CD40L generates immune responses in leukemia-bearing mice, an effect that is potentiated by IL-2. We studied the feasibility, safety, and immunologic efficacy of an IL-2– and CD40L-expressing recipient-derived tumor vaccine consisting of leukemic blasts admixed with skin fibroblasts transduced with adenoviral vectors encoding human IL-2 (hIL-2) and hCD40L. Ten patients (including 7 children) with high-risk acute myeloid (n = 4) or lymphoblastic (n = 6) leukemia in cytologic remission (after allogeneic stem cell transplantation [n = 9] or chemotherapy alone [n = 1]) received up to 6 subcutaneous injections of the IL-2/CD40L vaccine. None of the patients were receiving immunosuppressive drugs. No severe adverse reactions were noted. Immunization produced a 10- to 890-fold increase in the frequencies of major histocompatibility complex (MHC)–restricted T cells reactive against recipient-derived blasts. These leukemia-reactive T cells included both T-cytotoxic/T-helper 1 (Th1) and Th2 subclasses, as determined from their production of granzyme B, interferon-γ, and interleukin-5. Two patients produced systemic IgG antibodies that bound to their blasts. Eight patients remained disease free for 27 to 62 months after treatment (5-year overall survival, 90%). Thus, even in heavily treated patients, including recipients of allogeneic stem cell transplants, recipient-derived antileukemia vaccines can induce immune responses reactive against leukemic blasts. This approach may be worthy of further study, particularly in patients with a high risk of relapse.
Introduction
Acute leukemia cells can express a range of tumor-associated or tumor-specific antigens. Peptides derived from these antigens have shown promise in early-phase clinical studies of acute myeloid leukemia (AML).1 However, specific peptides may not be present in many subtypes of leukemia, they may be restricted to particular HLA phenotypes, or the immune response may select cells that have lost the single-peptide epitope to which the response is directed. An alternative approach is to use the entire leukemic cell as an immunogen. Both acute lymphoblastic leukemia (ALL) and AML are potentially well suited to this approach.
Cells from ALL patients express both class I and class II major histocompatibility complex (MHC) molecules, allowing the oncogene products to be directly presented to both CD8+ and CD4+ T cells. However, the leukemic cells generally do not express costimulatory surface molecules such as B7.1 (CD80) or B7.2 (CD86), which are necessary for induction of a T-cell response. Consequently, they induce specific T-cell anergy instead of specifically activating T cells.2-4 This lack of conventional costimulatory molecules notwithstanding, more than half of ALL cases express the CD40 antigen, the receptor for the CD40 ligand (CD40L), a potent costimulatory molecule in its own right.4 Engagement of CD40L augments the antigen-presenting function of normal and malignant B cells by up-regulating the expression of intercellular adhesion molecules, as well as the costimulatory molecule B7.1, MHC class I and II molecules, and T-cell chemokines.5-9 Direct stimulation through the CD40-CD40L pathway activates dendritic cells10 and bypasses the classic CD4+ helper-cell mechanism in activating specific cytotoxic T cells (CTLs).11 Activation of CD40+ leukemia and lymphoma cells by CD40L enables these cells to generate an antitumor immune response ex vivo.5-7,12-16
Costimulatory molecules also play an important role in generating a T-cell response against AML cells.17-19 CD40L directly stimulates CD4+ and CD8+ T cells that have become activated by engagement of tumor antigens on professional antigen-presenting cells (APCs), and cross-linking of CD40L on interleukin-2 (IL-2)–activated natural killer (NK) cells redirects their cytolysis to CD40L- target cells.20-26 Hence, transgenic expression of the CD40L molecule on leukemic blast cells may improve the presentation of leukemia-specific antigens so that antileukemic immune responses are produced through direct recruitment of effector T cells or activation of professional APCs. Human ex vivo and murine in vivo studies have shown that addition of IL-2 to these systems further enhances the effects of CD40L expression, most likely by expanding and sustaining the recruited effector cells.4,27,28
Unfortunately, direct transduction of primary human leukemic cells remains inefficient and unpredictable, posing a major obstacle to adequate expression of any transgene. We therefore expressed the costimulatory molecule CD40L and transgenic IL-2 in accessory cells (syngeneic fibroblasts) and administered them with the patient's own leukemic cells. In preclinical studies, this approach generated an antileukemic immune response in vivo27 while usually avoiding unwanted immune reactions against normal host tissues.29 We now demonstrate the feasibility and safety of administering this vaccine to patients in remission of high-risk leukemia following allogeneic stem cell transplantation (n = 9) or chemotherapy alone (n = 1). We find leukemia-reactive immune responses may be generated in the absence of autoimmune or graft-versus-host disease.
Patients, materials, and methods
Patients
The clinical protocol was approved by the Institutional Review Board of Baylor College of Medicine, by the Food and Drug Administration, and by the Recombinant DNA Advisory Committee of the National Institutes of Health. Informed consent was provided according to the Declaration of Helsinki. Patients were eligible for leukemic-cell collection and vaccine preparation if they were younger than 75 years at diagnosis and had primary or relapsed ALL (pre-B, B, T, B-cell precursor, or Burkitt type if bone marrow blasts > 20%), AML (M0 to M7), or myelodysplastic syndrome considered to be at high risk of relapse (predicted relapse risk for this group > 50% at 2 years). Patients were eligible for vaccine administration if their disease had entered complete or partial cytologic remission (< 20% blasts infiltrating the bone marrow) after a second or higher line of conventional and/or high-dose chemotherapy. Patients were enrolled a minimum of 100 days after allogeneic stem cell transplantation or a minimum of 1 week after their last course of chemotherapy and/or immunosuppressive drugs. They had recovered from the toxic effects of all prior chemotherapy before entering the study and had an absolute neutrophil count (ANC) greater than 0.5 × 109/L (500/μL), an absolute lymphocyte count (ALC) greater than 0.2 × 109/L (200/μL), and a platelet count greater than 50 × 109/L (50 000/μL). Exclusion criteria were as follows: active infection at the time of entry, acute or chronic graft-versus-host disease (GvHD), disease progression (bone marrow blasts > 20%), bilirubin level greater than 25.65 μM (1.5 mg/dL), creatinine level greater than 132.6 μM (1.5 mg/dL), an Eastern Cooperative Oncology Group (ECOG) performance status higher than 2, and a life expectancy of less than 10 weeks.
Adenoviral vectors
The human IL-2 (hIL-2)– and hCD40L-encoding adenoviral (Ad) vectors were constructed by recombination with the ClaI fragment of Ad-dl327, an E1a/b and E3 deletion mutant of human adenovirus serotype 5. Transgenes were under transcriptional control of the Rous sarcoma virus (RSV) promoter. The source of hCD40L cDNA was the pBluescriptII SK phagemid pBS-hCD40L-6A9 (American Type Culture Collection, Manassas, VA) cloned by Gauchat et al.30 The source of the hIL-2 cDNA is described elsewhere.31 Clinical-grade adenoviral vectors were made, propagated, and tested according to local and federal requirements. The adenoviral hIL-2 (AdhIL-2) and AdhCD40L vectors had titers of 6.6 × 1011 infectious units/mL and 7.5 × 1010 infectious units/mL, respectively. Both vectors had a viral particles (VPs)–infectious unit ratio of less than 30:1.
Recipient-derived vaccine preparation
All manufacturing procedures followed proposed Good Tissue Practices (GTPs) and were approved by the Food and Drug Administration under investigational new drug (IND) no. 8243. Recipient-derived leukemic cells were enriched from peripheral-blood or bone marrow mononuclear cells on a Lymphocyte Separation Medium gradient (ICN Pharmaceuticals, Costa Mesa, CA) and stored at -170°C in liquid nitrogen until further needed. Blasts were also obtained by therapeutic leukapheresis. Enrichment was controlled by quantifying the clone with use of leukemia-associated surface markers and was considered adequate if greater than 65% blast cells were present. Recipient-derived fibroblasts were collected by a 6-mm punch biopsy from the patient's own skin prior to the study and were cultured in RPMI 1640 medium (BioWhittaker, Walkersville, MD) with 10% fetal bovine serum (Hyclone, Logan, UT). After expansion of the fibroblasts to numbers adequate for the preparation of at least 6 injections, they were divided into 2 groups, each transduced with either AdhIL-2 at a multiplicity of infection (MOI) of 500 VPs/cell or Ad-hCD40L at an MOI of 1000 VPs/cell. Gene expression was considered adequate if CD40L expression was greater than 20% as measured by flow cytometry and IL-2 production was greater than 150 pg/106 cells/24 hours as measured by enzyme-linked immunosorbent assay (ELISA). Fibroblasts were frozen 24 hours after transduction and stored at -170°C in liquid nitrogen. All fibroblast preparations were negative for bacteria, fungi, Mycoplasma, adventitious viruses, and replication-competent adenoviruses and lacked measurable endotoxin. Neither irradiation (3000 cGy) nor a freeze-thaw cycle impaired transgene expression or fibroblast recovery/viability, but irradiated fibroblasts did not divide. Upon thawing, CD40L expression by fibroblasts was detected for up to 10 days, with a mean peak expression of 40% at 6 days after adenoviral gene transfer. Similarly, IL-2 secretion in the supernatant of transduced fibroblasts was detected for up to 10 days, with a mean peak secretion of 50 000 pg/106 cells per 24 hours on day 6.
Treatment
The treatment plan consisted of up to 6 subcutaneous injections of a fixed dose of irradiated (4000 cGy) recipient-derived leukemic blasts (2 × 107/injection) admixed with a fixed dose of IL-2–secreting irradiated (3000 cGy) recipient-derived skin fibroblasts (2 × 107/injection) and an escalating dose of CD40L-expressing irradiated (3000 cGy) fibroblasts, from 2 × 105/injection (dose-level 1) to 2 × 107/injection (dose-level 3). All injections were given subcutaneously in the upper arm in 1-mL volumes. The first 3 injections were given at weekly intervals, followed by a 2-week rest. If the first 3 injections were well tolerated, and if there was no evidence of tumor progression, patients received 3 additional injections of IL-2–secreting and CD40L-expressing fibroblasts admixed with leukemic blasts separated by 2-week intervals.
Evaluation of toxicity and antitumor responses
Patients were monitored for local and systemic toxicity by physical examination and blood chemistry analysis at weekly intervals. Toxic reactions were graded according to National Cancer Institute criteria. Antitumor immune responses were assessed at weekly intervals for 10 weeks after the first injection, then every other week for 6 weeks, then monthly for 1 year. At 12 weeks and 6 months after the first injection, the disease status of patients was determined by clinical evaluation according to the World Health Organization (WHO) classification32 and by bone marrow aspiration.
Phenotyping of local lesions
Injection-site skin biopsies were performed in all patients 1 week after the first and the second vaccine injections. Samples were immediately fixed in formalin and processed overnight. Immunohistochemical staining was done by a standard avidin/biotin technique used with the Optimax automated stainer (Biogenics, San Ramon, CA). The antibodies used included CD4 (clone OPD4; Dako, Carpinteria, CA) and CD8 (clone C8/144B; Dako) for lymphoid cells, S-100 (mono/polyclonal mix; Ventana, Tucson, AZ) for dendritic cells, and CD1a (clone O10; Immunotech, Westbrook, ME) for Langerhans cells. HLA-DR (clone TAL.1B5; Dako) was used as a marker for GvHD. CD68 (clone KP-1; Dako) and CD83 (clone 1H4b; Vision Biosystems, Norwell, MA) were used on biopsies from patients with discernible dendritic-cell infiltrate as a marker of dendritic-cell activation and maturation. Control skin biopsies were obtained from healthy volunteers on the research team.
Phenotyping of peripheral-blood mononuclear cells
Fresh peripheral-blood mononuclear cells (PBMCs) were phenotyped in all evaluable patients before and after each immunization by flow cytometric analysis (FACScan; Becton Dickinson, San Jose, CA) with antibodies to CD3, CD4, CD8, TCR-αβ, TCR-γδ, κ and λ chains, CD11b, CD15, CD16, CD19, CD20, CD25, CD27, CD45, CD45RA, CD45RO, CD56, CD57, CD69, HLA-DR (Becton Dickinson), and LAG-3 (clone 17B4; Alexis Biochemical, San Diego, CA). The values for patients were compared with those for matched historic controls (a cohort of nonvaccinated patients, at our institution, that received bone marrow transplants).
Assessment of cytotoxic-T and helper-T profile by GrB, IFN-γ, and IL-5 ELISPOT
When adequate numbers of PBMCs and target cells were available, we measured the profile of T cells responding to the immunizing blast cells using granzyme B (GrB), interferon-γ (IFN-γ), and IL-5 enzyme-linked immunospot (ELISPOT; Pharmingen/BD Bioscience, San Diego, CA). Frozen PBMCs obtained before and after immunization were thawed and seeded at 3 × 105 to 5 × 105 cells per well in a 96-well plate coated with monoclonal antibodies (mAbs) specific for GrB, IFN-γ, or IL-5 in RPMI 1640 (BioWhittaker) containing 5% heat-inactivated human AB serum (Gemini BioProducts, Woodland, CA) and 1% L-glutamine (BioWhittaker). When enough PBMCs were available, MHC restriction was determined by blocking CD4 or CD8 surface receptors on T cells by preincubating the PBMCs for 30 minutes at 37°C with either 10 μL of an anti-CD4 or an anti-CD8 azide-free mAb (Immunotech). Target cells consisted of unmodified recipient-derived leukemic blasts obtained prior to chemotherapy. Controls consisted of the NK-specific K562 myeloid cell line, recipient-derived skin fibroblasts, and phytohemagglutinin (PHA) blasts (obtained from each patient's prevaccination PBMCs). To control for nonspecific cytokine release during posttransplantation immune recovery, we assessed IFN-γ release by PBMCs from patients that received transplants in an ELISPOT assay specific for pp65 (Jerini Bio Tools GmbH, Berlin, Germany), a potent CMV antigen. Frozen target cells were thawed and plated at 1 × 105 to 3 × 105 cells per well. Plates were incubated at 37°C, 5% CO2 for 24 (IFN-γ and GrB) or 48 (IL-5) hours. Cells were then discarded and wells were washed thoroughly. Biotinylated secondary antibody to either GrB, IFN-γ, or IL-5 was then added to the wells, with all subsequent steps following the manufacturer's recommendations. Spot analysis was performed with a Series 1 ImmunoSpot Image Analyzer and software (Cellular Technologies, Cleveland, OH). The frequency of positive (GrB-, IFN-γ–, or IL-5–producing) cells compared with the total number of plated cells was calculated after the number of positive cells in the control wells had been subtracted from that of the experimental wells. These control wells contained nonstimulated PBMCs (spontaneous GrB, IFN-γ, or IL-5 release), target cells alone, or reagents alone (blanks).
Detection of circulating IgG against the immunizing cells
We studied the binding of antibodies produced after immunization with recipient-derived leukemic blasts and skin fibroblasts. Reactivity was also measured against several leukemia cell lines, including K562 (acute-phase chronic myeloid leukemia [CML]), CCL-120 (B-ALL, EBV positive), Daudi (Burkitt-like ALL), MOLT-3 (T-ALL), and an unrelated tumor cell line, IMR-32 (neuroblastoma, GD2+). The cells were incubated with 5 μL or 50 μL of autologous plasma (diluted 1:2 in PBS) that had been obtained immediately before vaccination, 1 to 2 weeks after the third and sixth vaccinations, or 6 and 12 months after the first vaccination. Negative controls consisted of pooled plasma obtained from multiple healthy donors. The method used to detect circulating IgG is described elsewhere.33 In brief, bound IgG was detected with biotinylated F(ab′)2 (heavy + light) fragments of donkey anti–human IgG (Jackson Immuno Research, West Grove, PA) followed by Neutralite–Avidin-R–PE (Southern Biotechnology Associates, Birmingham, AL). All detection steps were repeated once at room temperature for 10 minutes. More than 105 cells were analyzed with a FACScan flow cytometer (Becton Dickinson).
Statistical analyses
When warranted, results of preimmunization and postimmunization assays were compared by 2-tailed paired t test or Wilcoxon signed rank analysis and analysis of variance (one-way ANOVA) using SPSS software (SPSS, Chicago, IL).
Results
Patients
The ages of the patients at study entry ranged from 4 to 56 years with a median of 15.5 years (Table 1). They had all suffered a relapse of their acute leukemia, except for patients 5, 8, and 9, who were eligible for vaccination because of pre-B ALL with the Philadelphia chromosome, myelodysplastic syndrome preceding AML, or secondary AML following treatment for Hodgkin disease, respectively. Of the 44 patients from whom blast cells were collected, 10 received their vaccines. Twenty-seven of the remaining 34 patients did not receive their vaccine because they lacked enough blasts for vaccine preparation, 5 chose another treatment option, and in 2 patients vaccines became available after the maximal accrual was met. Overall, the completion rate was 23%.
All but one patient were in complete cytologic remission of their acute leukemia at the time of immunization. Patient 1, who was enrolled at dose-level 1 while in partial remission of Burkitt-like B leukemia, died of rapid disease progression before the third injection and was not evaluated for systemic immunologic responses. Patient 5 had insufficient leukemic blasts after vaccine preparation for follow-up studies of immune responses to the vaccine; all other patients were evaluable for this end point. At the time of immunization, none of the patients had received any cytotoxic or radiation therapy for at least 4 weeks prior to enrollment. All other immunosuppressive drugs had been stopped an average of 71 days prior to the first vaccine administration (median, 45 days; range, 14-195 days; Table 1). The mean neutrophil count at the time of the first vaccine injection was 0.322 × 109/L (322/μL; range, 0.171 × 109/L to 0.609 × 109/L [171-609/μL]) and the mean lymphocyte count was 0.521 × 109/L (521/μL; range, 0.158 × 109/L to 1.071 × 109/L [158-1071/μL]).
Toxicity
CD40L-expressing and IL-2–secreting skin fibroblasts produced clinically significant adverse effects in only 1 patient (no. 10), who developed a localized abscess at the site of injection 5. Surgical drainage revealed sterile pus; blood cultures were negative. There was no evidence of GvHD or autoantibodies in any of the posttransplantation patients after immunization.
Local responses to vaccine injection
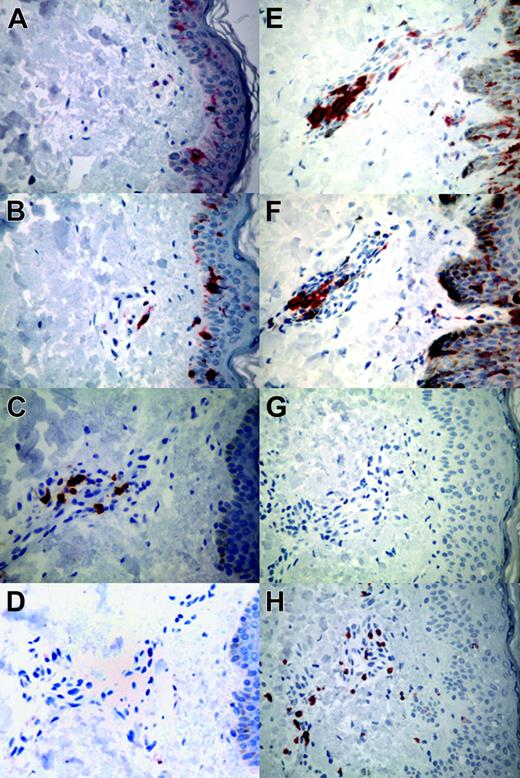
We observed mild, transient erythema at the injection sites in 3 patients (nos. 1, 6, and 9) after 2 or more injections. Twenty injection-site punch biopsies, performed on all 10 patients at 1 week after the first and second injections, were compared with skin biopsies from 2 healthy volunteers. None of the specimens contained leukemic cells. Inflammatory reactions were graded according to the highest density of lymphocytes in either the dermal perivascular or subcutaneous regions (mild, < 10 cells/high-power field [hpf]; moderate, 10-50/hpf; severe, > 50/hpf). Mild responses were noted in patients 8, 9, and 10; moderate in 1, 5, and 6; and severe in 2, 3, 4, and 7. The degree of inflammation increased from the first to the second biopsy in 5 patients: from moderate to severe in nos. 2, 3, 4, and 7 and from mild to moderate in no. 5. All patients showed a reversal of the normal 2:1 CD4-to-CD8 ratio, with nos. 2, 9, and 10 having a marked CD8 infiltrate (from 1:17 to 1:60; Figure 1G-H). Increased numbers of Langerhans cells in the perivascular areas of the dermis were noted in 8 of 10 patients (nos. 2-9; Figure 1E-F). In the 6 biopsies from the 3 patients with the most discernible dendritic-cell infiltrate, most cells were strongly CD1a+. Perinuclear areas stained strongly for HLA-DR whereas cell membranes were less positive. Cytoplasmic staining revealed a fine diffuse granular cytoplasmic pattern for CD68 whereas CD83 signal was negligible. These findings are consistent with activated, immature dendritic cells with no mature dendritic cells.
Systemic responses to injection
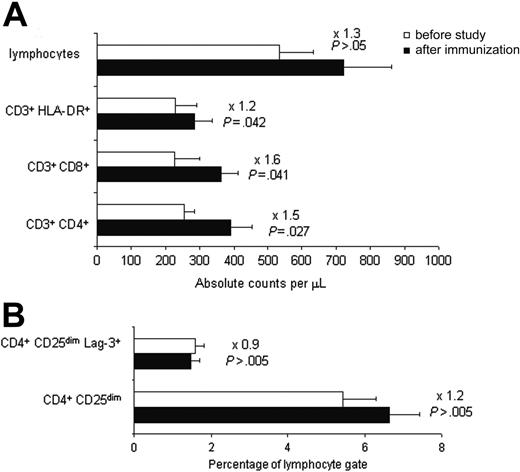
Mean (± SEM) peripheral-blood cell counts (absolute and percentages) were determined in all 8 evaluable patients before and 7 days after the completion of inoculations. There was a modest but nonsignificant increase in the absolute number of circulating lymphocytes (Figure 2; before immunization 0.533 × 109/L ± 0.099 × 109/L [533 ± 99/μL] vs after immunization 0.722 × 109/L ± 0.139 × 109/L [722 ± 139/μL], P > .05), whereas the proportion of lymphocytes in the total leukocyte population did not change (before immunization 0.28 ± 0.05 vs after immunization 0.33 ± 0.04, P > .05).
Systemic responses to injection: NK-cell and B- and T-lymphocyte populations
There was a 1.5-fold increase in the CD3+ CD4+ lymphocyte population (Figure 2; before immunization 0.254 × 109/L ± 0.031 × 109/L [254 ± 31/μL] vs after immunization 0.392 × 109/L ± 0.061 × 109/L [392 ± 61/μL], P = .027), a 1.6-fold increase (Figure 2; 0.227 × 109/L ± 0.072 × 109/L [227 ± 72/μL] vs 0.362 × 109/L ± 0.049 × 109/L [362 ± 49/μL], P = .041) in the absolute number of circulating CD3+ CD8+ lymphocytes, and a 1.2-fold increase (Figure 2; 0.229 × 109/L ± 0.061 × 109/L [229 ± 61/μL] vs 0.284 × 109/L ± 0.053 × 109/L [284 ± 53/μL], P = .042) in the absolute number of circulating CD3+ DR+-activated lymphocytes. The mean (± SEM) proportions of CD4+ and CD8+ cells before and after treatment were not significantly different (0.0551 ± 0.0098 and 0.0516 ± 0.0181 vs 0.069 ± 0.0078 and 0.0634 ± 0.0073, respectively; P > .05) nor were the mean (± SEM) proportions of DR+ cells among the CD3+ population (0.4675 ± 0.0736 vs 0.4182 ± 0.0651; P > .05). We did not observe any variation in the proportions of CD4+ CD25dim LAG-3+ regulatory T cells, which remained within normal ranges before and after treatment (Figure 2; 0.0159 ± 0.0024 vs 0.0146 ± 0.0025, P = .664). The absolute numbers of NK and B cells, as well as their proportions, remained statistically unchanged during treatment (data not shown).
Representative local responses to the autologous combination vaccine in punch biopsy specimens from the injection site. (A-D) Control tissues from healthy volunteers. (A) CD1a (O10 staining). (B) Dendritic cells (S-100 staining). (C) CD4 (CD45RO/OPD4 staining). (D) CD8 (CD45RO/C8/144B staining). (E-H) Representative tissues from patients. (E) Abundant dermal perivascular infiltration of Langerhans cells (O10 staining) with (F) retention of dendritic-cell infiltrate in patient 5 (S-100 staining). (G) Paucity of CD4+ lymphocytes (CD45RO/OPD4 staining) with (H) moderate infiltration of CD8+ lymphocytes in patient 2 (CD45RO/C8/144B staining). These studies were performed on all patients. For all panels, original magnification is × 20. Images were visualized using a Nikon Labophot-2 microscope equipped with a 20×/1.0 Plan objective lens (Nikon, Tokyo, Japan). A Spot Insight QE 4.2 camera (Diagnostic Instruments, Sterling Heights, MI) was used to capture images, and Adobe Photoshop Elements 2.0 software (Adobe Systems, San Jose, CA) was used to process them.
Representative local responses to the autologous combination vaccine in punch biopsy specimens from the injection site. (A-D) Control tissues from healthy volunteers. (A) CD1a (O10 staining). (B) Dendritic cells (S-100 staining). (C) CD4 (CD45RO/OPD4 staining). (D) CD8 (CD45RO/C8/144B staining). (E-H) Representative tissues from patients. (E) Abundant dermal perivascular infiltration of Langerhans cells (O10 staining) with (F) retention of dendritic-cell infiltrate in patient 5 (S-100 staining). (G) Paucity of CD4+ lymphocytes (CD45RO/OPD4 staining) with (H) moderate infiltration of CD8+ lymphocytes in patient 2 (CD45RO/C8/144B staining). These studies were performed on all patients. For all panels, original magnification is × 20. Images were visualized using a Nikon Labophot-2 microscope equipped with a 20×/1.0 Plan objective lens (Nikon, Tokyo, Japan). A Spot Insight QE 4.2 camera (Diagnostic Instruments, Sterling Heights, MI) was used to capture images, and Adobe Photoshop Elements 2.0 software (Adobe Systems, San Jose, CA) was used to process them.
Leukemia-reactive, T-cell–mediated cytolytic and helper responses
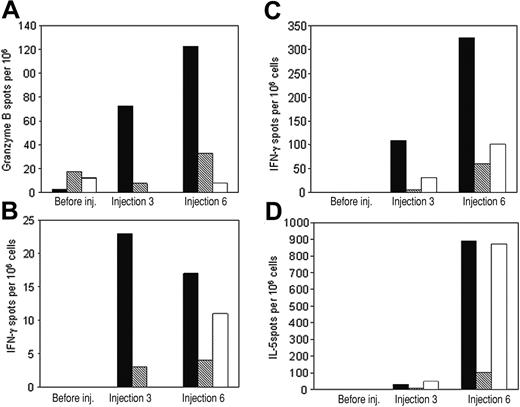
We determined the cytotoxic and helper T-cell activities against recipient-derived blasts in all evaluable patients before and 7 days after completion of immunizations, measuring GrB, IFN-γ, and IL-5 release after coculture with whole recipient-derived leukemia cells. Using the immunizing cell line (unmanipulated recipient-derived blasts) and freshly thawed, unmanipulated PBMCs collected before, during, or after immunizations, we detected systemic cytotoxic effector-cell activity against the immunizing, recipient-derived leukemic blasts in 5 of 8 patients (nos. 2, 3, 4, 9, and 10; Table 2; Figure 3A). The increase in GrB release was statistically significant after 3 immunizations compared with prevaccination values (P = .046). We also observed T-helper type 1 (Th1) and Th2 leukemia-reactive immune effectors in 4 patients (nos. 2, 4, 7, and 9; Table 2; Figure 3B) and 3 patients (nos. 2, 4, and 7; Table 2; Figure 3C), respectively. Increases in IFN-γ and IL-5 secretion were statistically significant after 6 immunizations compared with prevaccination values (P = .046 and .043, respectively). The cytotoxic- and Th1-mediated responses were associated with both CD4+ and CD8+ T cells, as demonstrated by blocking experiments with mAbs to these T-cell surface markers (Figure 3A-C). As expected, the Th2 responses were mediated solely by CD4+ T cells (Figure 3D). Limitations in the availability of patients' PBMCs at follow-up allowed only the assessment of IFN-γ release against recipient-derived PHA (T cell) blasts and fibroblasts. There was no significant increase in IFN-γ secretion after immunization compared with prevaccination values (P > .5 for skin fibroblasts, P > .345 for PHA blasts), and there was no correlation with the responses observed against recipient-derived leukemic blasts (Table 3). No patient developed autoimmunity clinically or from measurement of antinuclear (ANA) or anti–red-cell antibodies. Of note, unstimulated controls or samples stimulated with allogeneic blasts showed no change in GrB or cytokine secretion following immunization (data not shown). In transplant recipients whose bone marrow donors were CMV-seropositive, IFN-γ release by pp65-stimulated PBMCs collected before and after immunization remained unchanged (P > .26 between preimmunization and postimmunization values), indicating that the appearance of leukemia-reactive effector cells was not simply a reflection of the tempo of immune recovery after bone marrow transplantation (Table 4).
Leukemia-reactive humoral responses
In 2 patients (nos. 6 and 7), T-helper cell activity was also demonstrated by the development of IgG antibodies specific for the immunizing cell line in plasma samples (Table 5). Even though increases in specific IgG did not reach statistical significance (P > .091), patients 6 and 7 had a 2- to 3-fold increase compared with controls. These IgG antibodies reacted only with the immunizing recipient-derived blasts and did not cross-react against the unmodified recipient-derived skin fibroblasts, other leukemia cell lines (K562, CCL-120, MOLT-3, and Daudi), or other tumors (IMR-32; data not shown). Pooled sera from healthy donors showed no reactivity against leukemic or nonleukemic cells (data not shown).
Antileukemic responses
As summarized in Table 1, patient 1 had rapidly progressing Burkitt-like leukemia after the second immunization. He was taken off study and therefore was not eligible for evaluation of either tumor or systemic immunologic responses. He died of disease 33 days after entering the study (152 days after allogeneic bone marrow transplantation). Patient 3 relapsed 152 days after entering the study (470 days after allogeneic bone marrow transplantation). Relapse was isolated to the scalp. The patient was taken off study and is disease free 4 years after a third complete remission induced with conventional chemotherapy. All other patients remained free of measurable disease at the 6-month evaluation and are off all therapy without any evidence of disease at a median follow-up of 41 months (range, 27-61.6 months; 5-year overall survival, 90%).
Flow cytometric analysis of lymphocyte subpopulations before (□) or after (▪) administration of recipient-derived leukemia vaccines. (A) Absolute circulating lymphocyte counts showing cytotoxic (CD3+ CD8+), helper (CD3+ CD4+), and activated (CD3+ HLA-DR+) T-cell subpopulations. (B) Percentage of regulatory T cells (CD4+ CD25dim Lag-3+) among all lymphocytes. Values are means ± SEM; fold values are given above P values.
Flow cytometric analysis of lymphocyte subpopulations before (□) or after (▪) administration of recipient-derived leukemia vaccines. (A) Absolute circulating lymphocyte counts showing cytotoxic (CD3+ CD8+), helper (CD3+ CD4+), and activated (CD3+ HLA-DR+) T-cell subpopulations. (B) Percentage of regulatory T cells (CD4+ CD25dim Lag-3+) among all lymphocytes. Values are means ± SEM; fold values are given above P values.
Representative ELISPOT results showing granzyme B, IFN-γ, or IL-5 release from PBMCs obtained before or after 3 or 6 immunizations with recipient-derived leukemia vaccines. PBMCs were stimulated ex vivo against recipient-derived leukemic blasts alone (▪) or after preincubation with anti-CD4 ( ) or anti-CD8 (□) monoclonal antibodies. (A) Granzyme B, patient 4. (B) IFN-γ, patient 4. (C) IFN-γ, patient 7. (D) IL-5, patient 2. All values are mean spot numbers for duplicate wells minus background per 106 PBMCs.
) or anti-CD8 (□) monoclonal antibodies. (A) Granzyme B, patient 4. (B) IFN-γ, patient 4. (C) IFN-γ, patient 7. (D) IL-5, patient 2. All values are mean spot numbers for duplicate wells minus background per 106 PBMCs.
Representative ELISPOT results showing granzyme B, IFN-γ, or IL-5 release from PBMCs obtained before or after 3 or 6 immunizations with recipient-derived leukemia vaccines. PBMCs were stimulated ex vivo against recipient-derived leukemic blasts alone (▪) or after preincubation with anti-CD4 ( ) or anti-CD8 (□) monoclonal antibodies. (A) Granzyme B, patient 4. (B) IFN-γ, patient 4. (C) IFN-γ, patient 7. (D) IL-5, patient 2. All values are mean spot numbers for duplicate wells minus background per 106 PBMCs.
) or anti-CD8 (□) monoclonal antibodies. (A) Granzyme B, patient 4. (B) IFN-γ, patient 4. (C) IFN-γ, patient 7. (D) IL-5, patient 2. All values are mean spot numbers for duplicate wells minus background per 106 PBMCs.
Discussion
We have shown that immunogenic, recipient-derived leukemia vaccines can be prepared from unmanipulated leukemic blasts and IL2 and CD40LG gene–modified skin fibroblasts and can be readministered to patients after allogeneic bone marrow transplantation. The engineered immunizing cells recruited CD8+ and CD4+ T cells, as well as CD1a+ Langerhans cells, to the injection site. Systemically, there was a modest but consistent rise in circulating CD4+, CD8+ and activated CD3+ DR+ lymphocytes after immunization. More importantly, ELISPOT analysis showed a 10- to 890-fold rise in leukemia-reactive cytotoxic CD4+ and CD8+ T cells as well as Th1 and Th2 helper T cells that persisted up to 3 months after the last immunization. Both cytotoxic and Th1 lymphocytes were found in the CD4+ and CD8+ T-cell populations, whereas Th2 lymphocytes were exclusively from the CD4+ pool. Two patients made antibodies that bound to the recipient-derived leukemic blasts, reinforcing the concept that the vaccine had the ability to recruit and stimulate CD4+ Th2 cells. There was no evidence for vaccine-induced tolerance. We did not observe any increase in CD4+ CD25+ regulatory T cells, and all but 2 patients developed measurable leukemia-reactive cellular responses. Of the 2 apparent cellular nonresponders, 1 developed a leukemia-reactive antibody. Local responses and delayed-type hypersensitivity–like immune infiltrates at the sites of inoculation also suggest that anergy had been reversed.
Despite the generation of effector cells reactive to the recipient-derived leukemic blasts, immunization did not produce GvHD, even though none of the patients were receiving immunosuppressive drugs for a median time of 45 days prior to immunization. In the absence of a control group, we cannot exclude the possibility that local inflammatory changes induced by injection of fibroblasts alone or by adenoviral proteins could have induced an equivalent local and systemic effect, but prior ex vivo and in vivo studies suggest such an effect is unlikely.5-7,34-36 By contrast, CD40-CD40L interactions play a key role in B-cell activation and differentiation, and engagement of CD40L augments the antigen-presenting functions of normal and malignant B cells and of professional APCs10,20,37 by up-regulating the expression of intercellular adhesion molecules as well as the costimulatory molecule B7.1 and MHC class I and II molecules.5-7 Consequently, ex vivo activation of CD40+ leukemia and lymphoma cells by CD40L enables these cells to generate an antitumor response in vitro.5-7,12 This effect is complemented by the action of IL-2, which allows other cytotoxic precursor cells to bypass their requirement for dendritic-cell (DC)– or Th1-derived helper signals in the generation of a CTL response.34,38 Animal models indicate that expression of transgenic IL-2 augments immunogenicity across a broad range of tumors, including leukemic cell lines.35 IL-2, unlike many other T-cell stimulatory cytokines, rarely induces leukemic cell proliferation,36 even when these cells express IL-2 receptors.39-41 Murine models from our own and other groups demonstrate that combinations of immunomodulatory molecules that act on different levels of the immune response enhance antitumor responses, and the combination of IL-2–secreting cells injected concomitantly with CD40L-expressing cells was shown to be superior to each component alone.27,28 Thus, our observation that the CD40L and IL-2 combination can induce local and systemic CD4+ and CD8+ T cells specific to recipient-derived leukemic blasts is consistent with the known effects of this combination on normal as well as leukemic cells in vitro and in vivo.4,24,27,28,42,43
We do not know whether the leukemia reactivity we detected is attributable to recognition of tumor-specific or tumor-associated antigen, but the absence of autoimmune disease, clinical GvHD, and any correlation between leukemia reactivity and reactivity with patient PHA blasts or fibroblasts all suggest that the antigen(s) recognized is of restricted tissue distribution. While leukemic cells are susceptible to cytotoxic effector activities both in vitro and in vivo, it is yet not clear which components of the immune and innate responses are most critical for the effective destruction of the host leukemia in vivo.44-46 Experimental results confirm the pivotal role of CD4+ T cells in providing helper effects to CD8+ cytotoxic T lymphocytes or to B cells.47 Activated B cells, in turn, can produce specific antileukemic antibodies.48 Alternatively, activation of the CD40-CD40L pathway can cross-prime professional APCs and bypass the need for CD4 T-cell help.11,49-51 At present, we do not know if the recruitment of the local and circulating T-cell effectors we observed is the result of a direct effect of enhanced antigen presentation by leukemic blasts stimulated through the CD40-CD40L pathway, as previously suggested,15,43,49,52,53 or whether the favorable milieu generated by the gene-modified fibroblasts encourages cross-priming of intradermal professional APCs.54 Previous observations from our group support the activation of CD40+ leukemia cells when stimulated with CD40L-secreting fibroblasts.16 Alternatively, APCs stimulated through the CD40-CD40L pathway may take up leukemic cells or apoptotic bodies and present the processed antigen to T cells stimulated by IL-2–producing fibroblasts.55 Subsequently, the APCs may migrate to locoregional lymph nodes to recruit additional leukemia-reactive T cells.56,57 Our histologic and systemic immunologic analyses are consistent with either or both processes. Ideally, skin biopsies should also be performed within 1 or 2 days of immunization to assess the early events in the vaccination process, but this would remove the antigenic stimulus and likely prevent the production of the systemic effects we observed. Certainly by day 7 the injected tumor cells have disappeared, and this may explain why the residual infiltrating DCs are activated but not yet mature.
Despite efforts to develop vectors that will reliably and effectively transduce primary human acute leukemia cells, none have yet shown sufficient promise to enter clinic testing. Our strategy of using skin fibroblasts as “transfection partners” appears immunologically effective, producing an antileukemic but not an unwanted autoimmune response.29 Nonetheless, it would be desirable to develop a means to directly transduce the leukemic cells themselves. The availability of such a process would substantially simplify the logistics of the vaccine preparation.16,58,59 An alternative consists of ex vivo generation of CD40L-stimulated autologous ALL vaccines, which was tested in a recent phase 1 trial.15,52,60 While these vaccines failed to produce immune responses in patients with relapsed, active disease, they were potent APCs capable of stimulating allogeneic and peptide-specific T cells in vitro. Despite the lack of activity in vivo in the chosen setting, this approach may prove logistically feasible for a larger study in patients in complete remission. Even so, the complexity of any whole-cell tumor vaccine approach would greatly exceed that of alternative methods, particularly the use of peptides. For example, proteinase 1 (PR1) is a target for CML-specific CTLs, and vaccination with a PR1 peptide induces prolonged partial as well as complete clinical and molecular remissions in patients with myeloid malignancies.1,61,62 However, peptide epitopes, including those derived from PR1, are not available across the HLA repertoire, may be inadequate for lymphoblastic leukemia, and may lead to the selection of variants with loss of single-epitope tumor antigens. We would argue, therefore, that the added complexities of whole leukemia-cell vaccines can be offset by their ability to elicit a more broad-based and more robust immune response.
Most clinical trials of cancer vaccines or tumor-derived peptides have been conducted in patients with bulky disease. For many well-established reasons, this strategy reduces the likelihood of observing prolonged or complete tumor responses.63 Murine Cd40lg gene–expressing autologous B-cell chronic lymphocytic leukemia (B-CLL) cells injected intravenously have induced measurable antitumor responses in vivo in patients,64 but much of this activity may be due to the direct induction of apoptosis of CD40-expressing endogenous tumor cells. We therefore chose to immunize patients with only minimal residual disease, in whom tumor cell–mediated immune inhibition would be less effective and the selection of resistant tumor antigen–loss variants less likely. The size of our study and the intentional immunization of high-risk patients without measurable disease mean that we cannot directly link clinical outcome to the observed MHC-restricted cytotoxic as well as helper T-cell antileukemic immune responses. The recent observation that the memory T-cell compartment is the least affected by chemotherapy may allow for improved immunization by vaccinating prior to high-dose chemotherapy, subsequently using vaccine boosts to induce re-expansion of this memory T-cell compartment.65
In conclusion, our results demonstrate the feasibility of generating a recipient-derived leukemia vaccine combining transgenic CD40L with IL-2. The vaccine is not only well tolerated but also induces the same pattern of antileukemic immunity predicted from our murine model.27 Although the results are consistent with a therapeutic benefit, any estimate of the vaccine's true value will require more rigorous testing in a randomized trial.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-03-1259.
Supported in part by National Institutes of Health Core Grant no. 5RO1 CA78792. Baylor College of Medicine General Clinical Research Center is supported by National Institutes of Health Grant no. M01RR0188. R.F.R. was supported by grants from the Fondation de France-Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC) and the Hope Street Kids Foundation. E.B. was supported by a grant from the Comitato Maria Letizia Verga per lo studio e la cura della leucemia del bambino (Monza, Italy).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Tatiana Gotsolva for excellent technical assistance, Pat Alcoser and Sheryl Rodgers for their dedicated patient care, and Shannon Inman and Kimberly East for study coordination and data management. We are indebted to Persis Amrolia, Gian-Pietro Dotti, Régis Costello, and Jean-Charles Soria for their careful reading of the manuscript and their helpful comments. We would also like to thank John Gilbert for scientific editing and Debbie Graustein for editorial assistance. We are grateful to all of our medical colleagues who referred patients to this study. Finally, we thank the patients and their families for their trust and their commitment to medical research and, hopefully, progress.





 ) or anti-CD8 (□) monoclonal antibodies. (A) Granzyme B, patient 4. (B) IFN-γ, patient 4. (C) IFN-γ, patient 7. (D) IL-5, patient 2. All values are mean spot numbers for duplicate wells minus background per 106 PBMCs.
) or anti-CD8 (□) monoclonal antibodies. (A) Granzyme B, patient 4. (B) IFN-γ, patient 4. (C) IFN-γ, patient 7. (D) IL-5, patient 2. All values are mean spot numbers for duplicate wells minus background per 106 PBMCs.