Abstract
According to the prevailing paradigm, neutrophils are short-lived cells that undergo spontaneous apoptosis within 24 hours of their release from the bone marrow. However, neutrophil survival can be significantly prolonged within inflamed tissue by cytokines, inflammatory mediators, and hypoxia. During screening experiments aimed at identifying the effect of the adhesive microenvironment on neutrophil survival, we found that VCAM-1 (CD106) was able to delay both spontaneous and Fas-induced apoptosis. VCAM-1-mediated survival was as efficient as that induced by the cytokine IFN-β and provided an additive, increased delay in apoptosis when given in combination with IFN-β. VCAM-1 delivered its antiapoptotic effect through binding the integrin α9β1. The α9β1 signaling pathway shares significant features with the IFN-β survival signaling pathway, requiring PI3 kinase, NF-κB activation, as well as de novo protein synthesis, but the kinetics of NF-κB activation by VCAM-1 were slower and more sustained compared with IFN-β. This study demonstrates a novel functional role for α9β1 in neutrophil biology and suggests that adhesive signaling pathways provide an important extrinsic checkpoint for the resolution of inflammatory responses in tissues.
Introduction
Neutrophils are terminally differentiated cells with a short half life within the circulation in vivo and in culture in vitro.1,2 However, neutrophil survival can be extended by exposure to a wide variety of cytokines (interferon-α/β, granulocyte-macrophage colony-stimulating factor [GM-CSF]), inflammatory mediators (fibrinogen and C5a),3-5 as well as the physicochemical properties of the local environment such as oxygen tension.6 In some inflammatory conditions such as arthritis, peritonitis, and meningitis, neutrophils accumulate and survive for prolonged periods extending up to many days.7-9 Furthermore, recent studies have shown that tissue-infiltrating neutrophils are transcriptionally active, produce lymphocyte-activating cytokines such as the TNF family member BlyS/BAFF, and therefore provide an important link in the transition between innate and acquired immunity during the development of inflammatory reactions.10
During an inflammatory response, local mediators induce neutrophil migration from blood into tissue.2 The interaction of neutrophils with endothelium has been well characterized, and a number of adhesion molecule families are known to be involved, including selectins, integrins, and IgSF members.11-13 Whether these adhesive interactions provide signals that are capable of modulating cell survival as well as adhesion and migration remains unclear, but there have been reports that neutrophil apoptosis can be delayed following transendothelial migration and adhesion to subendothelial matrix components.14,15
Neutrophil integrins fall into 2 distinct groups: those that are constitutively expressed (αLβ2, αMβ2, αvβ3, and α9β1) and others that are up-regulated upon activation (α2β1, α3β1, α5β1, and αvβ3).11 In some cases the expression of constitutive integrins can be further increased upon stimulation, suggesting a dual role for these particular integrins. One such integrin is the α9β1 integrin, which is expressed on neutrophils, smooth and skeletal muscle, and epithelia and can bind VCAM-1 (CD106) and the extracellular matrix protein osteopontin.16,17 Although expression of α9β1 on neutrophils has been recognized for some time, the full range of biologic functions relevant to neutrophil behavior is unclear.
Integrin engagement in conjunction with chemokine-delivered signals regulates neutrophil navigation across endothelium and within tissues.18,19 Integrin engagement activates a wide range of signaling pathways such as integrin-linked kinase (ILK), focal adhesion kinase (FAK), phosphoinositol 3-kinase (PI3K), the serine/threonine kinase protein kinase B (PKB/AKT), and the extracellular signal-regulated kinase (ERK) pathway.20-25 Most of these pathways converge on and modulate NF-κB.26,27 Activation of a number of these pathways has also been demonstrated in neutrophils upon exposure to IFN-β, suggesting that integrin-mediated and cytokine survival signaling pathways might have the potential to interact with each other to modify neutrophil survival.3
In this study we describe a previously unrecognized survival pathway involving the integrin α9β1 and cell adhesion molecule VCAM-1. We show that this pathway activates NF-κB and is likely to be important for enhancing survival in inflammatory microenvironments where neutrophils are exposed to a wide variety of soluble cytokine, cell-cell, and cell-matrix-derived signals.
Materials and methods
Reagents and antibodies
All reagents were purchased from Sigma Aldrich (Poole, United Kingdom) unless otherwise stated. The recombinant soluble human proteins ICAM-1 (CD54), PECAM-1 (CD31), and VCAM-1 (CD106) were purchased from R&D Systems (Abingdon, United Kingdom). Human soluble recombinant IFN-β was purchased from Biosource (Nivelles, Belgium). The integrin antibodies used for flow cytometery were anti-α4 (clone HP2/1, IgG1; Serotec, Kidlington, United Kingdom), anti-α9 (clone Y9A2, IgG1; Chemicon, Chandlers Ford, United Kingdom), anti-αV (clone P3G8, IgG1; Chemicon), anti-β1 (clone S3S, IgG1; Serotec), anti-β2 (clone MHM23, IgG1; Dako, Glostrup, Denmark), and anti-β3 (clone RUU-PL7F12, IgG1; BD Pharmingen, San Diego, CA). Other antibodies used were CD16 (clone 3G8, IgG1; BD Pharmingen), CD3 (clone UCHT1, IgG1; Dako), CD4 (clone SK3, IgG1; BD Pharmingen), and an activating CD95 antibody (clone CH-11, IgM; Upstate Biotechnology, Lake Placid, NY). The secondary antibody used was goat F(ab′)2 anti-mouse IgG1-FITC (Southern Biotech, Birmingham, AL), and the irrelevant control was mouse IgG1 (Dako). For Western blotting, the primary antibodies were as those used for flow cytometry, and the secondary antibody was a sheep anti-mouse IgG-HRP detected using the ECL Plus chemiluminescent substrate (both from Amersham). Functional blocking antibodies to VCAM-1 (clone 1G11, IgG1) and α4β1 (clone Max68P, IgG1) were kind gifts from Dr Tony Shock, Celltech (Slough, United Kingdom).
Isolation and culture of human leukocytes
Venous blood from healthy volunteers was used to isolate neutrophils on Percoll density gradients as described previously.28 Approval was obtained from the South Birmingham Local Ethics Committee for these studies. Informed consent from all volunteers was provided according to the Declaration of Helsinki. Neutrophil preparations contained more than 97% neutrophils, and contaminating cells were mainly eosinophils. Neutrophils were resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum (Labtech International, Ringmer, United Kingdom), 1.64 mM glutamine, 40 U/mL penicillin, and 40 μg/mL streptomycin. Neutrophils were either used immediately as control samples or incubated in a humidified 5% CO2 atmosphere in the presence or absence of human soluble recombinant adhesion proteins at the concentrations indicated for 18 hours. For studies involving the PI3K inhibitor LY294002 or the inhibitor of NF-κB function SN50 (both purchased from Calbiochem, San Diego, CA), cells were incubated with the inhibitors for 30 minutes at the concentrations indicated before the addition of other factors. To determine the requirement for de novo protein synthesis, 1 μg/mL cycloheximide was added to cell cultures at the same time as other survival factors. CD4+ T cells were generated and cultured from peripheral blood as previously described and were used at passage 5, 7 days after restimulation with PHA.29
Quantification of apoptosis
To determine the effect of survival factors on spontaneous neutrophil apoptosis, mitochondrial permeability transition was detected using a method based upon the ability of intact mitochondria to take up and retain cationic lipophilic fluorescent dyes.30 Cells were loaded at 37°C for 30 minutes with 40 nM DiOC6 (Molecular Probes, Leiden, Netherlands) and then washed at 4°C with PBS, resuspended in ice-cold PBS, and analyzed by flow cytometry. DiOC6 is retained by cells with intact mitochondria but is lost when permeability transition occurs. Neutrophils were also analyzed for changes in morphology using cytospin preparations of fresh or cultured cells. Cytospins were then stained using a commercial May-Grunwald Giemsa stain (Diff-Quick; Gamidor, Abingdon, United Kingdom) and assessed for apoptotic morphology using a Zeiss Axiostar Plus microscope equipped with a 100×/1.25 objective lens (Carl Zeiss, Welwyn Garden City, United Kingdom). Micrograph images were visualized using a Zeiss Universal microscope equipped with a 40×/0.75 objective lens and captured with a SPOT-2 (Diagnostic Instruments, Sterling Heights, MI) digital camera and Image-Pro software (Media Cybernetics, Silver Spring, MD). Quantification of caspase activity was assessed by cleavage of a tagged caspase 3 substrate (DEVD-7-amino-4-methyl-coumarin [DEVD-AMC]) and the release of the fluorochrome AMC using a commercial kit (R&D Systems).
Flow cytometry
Freshly isolated neutrophils or short-term-cultured CD4 cells were checked for expression of potential VCAM-1-binding integrins by flow cytometery. Cells were washed twice with PBS and incubated on ice with either primary antibody or murine IgG1 control in PBS/BSA for 30 minutes. Cells were then washed twice with ice-cold PBS, incubated with the appropriate secondary antibody, and run on a Coulter XL flow cytometer (Beckman Coulter, High Wycombe, United Kingdom).
Immunoprecipitation and Western blots
Soluble recombinant VCAM-1-Fc protein (kind gift from Dr Tony Shock, Celltech) and human IgG Sepharose beads were prepared by covalently coupling VCAM-1-Fc to protein A-Sepharose beads (Amersham, United Kingdom) using dimethylpimelimidate as described.31 Freshly isolated neutrophils were resuspended at 20 × 106/mL and 500 μL added to duplicate eppendorfs. Twenty microliters protein A-sepharose bead slurry was added and the eppendorfs incubated for 20 minutes at 37°C; 500 μL the cross-linking agent DTSP (0.04%) was added to each eppendorf and incubated at 4°C for 20 minutes. The reaction was quenched with 10 μL 1-M glycine and incubated for a further 10 minutes at 4°C. The cells were then lysed for 20 to 30 minutes on ice with hypotonic lysis buffer (50 mM HEPES pH 7.4, 1 mM EDTA pH 8, 1 mM EGTA pH 8, 1% Triton X-100, and 10% protease inhibitor cocktail [Roche, Milan, Italy). The beads were spun down and washed several times with TBS/0.05% Tween 20 and then resuspended in lysis buffer with SDS sample buffer. Lysates were boiled, run on a reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to PVDF membrane, and Western blotted as normal, probing with the antibodies shown.
Neutrophil functional studies
To measure the functionality of neutrophils, superoxide release upon stimulation with 200 nM phorbol myristate acetate (PMA) was measured using the substrate cytochrome c. Neutrophils were incubated for 18 hours in the presence or absence of soluble factors, washed once in HBSS buffer (HBSS pH 7.3 plus 25 mM HEPES and 5 mM glucose), and resuspended in HBSS containing 1% human serum (HD Supplies, Aylesbury, United Kingdom). A total of 0.5 × 105 neutrophils was added to triplicate wells of a 96-well plate in the presence of PMA or HBSS as an unstimulated control. Release of superoxide was quantified by measuring the color change of cytochrome c. The positive control was complete reduction of cytochrome c using the reducing agent sodium dithionite (100 mM).
NF-κB activation
The activation of NF-κB was detected by electrophoretic mobility shift assay (EMSA) using a protocol that has already been described.3 Briefly, neutrophils were incubated with either 1000 U/mL IFN-β, 10 μg/mL VCAM-1, or both together for 30 minutes at 37°C. Cells were then disrupted by nitrogen cavitation and nuclear proteins collected. Protein concentration was measured by BCA assay (Pierce, Rockford, IL), and 10 ng 32P-end-labeled double-stranded oligonucleotide (sequence 5′-tcgaacggcaggggaattcccctctc-3′) was incubated with the extract as described.3 Binding of the probe to NF-κB was visualized by separation on a 5.5% polyacrylamide gel and detected by autoradiography. Bands were quantified by exposure of the blots to a phosphoimager screen, followed by analysis using a Typhoon 9200 variable mode imager and ImageQuant software (Amersham Biosciences).
Results
Soluble VCAM-1 delays spontaneous neutrophil apoptosis
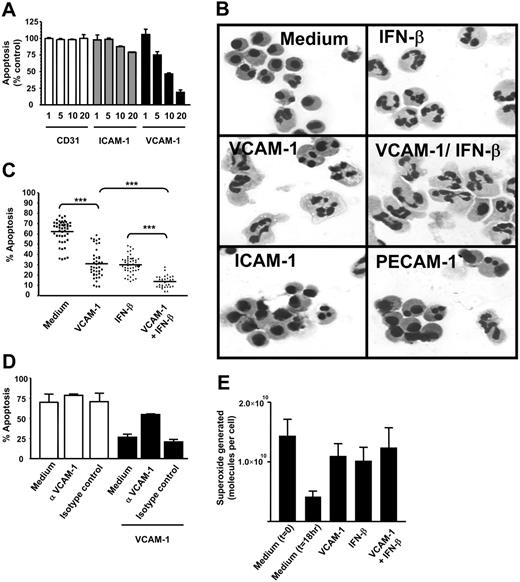
During a screen for soluble adhesion molecules that might affect neutrophil apoptosis, we discovered that recombinant VCAM-1 was able to delay spontaneous neutrophil apoptosis in a dose-dependent manner (Figure 1A). This was not a nonspecific effect of adhesion per se, because neither the adhesion molecule PECAM-1 (CD31) nor ICAM-1 (CD54), both of which can support neutrophil adhesion, significantly affected apoptosis. We confirmed the delay in apoptosis measured by DiOC6 staining using cell morphology (small shrunken cells with condensed nuclei) and found that the VCAM-1-induced cell survival was as efficient as and additive to IFN-β-(1000 U/mL) mediated survival (Figure 1B-C).
Soluble VCAM-1 delays the functional senescence of neutrophils. (A) Neutrophils were cultured in the presence or absence of soluble adhesion molecules for 18 hours at the concentrations stated (micrograms per milliliter). Apoptosis was measured by DiOC6 staining. Results are expressed as the percentage of apoptotic neutrophils compared with neutrophils cultured in medium alone; mean ± SD of 3 separate experiments. (B) Neutrophils were cultured for 18 hours in the presence or absence of soluble adhesion molecules (VCAM-1, ICAM-1, PECAM-1 all at 10 μg/mL) or IFN-β (1000 U/mL). Apoptosis is evident in medium and ICAM-1- and PECAM-1-treated cells by the presence of small shrunken cells with condensed nuclei. (C) Neutrophils were cultured in the presence or absence of 10 μg/mL sVCAM-1, 1000 U/mL IFN-β, or both together and apoptosis measured by DiOC6 staining. Each point is a single experiment. Bar is the mean; ***P < .001. (D) Neutrophils were incubated with VCAM-1 in the presence or absence of the anti-VCAM antibody 1G11 (10 μg/mL). Cells were cultured alone (□) or in the presence of VCAM-1 (▪). An isotype-matched control antibody is shown. Apoptosis was measured by DiOC6 staining. Data are the mean ± SD of 3 separate experiments. (E) VCAM-1 delays the functional senescence of neutrophils. Superoxide generation in response to PMA was determined by measuring oxidation of cytochrome c at the time of neutrophil isolation (t = 0) or following 18 hours of incubation with medium alone, 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together. Data are the mean ± SD of 3 separate experiments.
Soluble VCAM-1 delays the functional senescence of neutrophils. (A) Neutrophils were cultured in the presence or absence of soluble adhesion molecules for 18 hours at the concentrations stated (micrograms per milliliter). Apoptosis was measured by DiOC6 staining. Results are expressed as the percentage of apoptotic neutrophils compared with neutrophils cultured in medium alone; mean ± SD of 3 separate experiments. (B) Neutrophils were cultured for 18 hours in the presence or absence of soluble adhesion molecules (VCAM-1, ICAM-1, PECAM-1 all at 10 μg/mL) or IFN-β (1000 U/mL). Apoptosis is evident in medium and ICAM-1- and PECAM-1-treated cells by the presence of small shrunken cells with condensed nuclei. (C) Neutrophils were cultured in the presence or absence of 10 μg/mL sVCAM-1, 1000 U/mL IFN-β, or both together and apoptosis measured by DiOC6 staining. Each point is a single experiment. Bar is the mean; ***P < .001. (D) Neutrophils were incubated with VCAM-1 in the presence or absence of the anti-VCAM antibody 1G11 (10 μg/mL). Cells were cultured alone (□) or in the presence of VCAM-1 (▪). An isotype-matched control antibody is shown. Apoptosis was measured by DiOC6 staining. Data are the mean ± SD of 3 separate experiments. (E) VCAM-1 delays the functional senescence of neutrophils. Superoxide generation in response to PMA was determined by measuring oxidation of cytochrome c at the time of neutrophil isolation (t = 0) or following 18 hours of incubation with medium alone, 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together. Data are the mean ± SD of 3 separate experiments.
To confirm that VCAM-1-mediated survival was not attributable to an unknown factor in the preparations of recombinant human VCAM-1, neutrophils were incubated with VCAM-1 in the presence of a blocking anti-VCAM-1 antibody (1G11) (Figure 1D). Treatment of neutrophils with this antibody in the absence of VCAM-1 did not alter the levels of spontaneous apoptosis; neither did the use of an isotype-matched control antibody. However VCAM-1-mediated survival was abolished by the anti-VCAM-1 blocking antibody, confirming that the response was specific to VCAM-1. To determine whether VCAM-1-induced survival was functionally relevant, we confirmed that exposure to VCAM-1 led to functionally active neutrophils as measured by their ability to generate superoxide radicals (Figure 1E).
Soluble VCAM-1 inhibits death-receptor- (Fas) induced apoptosis
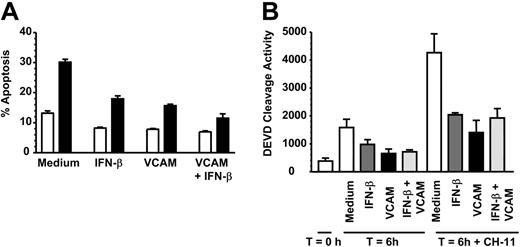
Neutrophils can undergo both spontaneous as well as death-induced apoptosis mediated via ligation of the TNF receptor family member Fas (CD95). We therefore tested whether VCAM-1 could inhibit death receptor-induced apoptosis, using the agonistic anti-CD95 (Fas) antibody CH-11. After 6 hours of incubation, VCAM-1 significantly inhibited CH-11-induced apoptosis as measured using 2 independent methods (Figure 2A-B). However, unlike the case for spontaneous apoptosis, there was no additive increase in survival observed between VCAM-1 and IFN-β. This suggests that there are likely to be additional VCAM-1-dependent signaling pathways that are required for inhibition of spontaneous neutrophil apoptosis but not Fas-induced apoptosis.
Immobilized VCAM-1 delays spontaneous neutrophil apoptosis
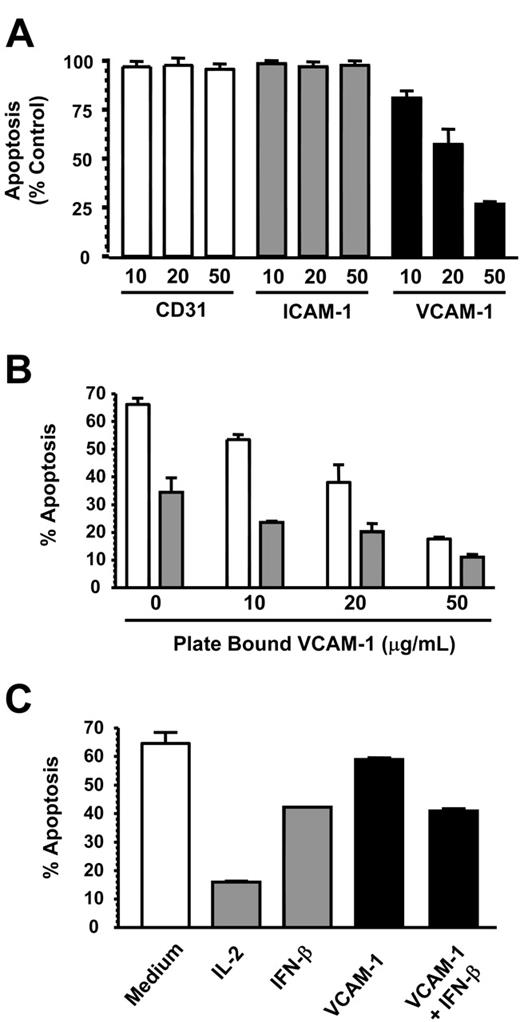
In inflammatory conditions, neutrophils may encounter both soluble and immobilized VCAM-1 on the surface of activated endothelial and other stromal cells such as smooth muscle cells and fibroblasts. We therefore tested the ability of immobilized VCAM-1 to delay spontaneous neutrophil apoptosis. Platebound VCAM-1, but not CD31 or ICAM-1, inhibited spontaneous neutrophil apoptosis in a dose-dependent manner, similar to that seen with VCAM-1 added in solution (Figure 3A). This suggests that the antiapoptotic effect of VCAM-1 is specific and not due to neutrophil adhesion in general. It also suggests that VCAM-1 induces survival through binding a cell surface receptor and does not need to be internalized (Figure 3A). Furthermore, the same additive survival-enhancing effect observed with soluble VCAM-1 and IFN-β (Figure 1C) was also observed with platebound VCAM-1 (Figure 3B).
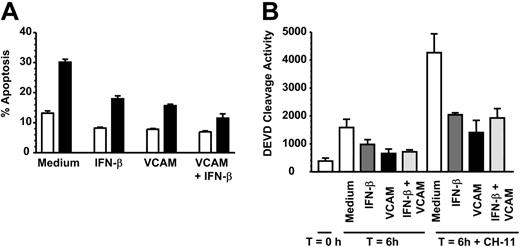
VCAM-1 inhibits FAS-induced neutrophil apoptosis. (A) Neutrophils were incubated with 10 μg/mL sVCAM-1, 1000 U/mL IFN-β, or both together in the absence (□) or presence (▪) of 50 ng/mL CD95 antagonist antibody CH-11 for 6 hours and apoptosis measured by DiOC6 staining. Data are the mean ± SD of 3 separate experiments. (B) Caspase 3 activation was measured in freshly isolated neutrophils (T = 0) or neutrophils incubated for 6 hours in medium alone (□) or presence of 1000 U/mL IFN-β (▪), 10 μg/mL soluble VCAM-1 (▪), or both together (▪) with or without 50 ng/mL of the CD95 activating antibody CH-11. Data are the mean ± SD of 3 separate experiments.
VCAM-1 inhibits FAS-induced neutrophil apoptosis. (A) Neutrophils were incubated with 10 μg/mL sVCAM-1, 1000 U/mL IFN-β, or both together in the absence (□) or presence (▪) of 50 ng/mL CD95 antagonist antibody CH-11 for 6 hours and apoptosis measured by DiOC6 staining. Data are the mean ± SD of 3 separate experiments. (B) Caspase 3 activation was measured in freshly isolated neutrophils (T = 0) or neutrophils incubated for 6 hours in medium alone (□) or presence of 1000 U/mL IFN-β (▪), 10 μg/mL soluble VCAM-1 (▪), or both together (▪) with or without 50 ng/mL of the CD95 activating antibody CH-11. Data are the mean ± SD of 3 separate experiments.
VCAM-1 does not inhibit T-cell apoptosis
We next investigated whether VCAM-1 could support the survival of other leukocyte cell types or whether it was specific to neutrophils. We chose CD4 T cells, which are known to be capable of binding VCAM-1 though the integrin α4β1.21 While IL-2 and IFN-β both inhibited T-cell apoptosis efficiently, VCAM-1 was unable to prevent T-cell apoptosis even at very high concentrations up to 100 μg/mL (Figure 3C). This suggested that the necessary receptors or signaling machinery for VCAM-1-mediated survival were not present in CD4 T cells.
VCAM-1-mediated inhibition of spontaneous neutrophil apoptosis is mediated by the α9β1 integrin
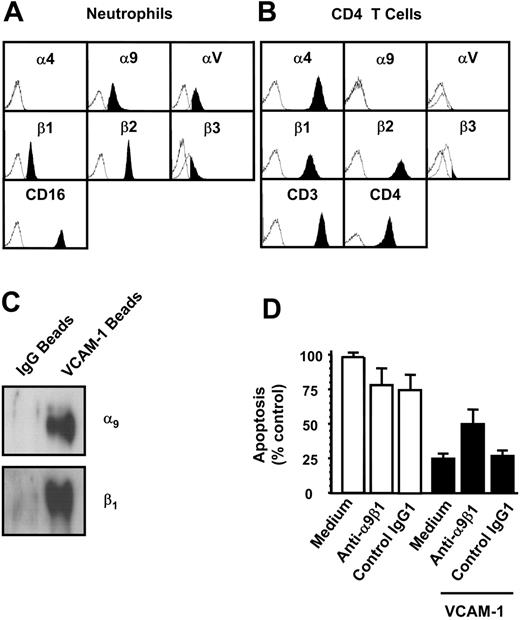
VCAM-1 is known to bind to two β1 integrins (α4β1 and α9β1) as well as the integrin α4β7. To identify whether neutrophils and T cells differed with respect to their expression of potential VCAM-1 receptors, we examined which integrin receptors were present on both cell types by flow cytometry (Figure 4A-B). Consistent with previous reports, neutrophils expressed the integrin α9β1 but neither of the α4 chain-containing integrins, α4β1 or α4β7. CD4 T cells, in contrast, expressed α4β1 but not α9β1.
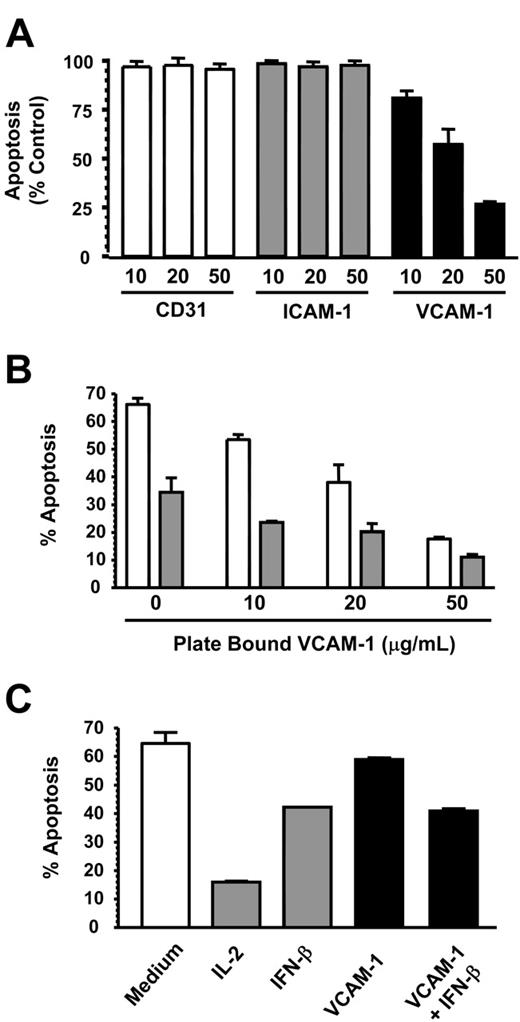
VCAM-1 inhibits neutrophil but not lymphocyte apoptosis. (A) CD31, ICAM-1, and VCAM-1 were immobilized at the concentrations shown (micrograms per milliliter) and neutrophils added for 18 hours before apoptosis was measured by DiOC6 staining. Results are expressed as the percentage of apoptotic neutrophils compared with neutrophils cultured in medium alone; mean ± SD of 3 separate experiments. (B) VCAM-1 was immobilized in a 96-well plate, and neutrophils were added in the absence (□) or presence (▪) of 1000 U/mL IFN-β. After 18 hours, apoptosis was measured by DiOC6 staining. (C) Cells from a CD4 T-cell line were incubated in the presence or absence of the survival factors IL-2 (50 U/mL), IFN-β (1000 U/mL), or sVCAM-1 (10 μg/mL). Apoptosis was measured by DiOC6 staining. Data in panels B-C are from a single experiment that is representative of 3 performed.
VCAM-1 inhibits neutrophil but not lymphocyte apoptosis. (A) CD31, ICAM-1, and VCAM-1 were immobilized at the concentrations shown (micrograms per milliliter) and neutrophils added for 18 hours before apoptosis was measured by DiOC6 staining. Results are expressed as the percentage of apoptotic neutrophils compared with neutrophils cultured in medium alone; mean ± SD of 3 separate experiments. (B) VCAM-1 was immobilized in a 96-well plate, and neutrophils were added in the absence (□) or presence (▪) of 1000 U/mL IFN-β. After 18 hours, apoptosis was measured by DiOC6 staining. (C) Cells from a CD4 T-cell line were incubated in the presence or absence of the survival factors IL-2 (50 U/mL), IFN-β (1000 U/mL), or sVCAM-1 (10 μg/mL). Apoptosis was measured by DiOC6 staining. Data in panels B-C are from a single experiment that is representative of 3 performed.
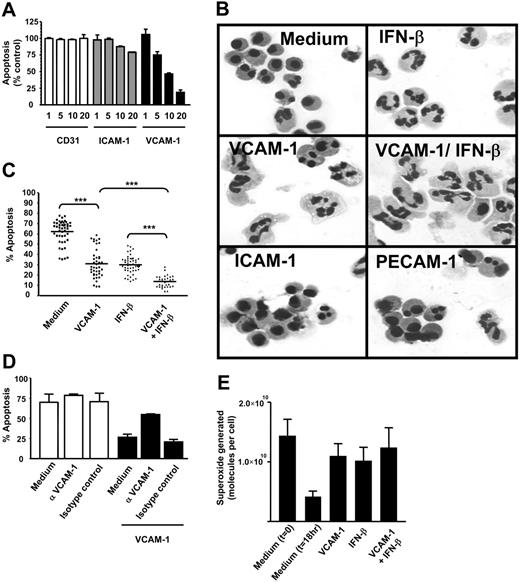
The integrin α9β1 is the VCAM-1-binding receptor mediating neutrophil survival. Potential VCAM-1-binding integrins expressed on neutrophils (A) or on CD4 T cells (B) were quantified using flow cytometry. (C) VCAM-1-coated beads were cross-linked to the cell surface of neutrophils, and beads were washed and run on a reducing PAGE gel as described. Control human IgG-coated beads (for bead conjugation, see “Immunoblots and Western blotting” under “Materials and methods”) were used to show nonspecific binding of surface molecules to the beads after cross-linking. Proteins were detected by Western blot with anti-α9 and anti-β1-specific antibodies. The blots shown are representative of 3 separate experiments performed. Neutrophils were incubated for 18 hours (D) in the presence or absence of the anti-α9β1 antibody Y9A2 (10 μg/mL). Cells were cultured alone (□) or in the presence of VCAM-1 (▪). An isotype-control antibody is shown. Apoptosis was measured by DiOC6 staining. Results are expressed as the percentage of apoptotic neutrophils compared with neutrophils cultured in medium alone with no VCAM-1; mean ± SD of 3 separate experiments.
The integrin α9β1 is the VCAM-1-binding receptor mediating neutrophil survival. Potential VCAM-1-binding integrins expressed on neutrophils (A) or on CD4 T cells (B) were quantified using flow cytometry. (C) VCAM-1-coated beads were cross-linked to the cell surface of neutrophils, and beads were washed and run on a reducing PAGE gel as described. Control human IgG-coated beads (for bead conjugation, see “Immunoblots and Western blotting” under “Materials and methods”) were used to show nonspecific binding of surface molecules to the beads after cross-linking. Proteins were detected by Western blot with anti-α9 and anti-β1-specific antibodies. The blots shown are representative of 3 separate experiments performed. Neutrophils were incubated for 18 hours (D) in the presence or absence of the anti-α9β1 antibody Y9A2 (10 μg/mL). Cells were cultured alone (□) or in the presence of VCAM-1 (▪). An isotype-control antibody is shown. Apoptosis was measured by DiOC6 staining. Results are expressed as the percentage of apoptotic neutrophils compared with neutrophils cultured in medium alone with no VCAM-1; mean ± SD of 3 separate experiments.
To determine whether VCAM-1 could bind α9β1 expressed on neutrophils, cells were exposed to VCAM-1-coated beads or control beads coated with human IgG, treated with a cross-linking agent, lysed, and the protein bound to the beads separated by electrophoresis. Western blotting revealed that both α9 and β1 chains associated with the VCAM-1-coated beads but not control beads (Figure 4C).
To determine if VCAM-1-α9β1 interactions were responsible for the inhibition of neutrophil apoptosis, we attempted to inhibit the interaction between VCAM-1 and α9β1 using the α9β1 blocking antibody Y9A2, which has been shown to inhibit α9β binding to VCAM-1.17 Y9A2 significantly inhibited VCAM-1-mediated survival (Figure 4D). Taken together, these data suggest that direct α9β1 ligation by VCAM-1 is responsible for delaying neutrophil apoptosis.
The VCAM-1-α9β1 survival signaling pathway activates NF-κB in neutrophils
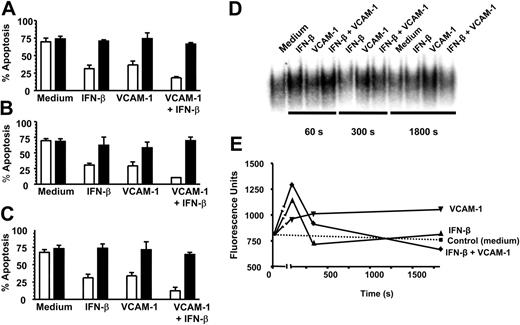
We have recently demonstrated that IFN-β promotes neutrophil survival in a PI3K-dependent manner requiring the induction of NF-κB-regulated genes and de novo protein synthesis.3 Because we observed an additive effect between VCAM-1 and IFN-β in regulating neutrophil survival, we hypothesized that the signaling pathways induced by these 2 treatments might be qualitatively similar. We found that the 2 rescue pathways shared similar sensitivities to a PI3K inhibitor (LY294002) (Figure 5A), an NF-κB translocation inhibitor (SN50) (Figure 5B), and both required de novo protein synthesis because survival was inhibited by the translation inhibitor cycloheximide (Figure 5C). However, the 2 pathways differed in their ability to activate NF-κB as measured using EMSAs on neutrophils treated with VCAM-1 and IFN-β either alone or in combination (Figure 5D). Both the initial intensity and duration of NF-κB activation differed between VCAM-1 and IFN-β, with VCAM-1 signaling leading to a slower, more prolonged activation compared with IFN-β. Interestingly, an additive effect was seen at early time points (60 and 300 seconds) with the combination of VCAM-1 and IFN-β, which was lost by 30 minutes. Taken together, this supports the concept that the 2 rescue pathways (VCAM-1 and IFN-β) prevent neutrophil apoptosis through distinct receptor-mediated signaling pathways that converge upstream of PI3K but use different, but overlapping, downstream signaling elements involving NF-κB activation.
Discussion
Prolonged neutrophil survival has been demonstrated following the binding of neutrophils to endothelium,14,15 fibrinogen,32 and cross-linking β2 integrin antibodies.26 In this study we found that the cell adhesion molecule VCAM-1 delays neutrophil apoptosis and maintains physiologic function though direct ligation of the integrin receptor α9β1 The VCAM-1-α9β1 signaling pathway intersects with the IFN-β antiapoptotic cytokine pathway, providing a biologic context for the observations that neutrophil adhesion delays apoptosis.
The downstream consequences of integrin engagement in leukocytes have been an area of growing interest. During the process of leukocyte transendothelial migration, integrin ligation induces a series of signaling pathways leading to the induction of cytoskeletal reorganization, gene transcription, and effects on cell proliferation and survival.33-35 These cellular effects are mediated through a range of protein families such as small GTPases, phosphatases, and kinases with effects on transcriptional factors such as NF-κB. While numerous studies have addressed the effect of integrin ligation on cell survival (reviewed by Giancotti and Ruoslahti,22 Damsky and Ilic,36 and Rossetti et al37 ), many of these studies have been performed on adherent cells and not on leukocytes, which circulate as nonadherent cells and only upon entry into tissues become adherent. The signaling consequences of ligation of α9β1 in neutrophils has not been previously explored, but in this study we have shown that this receptor plays an important role in protecting neutrophils from apoptosis via activation of NF-κB.
We have found that ligation of α9β1 with VCAM-1 uses elements of a common signaling pathway described for other soluble survival factors such as IFN-β. Our data suggest that ligation of α9β1 modulates the response from the IFN-β receptor in a manner reminiscent of that observed between the integrin αvβ3 and the PDGF receptor in fibroblasts.38 The precise molecular details of how IFN-β signals are integrated with signals from α9β1 remain to be examined. Several potential candidate mechanisms involved in integrin signaling have been reported in the literature. For example, ILK is known to be rapidly activated upon integrin ligation and can directly activate PKB/AKT and PI3K.20,39 We have recently shown that IFN-β signals through PI3K and does not activate PKB/AKT but instead uses PKC-δ as a downstream target.40 Modulation of the ERK has also been demonstrated in neutrophils to cause up-regulation of integrins as well as activating small GTPase proteins.25 It remains to be determined which of these potential candidates, if any, are used for VCAM-1-α9β1-mediated survival in neutrophils.
Interactions between survival signals emanating from both soluble cytokine and adhesion proteins may enhance the survival of neutrophils at sites of inflammation or even during the process of transmigration. In chronic persistent inflammatory diseases such as rheumatoid arthritis, large amounts of both IFN-β and VCAM-1 are found in the inflamed tissue, particularly on stromal cells such as fibroblasts and endothelium.41,42 It has been known for a number of years that neutrophils accumulate within the inflamed synovium and demonstrate marked resistance to apoptosis.9 Reports have also indicated that neutrophils isolated from synovial fluid are resistant to TNF-β-induced cell death and that this response requires NF-κB activation.43 We therefore propose that the presence of α9β1-specific ligands such as VCAM-1 at sites of inflammation and in the bone marrow may be one factor by which neutrophil survival is regulated by the stromal microenvironment.
This report describes a functional survival effect for interaction between VCAM-1 and the integrin α9β1 on neutrophils. Our data demonstrate that neutrophils have the potential to integrate survival signals from soluble factors with those from integrin ligation. Convergence of these pathways causes rapid activation of NF-κB and a greater delay in neutrophil apoptosis compared with either stimulus alone. Thus, at sites of inflammation where a wide range of potential survival factors are present, neutrophil life span may be significantly increased through combinations of stimuli from very different sources including cytokines, adhesion molecules, and oxygen tension.41 Our data suggest that the extended survival of neutrophils, mediated by both the inflammatory and adhesive properties of the local microenvironment, provides an important extrinsic checkpoint in the resolution of inflammatory responses.
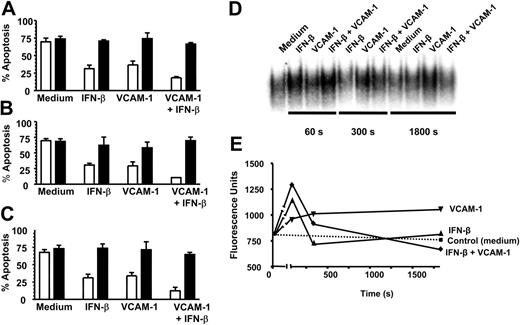
VCAM-1-mediated rescue shares similar signaling pathways to IFN-β and activates NF-κB. Neutrophils were cultured for 18 hours in the absence (□) or presence of 20 μM PI3K inhibitor LY294002 (▪) with or without 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together (A); in the absence (□) or presence of 100 μg/mL NF-κB inhibitor SN50 (▪) with or without 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together (B); and in the absence (□) or presence of 1 μg/mL cycloheximide (▪) with or without 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together (C). Apoptosis was measured by DiOC6 staining; mean ± SD of 3 separate experiments. (D) NF-κB activation was measured and (E) quantified (see “Materials and methods”) following the exposure of neutrophils to medium alone, 1000 U/mL IFN-β, 10 μg/mL VCAM-1, or both together after 60, 300, and 1800 seconds.
VCAM-1-mediated rescue shares similar signaling pathways to IFN-β and activates NF-κB. Neutrophils were cultured for 18 hours in the absence (□) or presence of 20 μM PI3K inhibitor LY294002 (▪) with or without 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together (A); in the absence (□) or presence of 100 μg/mL NF-κB inhibitor SN50 (▪) with or without 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together (B); and in the absence (□) or presence of 1 μg/mL cycloheximide (▪) with or without 1000 U/mL IFN-β, 10 μg/mL sVCAM-1, or both together (C). Apoptosis was measured by DiOC6 staining; mean ± SD of 3 separate experiments. (D) NF-κB activation was measured and (E) quantified (see “Materials and methods”) following the exposure of neutrophils to medium alone, 1000 U/mL IFN-β, 10 μg/mL VCAM-1, or both together after 60, 300, and 1800 seconds.
Prepublished online as Blood First Edition Paper, October 13, 2005; DOI 10.1182/blood-2005-07-2692.
Supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC), MRC, and Arthritis Research Campaign (ARC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Tony Shock (Celltech) for the generous gift of VCAM-1-Fc recombinant human protein as well as the antibodies 1G11 and Max68P, and thank Hema Chahal for technical assistance.