of CD45 and CD19 staining of peripheral blood from a control mouse (A, 0.1%) and a xenografted mouse (D, 32%). Splenocytes from this mouse were 65% human ALL (B), and bone marrow was 94% human ALL (C). The majority of xenografted animals had more than 90% replacement of bone marrow and more than 60% replacement of spleen with human ALL. Also pictured are photomicrographs of Wright-Giemsa-stained peripheral blood (E) with human lymphoblast (arrow) and murine granulocyte (shown) and a cytospin prepared from bone marrow nucleated cells showing a monomorphic population of lymphoblasts (F). Images were captured using a Zeiss Axiovert 40C light microscope (Carl Zeiss, Thornwood, NJ) equipped with an apochromatic 40 ×/0.60 NA objective lens and a Nikon 995 camera (Nikon, Melville, NY).
Efficacy of mTOR inhibitors in xenograft model
We hypothesized that mTOR inhibitors would be effective agents in adult human ALL because mTOR signaling is required for the survival of ALL blasts. To test our hypothesis in vivo, we generated sufficient numbers of xenografted mice from the 4 patient samples (Table 1), which developed sufficient quantities of blasts (> 5%) to evaluate CCI-779 as an antileukemic agent. After establishment of disease, mice were randomized to treatment with 5 to 10 mg/kg/d of CCI-779 or vehicle alone as control. Mice were treated 6 days per week. The dose of CCI-779 given was chosen based on unpublished mouse pharmacokinetic data obtained from Wyeth (L. Speicher, personal oral communication, October 2003). Weekly analysis of peripheral blood by CBC and flow cytometry was used to monitor ALL response. Ill-appearing mice were killed for multicompartmental analysis.
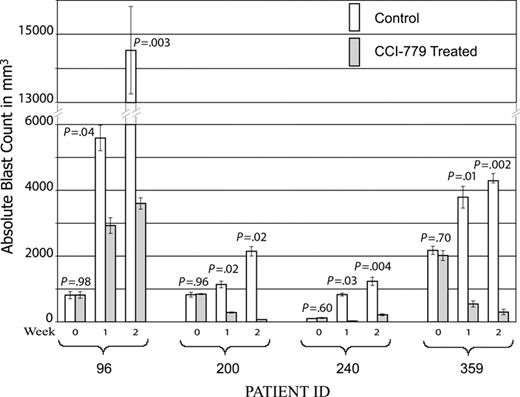
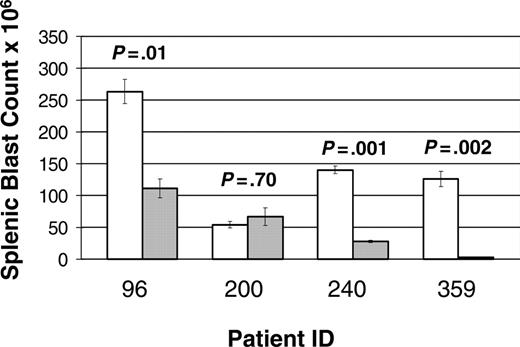
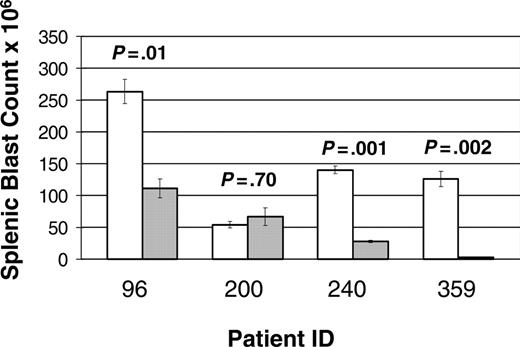
A total of 68 mice were entered in the study: 25 mice (13 treatments and 12 controls) from patient 96, 16 mice (9 treatments and 7 controls) from patient 200, 18 mice (9 treatment and 9 control) from patient 240, and 9 mice (5 treatment and 4 control) from patient 359. Of note, 3 mice (2 treatment and 1 control) xenografted from patient 200 succumbed to disease prior to first evaluation time point (1 week). A statistically significant improvement in disease burden was seen in all samples tested (Figure 3). Mice xenografted from 3 of the patients (96, 240, and 359) had a statistically significant decrease in splenic disease bulk (Figure 4). Mice generated from patient 200 demonstrated a decrease in peripheral blasts but did not demonstrate a decrease in splenic disease. These results demonstrate that mTOR signaling is required for the survival of ALL blasts in vivo and that CCI-779 is potentially an effective agent in adult human ALL.
To assess drug effect, it was necessary to wait until mice had advanced disease (> 5% blasts in the blood, with substantial disease burden in the spleen), by which point prolonged survival of these ill animals was not feasible. To show a survival difference, we also used a model of treatment earlier in the course of disease. Here we demonstrated a survival difference (average 43 vs 76 days after injection, P = .007) (not shown).
Toxicity of CCI-779 treatment
No appreciable toxicity was noted after treatment with CCI-779 except for modest thrombocytopenia. CCI-779 treatment did not impact hemoglobin levels, with a mean hemoglobin level of 143 g/L (normal range, 110-151 g/L) observed in treated mice and 139 g/L in control animals (P = .77). A modest impact on platelet counts was observed, with mean platelet count of 296 × 109/L(normal range, 592-2 972 × 109/L) observed in treated mice and 403 × 109/L in control mice. This difference was statistically significant (P < .001).
CCI-779 is efficacious in ALL xenografts. NOD/SCID mice were xenografted with human ALL from patient samples. After establishment of disease, defined as more than 5% blasts detected in peripheral blood, mice were randomized to treatment with 5 to 10 mg/kg/d of CCI-779 versus vehicle control. Disease was evaluated at weekly intervals by FACS analysis of peripheral blood, detecting human CD19+ and CD45+ cells. Graph depicts mean absolute blast counts (WBC × % blasts by FACS analysis) from mice generated from the 4 patient samples at weekly intervals, demonstrating statistically significant difference (P < .05) in all samples. Error bars depict standard error of the mean (SEM).
CCI-779 is efficacious in ALL xenografts. NOD/SCID mice were xenografted with human ALL from patient samples. After establishment of disease, defined as more than 5% blasts detected in peripheral blood, mice were randomized to treatment with 5 to 10 mg/kg/d of CCI-779 versus vehicle control. Disease was evaluated at weekly intervals by FACS analysis of peripheral blood, detecting human CD19+ and CD45+ cells. Graph depicts mean absolute blast counts (WBC × % blasts by FACS analysis) from mice generated from the 4 patient samples at weekly intervals, demonstrating statistically significant difference (P < .05) in all samples. Error bars depict standard error of the mean (SEM).
Evaluation of MTI signaling intermediates by immunoblot
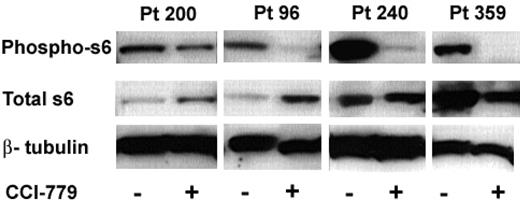
We hypothesized that the clinical response to CCI-779 in our xenografts would be associated with a biochemical response, indicating down-regulation of the mTOR signaling pathway. mTOR inhibitors impact protein synthesis through specific inhibition of 5′cap associated proteins.29-31 We have previously shown that activation of S6K1 (p70S6 kinase), an mTOR pathway intermediate, is down-regulated by rapamycin in murine B-cell lines.11 Similarly, other groups assessed phosphorylation of S6, the target of S6K1, in normal cells32 and other tumor types,33 as a marker of mTOR inhibition. In these experiments, we were attempting to validate S6 as a surrogate marker of mTOR inhibition in human ALL. We performed immunoblots on splenocytes obtained from treated and control xenografted mice to assess phosphorylation of S6. We performed immunoblots on mice treated with CCI-779 for both short (3-day) and long (3-week) duration. We were able to perform immunoblots from 3 patient samples (96, 200, and 240) at both time points; however, we only performed short-term (3-day) analysis of blasts from patient 359 because the mice were in remission after 2 weeks of treatment and had no blasts to study.
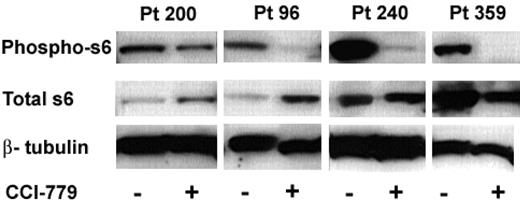
When mTOR is active, S6K1 phosphorylates S6, leading to ribosomal protein synthesis. Inhibition of mTOR results in dephosphorylation of S6 and inhibits this process. We consistently found down-regulation of the mTOR pathway as a consequence of these doses of CCI-779 in all samples (Figure 5).
Immunoblot analysis of ALL-cell lysates from CCI-779-treated mice demonstrated a 48% to more than 95% decrease in the amount of phospho-S6 compared to blasts from untreated control mice in all samples at both time points. We also noted changes in total S6 expression in the treated mice between time points from the 3 patients, where we were able to evaluate both time points. Total S6 was either increased (96 and 200) or unchanged (240) after short-term (3-day) treatment, but was decreased in all 3 patients after long-term treatment. These data suggest that treatment with an mTOR inhibitor initially results in dephosphorylation of S6 with a compensatory rise in total S6 production and with time and chronic mTOR inhibition, total S6 may be down-regulated.
Response of splenic disease to CCI-779 treatment. Xenografted mice with established disease (> 5% peripheral blasts) were randomized to treatment with 5 to 10 mg/kg/d of CCI-779 versus control (vehicle). Lymphoblasts in spleen in treated and untreated mice were compared at time of death. Graph represents number of blasts in millions (× 106), demonstrating statistically significant difference (P < .05) in 3 of 4 patient samples. Splenic blast counts were calculated by multiplying total number of cells in spleens by percent of anti-human CD19+ and anti-human CD45+ cells as determined by FACS analysis. Error bars depict SEM.
Response of splenic disease to CCI-779 treatment. Xenografted mice with established disease (> 5% peripheral blasts) were randomized to treatment with 5 to 10 mg/kg/d of CCI-779 versus control (vehicle). Lymphoblasts in spleen in treated and untreated mice were compared at time of death. Graph represents number of blasts in millions (× 106), demonstrating statistically significant difference (P < .05) in 3 of 4 patient samples. Splenic blast counts were calculated by multiplying total number of cells in spleens by percent of anti-human CD19+ and anti-human CD45+ cells as determined by FACS analysis. Error bars depict SEM.
In vivo we found all 4 patient samples had decreased peripheral blasts after exposure to CCI-779, and 3 of the 4 patient samples had decreased splenic bulk. While all patient samples had down-regulation of phospho-S6, the patient sample with the least response in vivo (patient 200) had the least robust down-regulation of phospho-S6. These results demonstrate that the clinical response we found in the xenografts is associated with a biochemical response in the mTOR signaling pathway. This in turn supports the potential use of the phospho-S6 assay in ALL cells as a biologic marker of mTOR inhibition.
Discussion
Precursor B ALL arises from transforming events that occur in B-cell progenitors. Although the highest incidence of ALL occurs in children, ALL also occurs in adults (0.4 per 100 000).34 Adults with ALL fare significantly worse, with 5-year disease-free survival rates of 28% to 39%.1 Prognostic differences exist between children and adults even when treated with similar therapies and when comparing populations with similar prognostic variables, including WBC at diagnosis, cytogenetics, and sex.34 Apparently, even though adult and childhood ALL are morphologically similar, they act as different diseases and require different treatment strategies. Thus, to determine if an agent is potentially effective in adult ALL, models are needed that correlate specifically to adult ALL.
Treatment with MTIs results in hypophosphorylated S6 in ALL. Splenocytes were harvested from mice treated with CCI-779 or control (vehicle). Immunoblot of phospho-S6 (ser235/236) of control and CCI-779 treated (top bands), total S6 (middle bands), and β-tubulin (bottom band; loading control) from 4 patient samples is depicted. We found a correlation between clinical response and biochemical response to CCI-779 in all samples. ALL cells from CCI-779-treated mice had 48% to more than 95% down-regulation of phospho-S6 compared to blasts from control mice.
Treatment with MTIs results in hypophosphorylated S6 in ALL. Splenocytes were harvested from mice treated with CCI-779 or control (vehicle). Immunoblot of phospho-S6 (ser235/236) of control and CCI-779 treated (top bands), total S6 (middle bands), and β-tubulin (bottom band; loading control) from 4 patient samples is depicted. We found a correlation between clinical response and biochemical response to CCI-779 in all samples. ALL cells from CCI-779-treated mice had 48% to more than 95% down-regulation of phospho-S6 compared to blasts from control mice.
We successfully established and expanded leukemia in NOD/SCID mice to test efficacy of CCI-779, a potential therapeutic agent for adult ALL. Here, we present convincing evidence that we can significantly reduce overall disease burden in 3 different patient samples in the mice and reduce peripheral blasts in a fourth. Lymphoblasts from all 4 patients responded to CCI-779 in vitro, in vivo, and within the targeted signal transduction pathway, as assessed by immunoblots detecting S6K1. The degree of response varied somewhat using our differing assessment models. Using these patient specimens, we found most comparable degrees of response to drug between the long-term culture model and the xenograft (SCID/NOD) model, while the short-term culture model allowed for the assessment of the apoptotic response to CCI-779. These data establish proof of principle that CCI-779 may be an effective agent for treating human adult ALL, since previous work20,22 has shown that xenografted ALL maintains its original phenotypic and genotypic characteristics and that response to therapy in the mice correlates to human disease. ALL is a heterogeneous disease, and evaluating the spectrum of ALL response will require future studies with more samples and, eventually, a clinical trial. A cooperative group phase 3 clinical trial testing the use of the mTOR inhibitor rapamycin after bone marrow transplantation to prevent ALL relapse has been approved by the cancer therapy evaluation program.
We have also used a bone marrow stroma-supported culture system to demonstrate that CCI-779 caused both growth inhibition and induction of apoptosis in human adult ALL cells. We have evidence suggesting CCI-779 decreased proliferation of cultured lymphoblasts. The majority of leukemic blasts underwent apoptosis within 24 hours of treatment with CCI-779. As we have previously shown,25 cultured lymphoblasts from patients with ALL (including patients evaluated in the work) divide every 48 to 96 hours in stromal culture. Thus, many of the cells may be undergoing apoptosis from CCI-779 exposure prior to cell division.
One of the difficulties in treating adults with ALL is that many patients have difficulty tolerating current therapies.1 Current chemotherapeutic protocols for adult ALL include extremely myelosuppressive chemotherapy with or without hematopoietic stem cell transplantation. These protocols may be poorly tolerated, or dosing may be attenuated. MTIs, including CCI-779, are well tolerated in humans and have a side-effect profile more favorable than most current ALL therapies. The most common side effects of MTIs are hyperlipidemia, mild myelosuppression, and immunosuppression when dosed on a daily basis.35 Thus, effective agents that are less toxic and myelosuppressive, such as CCI-779, may be beneficial.
CCI-779, as single-agent therapy, would most likely be ineffective in curing patients with ALL; however, CCI-779 may add synergy and improve survival when combined with conventional cytotoxic agents. Recently, it was shown that FLT3 inhibitors, when added with conventional cytotoxic agents, were synergistic if added concomitantly but antagonistic if added prior to conventional therapy.36 Presumably, the FLT3 inhibitors induce cell-cycle arrest, and nondividing cells are less susceptible to cell-cycle-specific cytotoxic chemotherapeutics. MTIs cause G1 arrest,3 and a similar phenomenon may be found when combining MTIs with conventional agents. Thus, we will focus future work on preclinical testing of MTIs in combination with other agents in both of our preclinical models to determine optimal schedule of administration.
Most studies evaluating chemotherapeutics in NOD/SCID mice use mortality as a crude measurement of drug efficacy. Only a few studies22,37,38 have used serial blood sampling to follow disease response to a given therapy, and only 2 studies22,38 have used a model of evaluating response after establishment of disease. A model of treatment after establishment of disease correlates most directly with treatment in humans, and this type of model allows for testing of various combinations of multiagent therapies.
Another exciting potential of the NOD/SCID xenograft model is the ability to expand leukemic cells for biochemical and functional assays. The number of lymphoblasts obtained from patients that are available for study is often limited and, once used, they are not replaceable. The NOD/SCID model allows for very significant expansion of human ALL. For example, we have expanded lymphoblasts from patient sample 96 from original 5 × 107 cells injected into 5 mice into 3.2 × 109 cells (a 64-fold increase) available for further studies or subsequent re-injection.
While the xenograft model is an exciting in vivo model, many biochemical and mechanistic questions, including assessment of apoptosis, are difficult to address in vivo. The bone marrow stromal culture system affords us the opportunity to answer these questions on primary human lymphoblasts in vitro and gain insight into the effect of mTOR inhibition on apoptosis.
Clinical trials and in vivo experiments require a reliable assay for mTOR inhibition, such as a surrogate marker of in vivo biologic activity. We hypothesized that phosphorylation of S6 by S6K1 will serve as a surrogate marker for mTOR inhibition. Our data show that this target is hypophosphorylated after MTI exposure in tumor tissue, validating this marker as a surrogate of biochemical response and mTOR pathway inhibition in clinical trials of these agents. In future experiments, we will continue to study the mechanism of action of MTIs in NOD/SCID xenografts by investigating post-translational regulation of a number of signaling intermediates, hoping to better understand the mechanisms of MTI resistance, comparing sensitive to resistant ALL specimens. Because of the vast expansion of cell numbers in these xenografts, they are particularly suited to these biochemical experiments.
In summary, the NOD/SCID xenograft model and bone marrow stromal-cell culture provide powerful tools for preclinical testing of drug efficacy. In addition, because of the capacity to vastly expand lymphoblasts while potentially maintaining functional and phenotypic integrity, the xenograft model is particularly suited to the performance of biochemical experiments. These experiments will allow characterization of the role of the mTOR pathway in leukemia and the mechanism(s) through which ALL may become resistant to mTOR inhibition. We have shown that mTOR inhibitors can inhibit the growth of primary adult human ALL, and, therefore, mTOR inhibitors deserve close examination as therapeutic agents against a disease that has low cure rates with current therapy.
Prepublished online as Blood First Edition Paper, September 29, 2005; DOI 10.1182/blood-2005-05-1935.
Supported by grants CA82156, CA98543 (through Children's Oncology Group), the Sanford Chair of the Children's Hospital of Philadelphia (S.A.G.), and CA96696 (P.J.H.), and an American Society of Clinical Oncology Young Investigator Award (D.T.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Junior Hall and Anne Chapman for their help with the animal experiments, and Marybeth Helfrich for her help with staining and analyzing peripheral blood smears and generating bone marrow cytospins.