Abstract
Protease-activated receptors (PARs) activate Gq and G12/13 pathways, as well as Akt (protein kinase B [PKB/Akt]) in platelets. However, the relative contribution of different G-protein pathways to Akt phosphorylation has not been elucidated. We investigated the contribution of Gq and G12/13 to Gi/Gz-mediated Akt phosphorylation downstream of PAR activation. Selective G12/13 activation failed to cause Akt phosphorylation in human and Gαq-deficient mouse platelets. However, supplementing Gi/Gz signaling to G12/13 caused significant increase in Akt phosphorylation, confirming that G12/13 potentiates Akt phosphorylation. Inhibition of PAR-mediated Akt phosphorylation in the presence of the Gq-selective inhibitor YM-254890 was restored to the normal extent achieved by PAR agonists if supplemented with Gi signaling, indicating that Gq does not have any direct effect on Akt phosphorylation. Selective G12/13 activation resulted in Src kinase activation, and Akt phosphorylation induced by costimulation of G12/13 and Gi/Gz was inhibited by a Src kinase inhibitor but not by a Rho kinase inhibitor. These data demonstrate that G12/13, but not Gq, is essential for thrombin-induced Akt phosphorylation in platelets, whereas Gq indirectly contributes to Akt phosphorylation through Gi stimulation by secreted ADP. G12/13 activation might mediate its potentiating effect through Src activation, and Src kinases play an important role in thrombin-mediated Akt phosphorylation.
Introduction
Thrombin, generated at the site of vascular damage by extrinsic and intrinsic coagulation cascades, is an important agonist for platelet activation. Thrombin mediates its cellular effects primarily through a family of G-protein-coupled protease-activated receptors (PARs). It is known that PARs couple to Gq and G12/13 pathways,1 and we have demonstrated that thrombin and thrombin receptor-activating peptides cause Gi stimulation through P2Y12 receptor activation by secreted ADP.2 It has been shown that stimulation of platelets with thrombin results in Akt activation, and P2Y12 ADP receptor is responsible for a significant proportion of Akt activation.3
Protein kinase B (PKB; also known as Akt) is a 57-kDa serine/threonine kinase4 that contains a pleckstrin homology (PH) domain adjacent to a centrally located catalytic domain that is connected to a short C-terminal tail.5 The catalytic domain of Akt contains a Thr308 phosphorylation site and a regulatory domain located in the C-terminal tail contains a Ser473 phosphorylation site.5 There are at least 3 different Akt isoforms identified in humans, which display more than 80% sequence homology and are named Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ).6 It has been shown that the major Akt subtype in human platelets is Akt1 rather than Akt2.7 The Akt1 phosphorylation sites are Thr308/Ser,473 and both translocation of Akt to cell membranes and phosphorylation of both Thr308 and Ser473 are required for full enzyme activity. Phosphatidylinositol (PI) 3-kinase has been shown to be implicated in fibrinogen receptor activation,8 and Akt functions as one of several downstream effectors of PI 3-kinase.9,10 PI 3-kinase products PtdIns3,4 P2 and PtdIns3-5 P3 trigger the simultaneous phosphorylation of Akt by phosphatidylinositol-dependent kinase 1 (PDK1) and 2. Membrane attachment of Akt is a prerequisite for phosphorylation by PDK1 and PDK2, and PDK1 has been shown to phosphorylate Thr308 of Akt1 and the phosphorylation of Ser473 is independent of Thr308.11
ADP-induced platelet aggregation requires coactivation of both the P2Y1 and P2Y12 receptors that couple to Gq and Gi, respectively, and concomitant signaling from Gq and Gi is sufficient and necessary for ADP-induced platelet aggregation.12-14 Epinephrine binds to the α2A-adrenergic receptor and causes activation of the Gz pathway that leads to the pertussis toxin-insensitive inhibition of adenylyl cyclase.15,16 It has been shown that activated thrombin receptors in platelets couple to G12/13 pathways,1 which are involved in p160ROCK activation and the subsequent shape change.17,18 Y-27632, a specific inhibitor of the Rho-associated protein kinase p160ROCK,19 blocks G12/13-induced platelet shape change that is independent of an increase of intracellular calcium, indicating that p160ROCK plays a central role downstream of G12/13 for mediating shape change.20
Data from our own studies and others show that Akt is activated by various agonists including ADP, epinephrine, thrombin, and thrombin receptor-activating peptides.3,7,21 It has been shown that Akt phosphorylation stimulated by ADP was preserved in the platelets from Gq knockout mice, whereas thrombin-induced Akt phosphorylation was abolished.3,22 However, it is controversial whether thrombin-induced Akt phosphorylation is dependent on Gq or Gi signaling pathways. Woulfe et al22 reported that the activation of Akt by thrombin occurs in a Gq-dependent manner in which secreted ADP can only amplify Akt activation. We have suggested that thrombin-mediated Akt phosphorylation is largely dependent on Gi stimulation through P2Y12 receptor activation by secreted ADP. Even though thrombin-induced Akt phosphorylation depends on secretion, Akt phosphorylation mediated by ADP and epinephrine is much weaker than that caused by thrombin. These findings raised the possibility that there is a potentiating contribution of other G-protein signaling to Akt phosphorylation mediated by Gi or Gz signaling pathways. The relative contribution of Gq or G12/13 pathways to Akt phosphorylation and the downstream signaling events from G12/13 activation have not been fully understood. Therefore, we sought to define the contribution of Gq or G12/13 pathways to Akt phosphorylation mediated by Gi/Gz pathways in platelets.
In this study, we show that selective activation of G12/13 alone is not sufficient to cause Akt phosphorylation in platelets, but activation of G12/13 in combination with selective activation of Gi/Gz signaling in the absence of Gq can restore the Akt phosphorylation to the level achieved by PAR agonists in normal platelets, thus indicating the essential role of G12/13 pathways in potentiation of Akt phosphorylation downstream of PAR receptor activation. There has been an unresolved question of whether coupling of PAR1 to Gi is direct or indirect.23 We have previously demonstrated that PAR1 activation does not lead to Gi stimulation in platelets using cAMP levels as an assay.2 In this study, we confirm this important finding using Akt phosphorylation as the readout. The results also show that Gq-dependent pathways do not have any direct effect on PAR-mediated Akt phosphorylation and confirm our previous study that thrombin requires secreted ADP to induce Akt phosphorylation. We also show for the first time that G12/13 activation downstream of PARs leads to Src family kinase activation, and G12/13 might mediate its potentiating effect on Akt phosphorylation through Src family kinase.
Materials and methods
Approval for this study was obtained from the Institutional Review Board of Temple University (Philadelphia, PA). Informed consent was provided according to the Declaration of Helsinki.
Materials
Thrombin, 2-MeSADP, epinephrine, MRS-2179, apyrase (type V), and bovine serum albumin (fraction V) were purchased from Sigma (St Louis, MO). YFLLRNP was custom synthesized by New England Biolabs (Beverly, MA), and SFLLRN and AYPGKF were custom synthesized by Invitrogen (Carlsbad, CA). Anti-phospho-Akt (Ser473 and Thr308) and anti-phospho-Src (Tyr416) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Alkaline phosphatase (AP)-labeled secondary antibody was from Kirkegaard & Perry Laboratories (Gaithersburg, MD). CDP-Star chemiluminescent substrates were purchased from Applied Biosystems (Foster City, CA). Y-27632, LY294002, and PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazole[3,4-D]pyrimidine) were purchased from Biomol Research Laboratories (Plymouth Meeting, PA). PP3 (4-amino-7-phenylpyrazol[3,4-D]pyrimidine) was purchased from Calbiochem (San Diego, CA). AR-C69931MX was a gift from AstraZeneca (Loughborough, United Kingdom). YM-254890 was a generous gift from Yamanouchi Pharmaceutical (Ibaraki, Japan). All other reagents were reagent grade, and deionized water was used throughout.
Animals
Gαq-deficient mice were obtained from T. Kent Gartner,24 with permission from Stefan Offermanns (University of Heidelberg, Heidelberg, Germany).
Preparation of human platelets
Whole blood was obtained from a pool of healthy volunteers in a one-sixth volume of ACD (2.5 g sodium citrate, 1.5 g citric acid, and 2.0 g glucose in 100 mL of H2O). Platelet-rich plasma (PRP) was prepared by centrifugation of citrated blood at 230 rcf (g) for 20 minutes at room temperature (RT). Acetylsalicylic acid was added to PRP to a final concentration of 1 mM, and the preparation was incubated for 45 minutes at 37°C followed by centrifugation at 980 rcf (g) for 10 minutes at RT. The platelet pellet was resuspended in Tyrode buffer (138 mM NaCl; 2.7 mM KCl; 2 mM MgCl2; 0.42 mM NaH2PO4; 5 mM glucose; 10 mM HEPES, pH 7.4; and 0.2% bovine serum albumin) containing 0.05 unit/mL apyrase. This low concentration of apyrase is not enough to block responses to ADP but will prolong the responsiveness of platelets to ADP by preventing desensitization of the P2Y receptors. These conditions have been standardized in the laboratory. The platelet count was adjusted to 2 × 108 cells/mL.
Preparation of mouse platelets
Blood was collected from the vena cava of anesthetized mice into syringes containing one-tenth blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100 rcf (g) for 10 minutes at RT. PRP was recovered and platelets were pelleted at 400 rcf (g) for 10 minutes at RT. The platelet pellet was resuspended in Tyrode buffer (pH 7.4) containing 0.05 unit/mL apyrase.
Western blotting
Platelets were stimulated with agonists under nonstirring conditions for the appropriate time, and the reaction was stopped by the addition of 3 × sodium dodecyl sulfate (SDS) sample buffer. In some experiments, Y-27632 (10 μM), a p160ROCK inhibitor, and PP2 (10 μM), a Src family kinase inhibitor, were added and incubated for 5 minutes at 37°C without stirring before agonist stimulation. Levels of phospho-Akt and phospho-Src were determined after immunoblotting with anti-phospho-Akt (Ser473 and Thr308) and anti-phospho-Src (Tyr416) antibodies. Platelet samples were boiled for 5 minutes and proteins were separated on 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride membrane. Nonspecific binding sites were blocked by incubation in Tris-buffered saline/Tween (TBST; 20 mM Tris, 140 mM NaCL, 0.1% [v/v] Tween 20) containing 0.5% (w/v) milk protein and 3% (w/v) bovine serum albumin (BSA) for 30 minutes at RT, and membranes were incubated overnight at 4°C with primary antibody (1:1000 in TBST/2% BSA) with gentle agitation. After 3 washes for 5 minutes each with TBST, the membranes were probed with AP-labeled goat anti-rabbit IgG (1:5000 in TBST/2% BSA) for 1 hour at RT. After additional washing steps, membranes were then incubated with CDP-Star chemiluminescent substrates for 10 minutes at RT and immunoreactivity was detected using Fujifilm Luminescent Image Analyzer (model LAS-3000 CH; Tokyo, Japan).
Human platelet aggregation
Agonist-induced platelet aggregation was measured using a lumiaggregometer (Chrono-Log, Havertown, PA) at 37°C with stirring (900 rpm). A 0.5-mL sample of aspirin-treated washed platelets was stimulated with agonist, and change in light transmission was measured. Agonists were added simultaneously for platelet stimulation, and platelets were preincubated with 10 μM Y-27632 or 50 nM YM-254890 for 5 minutes at 37°C before agonist stimulation in some experiments. Each sample was allowed to aggregate for at least 5 minutes. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/s.
Results
Characterization of YFLLRNP-mediated Akt phosphorylation in human platelets
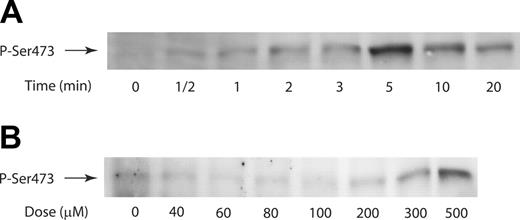
It has been shown that activated thrombin receptors in platelets couple to G12/13 pathways1 that are involved in Rho kinase p160ROCK activation and the subsequent shape change.17,18,25 Low concentrations of YFLLRNP, a heptapeptide binding to PAR1, causes shape change without calcium mobilization in platelets.26 This shape change is mediated by the activation of G12/13 pathways without activation of the Gq pathways. In human platelets, we used this selective agonist of G12/13 pathways in combination with selective activation of Gi/Gz pathways to demonstrate the contribution of G12/13 signaling to Akt phosphorylation. To determine the kinetics of Akt phosphorylation, Ser473 phosphorylation was monitored over a time range of 0.5 to 20 minutes. Figure 1A shows that an increase in Ser473 phosphorylation in response to YFLLRNP (500 μM) was detectable within 1 minute of stimulation, and the level of phosphorylation peaked at around 5 minutes of stimulation. We exposed platelets to different concentrations of YFLLRNP ranging from 40 to 500 μM, and Ser473 phosphorylation was measured at 5 minutes after stimulation. Figure 1B illustrates that YFLLRNP induced a concentration-dependent increase in Akt phosphorylation. An increase in Ser473 phosphorylation was detectable at concentrations above 200 μM YFLLRNP, suggesting that higher concentrations of YFLLRNP activate both Gq and G12/13 signaling. However, when human platelets were stimulated with low concentrations of YFLLRNP that activate only the G12/13 signaling cascade, YFLLRNP failed to cause Akt phosphorylation, suggesting that G12/13 signaling alone is not capable of causing Akt phosphorylation in human platelets.
Contribution of G12/13 signaling to Akt phosphorylation in human platelets
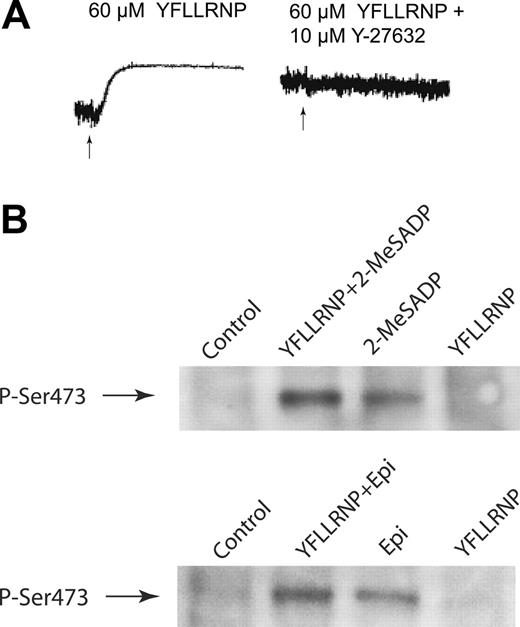
Activation of G12/13 pathways results in platelet shape change that involves Rho kinase.18,25 As shown in Figure 2A, YFLLRNP-induced (60 μM) platelet shape change was completely inhibited in the presence of 10 μM Y-27632,25 Rho kinase p160ROCK inhibitor, suggesting that a low dose of YFLLRNP causes selective activation of G12/13 pathways. Immunoblot analysis revealed that YFLLRNP (60 μM) failed to phosphorylate Akt at Ser473 in human platelets, whereas stimulation of the Gi-coupled P2Y12 receptor or Gz-coupled α2A-adrenergic receptor by 2-MeSADP and epinephrine, respectively, were able to cause Akt phosphorylation (Figure 2B). However, YFLLRNP-mediated G12/13 signaling in combination with Gi/Gz signaling caused a 1.92- to 2.37-fold increase in Akt phosphorylation compared with those obtained with epinephrine or 2-MeSADP alone (Figure 2B). These results suggest that G12/13 signaling has a potentiating effect on Akt phosphorylation when combined with selective Gi or Gz signaling in human platelets.
YFLLRNP-induced Akt phosphorylation in human platelets. (A) Time-dependent phosphorylation (P) of Akt at Ser473 with YFLLRNP. Washed platelets were stimulated at 37°C for the time points indicated with YFLLRNP (500 μM) without stirring. (B) Washed platelets were incubated with different concentrations of YFLLRNP for 5 minutes at 37°C without stirring. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with anti-phospho-Akt (Ser473) antibody. Results are representative of 3 experiments.
YFLLRNP-induced Akt phosphorylation in human platelets. (A) Time-dependent phosphorylation (P) of Akt at Ser473 with YFLLRNP. Washed platelets were stimulated at 37°C for the time points indicated with YFLLRNP (500 μM) without stirring. (B) Washed platelets were incubated with different concentrations of YFLLRNP for 5 minutes at 37°C without stirring. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with anti-phospho-Akt (Ser473) antibody. Results are representative of 3 experiments.
G12/13 signaling potentiates Akt phosphorylation mediated by Gi or Gz signaling in human platelets. (A) Aspirin-treated, washed human platelets preincubated with 10 μM Y-27632 were stimulated with 60 μM YFLLRNP, and platelet aggregation was measured as described in “Materials and methods.” Arrows indicate the addition of agonist. (B) Washed platelets were stimulated at 37°C for 5 minutes with YFLLRNP (60 μM), 2-MeSADP (1 μM), or epinephrine (Epi; 10 μM). Platelets stimulated with 2-MeSADP were preincubated with 10 μM MRS-2179. The addition of YFLLRNP (60 μM) was made as indicated. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. The data shown are representative of 3 experiments.
G12/13 signaling potentiates Akt phosphorylation mediated by Gi or Gz signaling in human platelets. (A) Aspirin-treated, washed human platelets preincubated with 10 μM Y-27632 were stimulated with 60 μM YFLLRNP, and platelet aggregation was measured as described in “Materials and methods.” Arrows indicate the addition of agonist. (B) Washed platelets were stimulated at 37°C for 5 minutes with YFLLRNP (60 μM), 2-MeSADP (1 μM), or epinephrine (Epi; 10 μM). Platelets stimulated with 2-MeSADP were preincubated with 10 μM MRS-2179. The addition of YFLLRNP (60 μM) was made as indicated. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. The data shown are representative of 3 experiments.
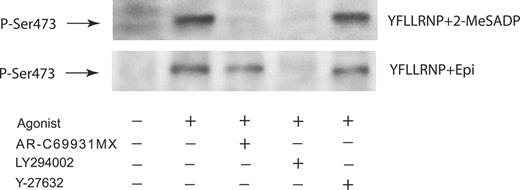
Role of Rho kinase in Akt phosphorylation induced by combined G12/13 and Gi/Gz signaling in platelets. Washed platelets preincubated with 1 μM AR-C69931MX, 25 μM LY294002, or 10 μM Y-27632 were stimulated at 37°C for 5 minutes with either YFLLRNP (60 μM) + 2-MeSADP (1 μM) or YFLLRNP (60 μM) + epinephrine (10 μM). Platelets stimulated with 2-MeSADP were preincubated with 10 μM MRS-2179. The reaction was stopped by the addition of 3 × SDS sample buffer. Equal amounts of proteins were analyzed by Western blot analysis with anti-Ser(P)473 antibody. The Western blot analysis shown is a representative of 3 independent experiments.
Role of Rho kinase in Akt phosphorylation induced by combined G12/13 and Gi/Gz signaling in platelets. Washed platelets preincubated with 1 μM AR-C69931MX, 25 μM LY294002, or 10 μM Y-27632 were stimulated at 37°C for 5 minutes with either YFLLRNP (60 μM) + 2-MeSADP (1 μM) or YFLLRNP (60 μM) + epinephrine (10 μM). Platelets stimulated with 2-MeSADP were preincubated with 10 μM MRS-2179. The reaction was stopped by the addition of 3 × SDS sample buffer. Equal amounts of proteins were analyzed by Western blot analysis with anti-Ser(P)473 antibody. The Western blot analysis shown is a representative of 3 independent experiments.
Effect of Rho kinase in G12/13- and Gi/Gz-mediated Akt phosphorylation
It has been shown that G12/13-mediated platelet shape change is inhibited by the p160ROCK inhibitor Y-27632 and is independent of PLC/Ca2+/calmodulin myosin light-chain kinase signaling.18,20 Thus, it is suggested that Rho kinase p160ROCK is a key signaling molecule downstream of G12/13 pathways, and we investigated its role in the potentiation of Akt phosphorylation. Since G12/13-induced platelet shape change was blocked by the Rho kinase inhibitor Y-27632, we tested the effect of Y-27632 on Akt phosphorylation induced by G12/13 and Gi/Gz pathways. Figure 3 shows that Y-27632 has no effect on Akt phosphorylation induced by costimulation of G12/13 and Gi/Gz signaling, indicating that Rho/Rho kinase does not have any role in potentiation of Akt phosphorylation. However, AR-C69931MX, an antagonist at the Gi-coupled P2Y12 receptor, inhibited concomitant G12/13- and Gi-mediated Akt phosphorylation. This result was substantiated in experiments where epinephrine reversed the inhibitory effect of AR-C69931MX on Akt phosphorylation. PI 3-kinase has been shown to be a key signaling molecule for Akt phosphorylation.9 LY294002, a PI 3-kinase inhibitor,27 blocked Akt phosphorylation caused by costimulation of G12/13 and Gi/Gz signaling, suggesting the important role of PI 3-kinase in this Akt phosphorylation. It appears that PI 3-kinase mediates its effect through the P2Y12- or α2A-adrenergic-stimulated Gi/Gz signaling pathways.
Contribution of G12/13 signaling to Akt phosphorylation in mouse platelets
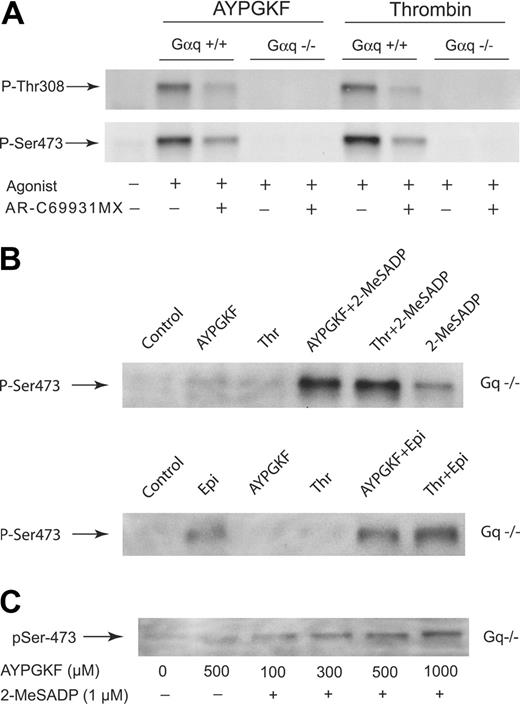
We compared Akt phosphorylation in response to AYPGKF, a PAR4-activating peptide, and thrombin in platelets from wild-type mice as well as from mice lacking Gαq. Immunoblot analysis revealed that AYPGKF and thrombin failed to phosphorylate Akt at Ser473 and Thr308 in platelets from Gαq knockout mice (Figure 4A). However, AYPGKF and thrombin caused phosphorylation of Ser473 and Thr308 in wild-type littermates, which was partially inhibited in the presence of AR-C69931MX. We have shown that thrombin receptors fail to directly couple to Gi in platelets and depend on secreted ADP for Gi stimulation2 and that thrombin-induced Akt phosphorylation depends on Gi pathways.3 Therefore, our data suggest the possible contribution of other G-protein-coupled signaling to Akt phosphorylation mediated by thrombin in platelets.
Role of G12/13 signaling in potentiation of Akt phosphorylation in Gαq-/- mice. (A) Akt phosphorylation in response to AYPGKF and thrombin in Gαq-/- mice. Wild-type platelets and Gαq-deficient platelets preincubated in the absence and presence of 1 μM AR-C69931MX were stimulated at 37°C for 5 minutes with either AYPGKF (500 μM) or thrombin (0.5 unit/mL). The reaction was stopped by the addition of 3 × SDS sample buffer. Equal amounts of proteins were analyzed by Western blot analysis with anti-Thr(P)308 or anti-Ser(P)473. The data shown are representative of 3 experiments. (B) G12/13 signaling potentiates Akt phosphorylation in Gαq-deficient platelets. Gαq-deficient platelets were stimulated at 37°C for 5 minutes with either AYPGKF (500 μM) or thrombin (Thr; 0.5 unit/mL) without stirring. The addition of 2-MeSADP (1 μM) or epinephrine (10 μM) was made as indicated. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. The data shown are representative of 3 experiments. (C) Concentration-dependent Akt phosphorylation induced by combined G12/13 and Gi signaling in Gαq-deficient platelets. Gαq-deficient platelets were stimulated at 37°C for 5 minutes with different concentrations of AYPGKF in the presence of 2-MeSADP (1 μM). The reaction was stopped by the addition of 3 × SDS sample buffer. Platelet proteins were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody.
Role of G12/13 signaling in potentiation of Akt phosphorylation in Gαq-/- mice. (A) Akt phosphorylation in response to AYPGKF and thrombin in Gαq-/- mice. Wild-type platelets and Gαq-deficient platelets preincubated in the absence and presence of 1 μM AR-C69931MX were stimulated at 37°C for 5 minutes with either AYPGKF (500 μM) or thrombin (0.5 unit/mL). The reaction was stopped by the addition of 3 × SDS sample buffer. Equal amounts of proteins were analyzed by Western blot analysis with anti-Thr(P)308 or anti-Ser(P)473. The data shown are representative of 3 experiments. (B) G12/13 signaling potentiates Akt phosphorylation in Gαq-deficient platelets. Gαq-deficient platelets were stimulated at 37°C for 5 minutes with either AYPGKF (500 μM) or thrombin (Thr; 0.5 unit/mL) without stirring. The addition of 2-MeSADP (1 μM) or epinephrine (10 μM) was made as indicated. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. The data shown are representative of 3 experiments. (C) Concentration-dependent Akt phosphorylation induced by combined G12/13 and Gi signaling in Gαq-deficient platelets. Gαq-deficient platelets were stimulated at 37°C for 5 minutes with different concentrations of AYPGKF in the presence of 2-MeSADP (1 μM). The reaction was stopped by the addition of 3 × SDS sample buffer. Platelet proteins were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody.
It has been shown that thrombin receptors couple to Gq and G12/13 pathways.1 To determine the contribution of G12/13 pathways to Akt phosphorylation in mouse platelets, we selectively supplemented Gi or Gz stimulation to AYPGKF- and thrombin-induced Akt phosphorylation in Gαq-deficient mouse platelets. Figure 4B illustrates that the activation of PAR receptors with thrombin and AYPGKF in Gαq-deficient platelets, which couple to G12/13 pathways, did not induce Akt phosphorylation. Simultaneous addition of either 2-MeSADP or epinephrine to thrombin- or AYPGKF-stimulated Gαq-deficient platelets resulted in a significant increase (3.2- to 4.5-fold) in Akt phosphorylation compared with that induced by 2-MeSADP or epinephrine alone. These results suggest that G12/13 signaling from thrombin receptors is capable of potentiating Akt phosphorylation mediated by Gi or Gz pathways in mouse platelets.
To confirm the contribution of G12/13 signaling to potentiation of Akt phosphorylation, we exposed Gαq-deficient platelets to different concentrations of AYPGKF in the presence of 2-MeSADP. Figure 4C shows that selective activation of G12/13 signaling by AYPGKF induced a concentration-dependent increase in Akt phosphorylation mediated by 2-MeSADP-induced Gi stimulation, confirming the role of G12/13 signaling in potentiation of Akt phosphorylation when combined with Gi signaling. Taken together with our data showing that potentiation of Akt phosphorylation induced by AYPGKF and thrombin in Gαq-deficient platelets was much stronger than that induced by a low dose of YFLLRNP in human platelets, these results suggest that AYPGKF and thrombin in combination with Gi/Gz in Gαq-deficient platelets induced more robust Akt phosphorylation through strong stimulation of G12/13 pathways.
Contribution of Gq versus G12/13 pathways to Akt phosphorylation
YM-254890, which is isolated from culture broth of Chromobacterium species, has been shown to inhibit ADP-induced platelet aggregation by selectively inhibiting Gq activation.28 In order to compare the relative contribution of Gq and G12/13 pathways, we compared Akt phosphorylation in the presence and absence of the Gq-selective inhibitor YM-254890.
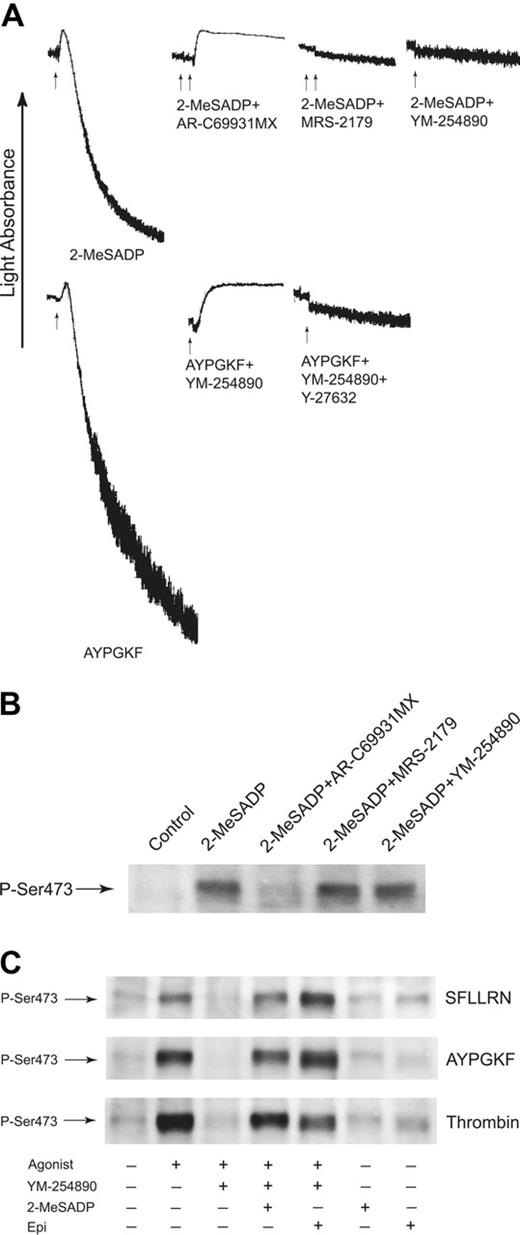
To ensure that YM-254890 inhibits the Gq pathway specifically, we measured agonist-induced platelet aggregation in the presence and absence of 50 nM YM-254890. YM-254890 or MRS-2179, a Gq-coupled P2Y1 receptor antagonist,29 blocked 2-MeSADP-induced platelet shape change and aggregation (Figure 5A). However, YM-254890 did not block the 2-MeSADP-induced inhibition of cAMP levels in platelets (data not shown). AYPGKF, which couples to Gq and G12/13 pathways, caused platelet shape change in the presence of YM-254890. This shape change was abolished by the addition of Rho kinase inhibitor Y-27632, which has been shown to block G12/13-induced shape change (Figure 5A). These data indicate that YM-254890 selectively inhibits Gq signaling in platelets.
It has been shown that ADP depends on stimulation of Gi, but not Gq, to activate Akt.3,22 Immunoblot analysis revealed that 2-MeSADP caused Akt phosphorylation in the presence and absence of MRS-2179 or YM-254890, whereas 2-MeSADP-induced Akt phosphorylation was abolished by AR-C69931MX (Figure 5B), confirming that YM-254890 is a Gq-selective inhibitor and does not have a nonselective effect on Akt phosphorylation.
G12/13 signaling is required for thrombin- and thrombin receptor-activating peptide-induced Akt phosphorylation. (A) YM-254890 does not inhibit G12/13-induced platelet shape change. Aspirin-treated, washed human platelets preincubated with 10 μM Y-27632 or 50 nM YM-2548890 were stimulated with agonists as noted, and platelet aggregation was measured as described in “Materials and methods.” The arrows indicate the addition of agonists and antagonists. Ten micromolar MRS-2179 and 1 μM AR-C69931MX was added to samples prior to stimulation with agonists. Tracings are representative of 3 experiments from 3 different donors. (B) Effect of YM-254890 on 2-MeSADP-induced Akt phosphorylation. Washed human platelets were stimulated with 1 μM 2-MeSADP in the presence or absence of reagents as indicated. Platelet proteins were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. (C) Selective G12/13 signaling restores PAR agonist-induced Akt phosphorylation in the presence of Gi/Gz signaling. Washed human platelets preincubated in the absence and presence of 50 nM YM-254890 were stimulated at 37°C for 5 minutes with either SFLLRN (10 μM), AYPGKF (500 μM), or thrombin (0.5 unit/mL). The addition of 1 μM 2-MeSADP or 10 μM epinephrine was made as indicated. Samples were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. Results are representative of 3 experiments.
G12/13 signaling is required for thrombin- and thrombin receptor-activating peptide-induced Akt phosphorylation. (A) YM-254890 does not inhibit G12/13-induced platelet shape change. Aspirin-treated, washed human platelets preincubated with 10 μM Y-27632 or 50 nM YM-2548890 were stimulated with agonists as noted, and platelet aggregation was measured as described in “Materials and methods.” The arrows indicate the addition of agonists and antagonists. Ten micromolar MRS-2179 and 1 μM AR-C69931MX was added to samples prior to stimulation with agonists. Tracings are representative of 3 experiments from 3 different donors. (B) Effect of YM-254890 on 2-MeSADP-induced Akt phosphorylation. Washed human platelets were stimulated with 1 μM 2-MeSADP in the presence or absence of reagents as indicated. Platelet proteins were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. (C) Selective G12/13 signaling restores PAR agonist-induced Akt phosphorylation in the presence of Gi/Gz signaling. Washed human platelets preincubated in the absence and presence of 50 nM YM-254890 were stimulated at 37°C for 5 minutes with either SFLLRN (10 μM), AYPGKF (500 μM), or thrombin (0.5 unit/mL). The addition of 1 μM 2-MeSADP or 10 μM epinephrine was made as indicated. Samples were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. Results are representative of 3 experiments.
In order to verify the relative contribution of Gq and G12/13 pathways to PAR-induced Akt phosphorylation, we investigated the effect of combined activation of G12/13 and Gi/Gz signaling in the presence of YM-254890. As shown in Figure 5C, SFLLRN-, AYPGKF-, and thrombin-induced Akt phosphorylation was abolished in the presence of YM-254890, confirming our previous study that PAR receptors cannot directly couple to Gi signaling.2 However, supplemental Gi or Gz signaling by 2-MeSADP or epinephrine in the presence of YM-254890 restored Akt phosphorylation to the extent achieved by these agonists. These results provide further evidence that Gq signaling does not have any direct effect on Akt phosphorylation and that G12/13 signaling downstream of PARs is essential to achieve normal extent of Akt phosphorylation by thrombin and thrombin receptor-activating peptides in platelets.
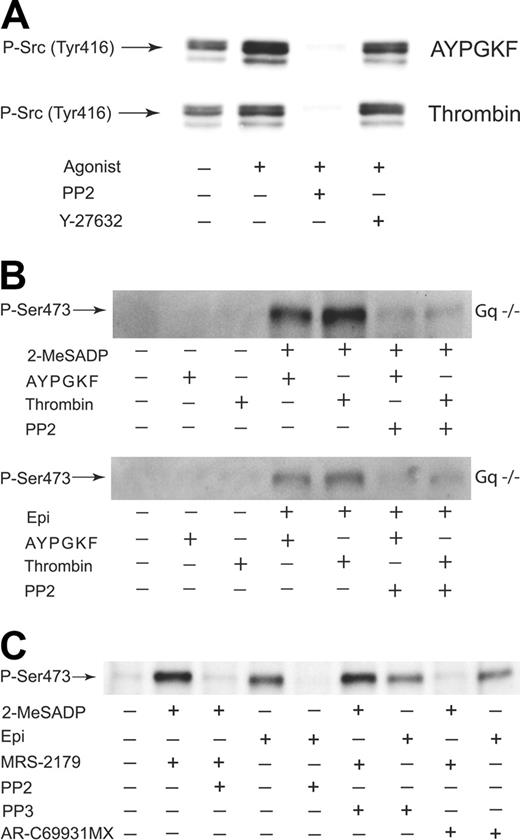
Src kinase-dependent Akt phosphorylation induced by combined G12/13 and Gi/Gz signaling in platelets. (A) Gαq-deficient platelets preincubated with either 10 μM Y-27632 or 10 μM PP2 were stimulated at 37°C for 2 minutes with either AYPGKF (500 μM) or thrombin (0.5 unit/mL). Platelet proteins were separated by SDS-PAGE, Western blotted, and probed for phospho-Tyr416 Src. (B) Gαq-deficient platelets preincubated in the absence and presence of 10 μM PP2 were stimulated at 37°C for 5 minutes with agonists as noted. The reaction was stopped by the addition of 3 × SDS sample buffer. Platelet proteins were analyzed by Western blot analysis with anti-Ser(P)473 antibody. (C) Washed human platelets preincubated with 1 μM AR-C69931MX, 10 μM PP2, or 10 μM PP3 were stimulated at 37°C for 5 minutes with either 2-MeSADP (1 μM) or epinephrine (10 μM). Platelets stimulated with 2-MeSADP were preincubated with 10 μM MRS-2179. Platelet proteins were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. The Western blot analysis shown is a representative of 3 independent experiments.
Src kinase-dependent Akt phosphorylation induced by combined G12/13 and Gi/Gz signaling in platelets. (A) Gαq-deficient platelets preincubated with either 10 μM Y-27632 or 10 μM PP2 were stimulated at 37°C for 2 minutes with either AYPGKF (500 μM) or thrombin (0.5 unit/mL). Platelet proteins were separated by SDS-PAGE, Western blotted, and probed for phospho-Tyr416 Src. (B) Gαq-deficient platelets preincubated in the absence and presence of 10 μM PP2 were stimulated at 37°C for 5 minutes with agonists as noted. The reaction was stopped by the addition of 3 × SDS sample buffer. Platelet proteins were analyzed by Western blot analysis with anti-Ser(P)473 antibody. (C) Washed human platelets preincubated with 1 μM AR-C69931MX, 10 μM PP2, or 10 μM PP3 were stimulated at 37°C for 5 minutes with either 2-MeSADP (1 μM) or epinephrine (10 μM). Platelets stimulated with 2-MeSADP were preincubated with 10 μM MRS-2179. Platelet proteins were separated by SDS-PAGE, Western blotted, and probed with anti-phospho-Akt (Ser473) antibody. The Western blot analysis shown is a representative of 3 independent experiments.
Role of Src family kinase in G12/13- and Gi/Gz-mediated Akt phosphorylation
As the Rho kinase inhibitor Y-27632 did not block Akt phosphorylation, we suggest that there is a separate pathway downstream of G12/13 stimulation that contributes to potentiation of Akt phosphorylation. Immunoblot analysis of the phosphorylation of Src tyrosine 416, which correlates with Src activity, revealed that AYPGKF and thrombin were able to induce an increase in Src phosphorylation in Gαq-deficient platelets (Figure 6A), suggesting that selective G12/13 activation results in activation of Src family kinase. SFLLRN, AYPGKF, and thrombin were also able to induce an increase in Src phosphorylation in the presence of 50 nM YM-254890 in human platelets (data not shown). Y-27632 had no effect on G12/13-induced Src phosphorylation, which was blocked by a selective inhibitor of Src family tyrosine kinase PP2,30 indicating that Rho kinase does not have any role in G12/13-induced Src phosphorylation. To determine the role of Src kinase in Akt phosphorylation, we measured the effects of PP2 on Akt phosphorylation induced by combined G12/13 and Gi/Gz stimulation. As shown in Figure 6B, Akt phosphorylation induced by simultaneous addition of either 2-MeSADP or epinephrine to thrombin or AYPGKF in Gαq-deficient platelets was abolished in the presence of PP2. This suggests that a Src-dependent pathway in the G12/13 signaling pathway plays an important role in this potentiation of Akt phosphorylation in the presence of Gi/Gz signaling in platelets.
Recently, we have suggested that both Gi and Gz signaling can cause Src activation in platelets.31 In order to verify the role of Src in Akt phosphorylation mediated by Gi/Gz stimulation, we tested the effect of PP2 and PP3, a control compound that does not inhibit tyrosine kinase activity, on Akt phosphorylation induced by Gi or Gz alone. Whereas PP2 abolished Akt phosphorylation induced by Gi and Gz signaling, the control compound PP3 had no effect (Figure 6C), indicating that Src activity plays a role in Akt phosphorylation mediated by selective Gi or Gz signaling.
Discussion
It has been shown that Akt is activated by various agonists including ADP, epinephrine, and thrombin in platelets.3,7,21,22 We have shown that ADP causes Akt phosphorylation independently of Gq stimulation, that thrombin- and thrombin receptor-activating peptide-induced Akt phosphorylation depends predominantly on Gi stimulation through P2Y12 receptor activation by secreted ADP, and that epinephrine substitutes for the P2Y12 receptor-mediated effects through Gz signaling cascade.3 However, the activation of P2Y12 or α2A-adrenergic receptors with 2-MeSADP and epinephrine, which couple to Gi and Gz, respectively, induces a much smaller extent of Akt phosphorylation than thrombin or thrombin receptor-activating peptides in platelets. Akt phosphorylation in stirred platelets has been shown to be biphasic, first through inside-out signaling and then through outside-in signaling.32 Thus, when aggregation is allowed to occur, Akt phosphorylation caused by thrombin could be stronger because thrombin can cause platelet aggregation to a greater extent than ADP and hence could cause stronger Akt phosphorylation. However, platelets were prevented from aggregating in our study to eliminate the contribution of outside-in signaling, and yet thrombin causes more Akt phosphorylation than ADP. Hence, it is conceivable that there might be some potentiating contribution of other G-protein signaling to Akt phosphorylation mediated by Gi or Gz pathways. In particular, we have focused on the potentiating effect of G12/13 pathways on the Akt phosphorylation mediated by Gi/Gz pathways.
It has been shown that thrombin receptors couple to Gq and G12/13 pathways.1 YFLLRNP binds to PAR1 and causes shape change without activating the Gq pathways when used at low concentrations, and this shape change is mediated by the G12/13 pathways. When human platelets are stimulated with a low concentration of YFLLRNP, YFLLRNP fails to cause Akt phosphorylation, suggesting that G12/13 signaling is unable to induce Akt phosphorylation independently. However, YFLLRNP significantly increases the Akt phosphorylation in combination with selective activation of either Gi or Gz pathways, suggesting that G12/13 pathways play an important role in potentiating Akt phosphorylation mediated by Gi/Gz pathways in human platelets. In addition, AYPGKF and thrombin fail to induce Akt phosphorylation in Gαq-deficient platelets, confirming that G12/13 signaling alone does not have any role in Akt phosphorylation in mouse platelets. When we selectively supplemented Gi or Gz signaling to AYPGKF- and thrombin-induced Akt phosphorylation in Gαq-deficient platelets, Akt phosphorylation was dramatically potentiated. These results further confirm that G12/13 signaling has a potentiating contribution to Gi/Gz-induced Akt phosphorylation in both human and mouse platelets.
Even though Gi signaling represents the main signaling pathway leading to Akt phosphorylation, some degree of thrombin-induced Akt phosphorylation has been shown in platelets even in the absence of P2Y12 receptor activation. Since thrombin receptors directly couple to Gq and activate Gi by secreted ADP,2 it has raised the speculation that thrombin can mediate Akt phosphorylation by the Gq-dependent pathways that do not require secreted ADP.22 This may be due to the fact that they did not consider the contribution of G12/13 signaling to Akt phosphorylation. Platelet granules contain several other agonists, such as chemokines and epinephrine, that could activate Gi/Gz pathways. Hence, thrombin could activate Gi/Gz pathways even in the absence of P2Y12 receptor by these other granule contents. Taken together with our data showing that Akt phosphorylation mediated by Gz signaling with epinephrine was potentiated by selective activation of G12/13 signaling, our study strongly suggests that this partial Akt phosphorylation that is independent of ADP secretion may not be due to the direct effect of Gq but to potentiating contribution of G12/13 signaling to Akt phosphorylation mediated by Gz signaling. Our study also reveals that the level of PAR-induced Akt phosphorylation is not affected by the Gq-selective inhibitor YM-254890 when G12/13 is supplemented with Gi/Gz signaling. These results further confirm that G12/13, but not Gq, signaling downstream of PARs is essential to achieve a normal extent of Akt phosphorylation. It also confirms that the absence of Akt phosphorylation in the absence of Gq signaling is due not to the direct effect of Gq on Akt phosphorylation, but to the absence of Gi/Gz stimulation by secretion.
It is controversial whether coupling of PAR1 to Gi is direct or indirect.23 Several groups have shown that PAR1 activates both Gq and Gi pathways whereas PAR4 couples to only the Gq pathway.33-35 If PAR1 can directly couple to Gi pathways independently of secretion, PAR1 could activate both G12/13 and Gi pathways in the absence of Gq pathways in human platelets. Thus, we expect the normal Akt phosphorylation in response to PAR1 agonist SFLLRN in the absence of Gq pathways because coactivation of both G12/13 and Gi pathways leads to normal Akt phosphorylation. In our hands, SFLLRN failed to induce Akt phosphorylation in the presence of YM-254890 (Figure 5C), suggesting that PAR1 can only couple to G12/13 and not Gi pathways in the absence of Gq in human platelets. Hence, this study confirms our previous findings2 and, using a different downstream signaling molecule, clearly demonstrates that PAR1 does not directly stimulate Gi pathways in platelets.
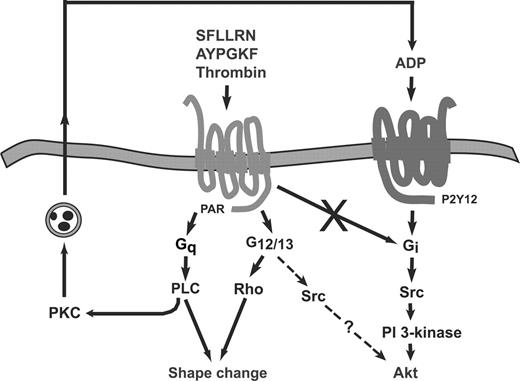
Outline of the signaling events leading to PAR-mediated protein kinase B/Akt activation in platelets. Thrombin and thrombin receptor-activating peptides induce Akt activation in platelets through secreted ADP that stimulates Gi signaling pathways in which Src kinase and PI 3-kinase play an important role. G12/13 signaling potentiates Akt phosphorylation mediated by Gi pathways, and costimulation of G12/13 and Gi signaling can induce the same extent of Akt phosphorylation achieved by PAR agonists in normal platelets. Activation of G12/13 signaling might mediate its potentiating effects through Src kinase activation, and Src kinase plays an important role in ADP- and thrombin-mediated Akt phosphorylation. The mechanism and the specific member of Src family kinases contributing to Akt phosphorylation are not clear. Note that consistent with our previous observations, PAR stimulation in platelets does not lead to direct Gi activation.2
Outline of the signaling events leading to PAR-mediated protein kinase B/Akt activation in platelets. Thrombin and thrombin receptor-activating peptides induce Akt activation in platelets through secreted ADP that stimulates Gi signaling pathways in which Src kinase and PI 3-kinase play an important role. G12/13 signaling potentiates Akt phosphorylation mediated by Gi pathways, and costimulation of G12/13 and Gi signaling can induce the same extent of Akt phosphorylation achieved by PAR agonists in normal platelets. Activation of G12/13 signaling might mediate its potentiating effects through Src kinase activation, and Src kinase plays an important role in ADP- and thrombin-mediated Akt phosphorylation. The mechanism and the specific member of Src family kinases contributing to Akt phosphorylation are not clear. Note that consistent with our previous observations, PAR stimulation in platelets does not lead to direct Gi activation.2
It has been shown that the Rho kinase p160ROCK is an important signaling molecule involved in G12/13-induced shape change.18 Although the p160ROCK inhibitor Y-27632 can block G12/13-mediated platelet shape change, our study reveals that Y-27632 fails to inhibit Akt phosphorylation caused by concomitant G12/13 and Gi/Gz signaling, suggesting that the Rho A/Rho kinase (p160ROCK)-dependent signaling cascade does not play any significant role in potentiating Akt phosphorylation although most G12/13 to date has involved the Rho A/Rho kinase (p160ROCK)-dependent pathway. Furthermore, it suggests that there is a separate pathway downstream of G12/13 contributing to potentiation of Akt phosphorylation that is independent of the Rho A/Rho kinase pathway.
Src family tyrosine kinases participate in the signal transduction pathways of various hematopoietic cells.36 Six of 8 members of Src family kinases are present in platelets.37 It has been shown that the Src family kinases Lyn and Fyn play a role in glycoprotein VI-mediated platelet activation.38 It has also been described that stimulation of G-protein-coupled receptors induces activation of Src kinases.39 However, the functional roles of Src family kinases in platelet activation have not been well defined. We show here that thrombin receptor-mediated activation of G12/13 leads to tyrosine phosphorylation of Src family kinase in platelets, suggesting that Src kinase is an important downstream molecule of G12/13 signaling. Consistent with our observations, it has been shown that constitutively active Gα12-expressing MDCK (Madin-Darby canine kidney) cells have an increased Src tyrosine kinase activity.40 It is also shown that Gα13 stimulates Src kinase activity in Rat-1 fibroblasts, suggesting the involvement of a Src-dependent pathway in Gα13-mediated cell proliferation and transformation.41 We then measured the effect of the Src kinase inhibitor PP2 on concomitant G12/13- and Gi/Gz-mediated Akt phosphorylation in Gαq-deficient platelets and found that PP2 blocked Akt phosphorylation induced by selective activation of either Gi or Gz signaling in the presence of G12/13 pathways. It has recently been reported that Src kinase is activated primarily by the Gq-coupled P2Y1 receptor in platelets,42 but Gq-mediated Src activation may not have any contribution to Akt phosphorylation since Gq signaling does not have any role in Akt phosphorylation in platelets. Our previous work has shown that both Gi and Gz signaling can cause Src activation,31 and we have found that the Src kinase inhibitor PP2 inhibits Gi/Gz-mediated Akt phosphorylation. Thus, our study demonstrates that Src kinase downstream of Gi and G12/13, but not Gq, is a key signaling molecule in the thrombin-mediated Akt phosphorylation, and G12/13 signaling cascade might mediate its potentiating effect on Akt phosphorylation through Src-dependent pathways (Figure 7). Further studies are required to identify which member of Src family kinases plays a role in this Akt phosphorylation.
In conclusion, we demonstrate that G12/13, but not Gq, signaling downstream of PARs is essential to achieve normal Akt phosphorylation by thrombin and thrombin receptor-activating peptides in platelets. While Gq signaling does not have a direct effect on Akt phosphorylation, it contributes to Gi stimulation by secreted ADP that is also required for thrombin-induced Akt phosphorylation. Furthermore, Src family kinases are essential for ADP- and thrombin-induced Akt phosphorylation in platelets.
Prepublished online as Blood First Edition Paper, October 13, 2005; DOI 10.1182/blood-2005-07-3040.
Supported by research grants HL60683 and HL80444 from the National Institutes of Health (S.P.K.).
S.K. wrote the manuscript, designed experiments, performed research, and analyzed data; J.J. designed experiments, performed experiments, and analyzed data; and S.P.K. designed experiments, analyzed data, and provided overall direction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.