In the present study, we demonstrated that the antiapoptotic function of Bcl-2 in mast cells is significantly dependent on its association with the heat shock protein 90β (Hsp90β). Dissociation of these 2 proteins inhibits the antiapoptotic activity of Bcl-2 by initiating the release of cytochrome c from mitochondria into cytosol and increasing the activity of caspase 3 and caspase 7, resulting in mast-cell apoptosis. The antiapoptotic activity of Bcl-2 was greatly affected by knocking-out specifically Hsp90β using the RNA interference approach. Thus, for the first time, it has been shown that Hsp90β might modulate the antiapoptotic activity of Bcl-2 at least in mast cells. These findings could have implications for a novel strategy of regulating apoptosis in patients with mastocytosis and other mast cell–associated diseases.
Introduction
Mast cells, the major cellular players in allergy, have been found to exist at a constant number in tissues under normal conditions. This probably reflects an equilibrium between cell migration, proliferation, and apoptosis.1,2 The biochemical events leading to apoptosis in this cell type have just started to be explored.2,3 We recently demonstrated the role of Bcl-2 in the prevention of mast-cell apoptosis using a novel antibody originated from phage display library.4 We constructed an anti–Bcl-2 single-chain Fv connected to 11 amino acids derived from TAT protein, which enabled the antibody to penetrate the cell membrane. The anti–Bcl-2 antibody bound specifically to endogenous Bcl-2, leading to the initiation of the apoptosis cascade.4
The Bcl2 gene family encodes a series of proteins involved in the regulation of programmed cell death.5 Members of the family can be grouped into 2 distinct sets with antagonistic functions. Bcl-2, BclXL, Mcl-1, and A1/Bfl-1 have antiapoptotic, protective functions and prevent the activation of downstream death-effector caspase proteases. In contrast, proteins such as Bax, Bak, Bad, and Bid have proapoptotic roles and can antagonize the cell-protective functions of Bcl-2.5,6
Although it is not fully understood how Bcl-2 family proteins regulate apoptotic pathways, one possible mechanism is that members of this family engage in various protein–protein interactions to form homotypic and heterotypic dimers important for their biologic functions.7,8 Association of Bcl-2 with other antiapoptotic proteins through Bcl-2 homology (BH) domains prevents changes in mitochondrial membrane potential (ΔΨm) and the release of cytochrome c from mitochondria.9
The ability of many Bcl-2 family members to form homodimers and heterodimers through their BH domains is important for the activation of specific functions, such as initiating and altering the process of apoptosis and neutralizing these functions in the cells.10 It was, therefore, postulated that the relative ratio of antiapoptotic to proapoptotic dimers plays a pivotal role in determining the resistance of a cell to apoptosis. However, the function of Bcl-2 has been found to be affected by its association with proteins not belonging to the Bcl-2 family. Recently, it was observed that Bcl-2 is able to act as a “cell-killer” on binding to the nuclear orphan receptor Nur77/TR3.11
Heat shock protein 90 (Hsp90) belongs to a large family of heat shock proteins, classified according to their molecular size, such as Hsp100, Hsp90, Hsp70, and others. An increasing number of reports reveal that the intracellular pathways leading to apoptosis and the stress response are linked. For example, Hsp70 has been proposed to act on the apoptotic pathway at earlier steps because it serves as a natural inhibitor of JNK112 and it also interacts with BAG-1,13 which functions as a co-chaperone of Hsp70. Whether the Hsp70–BAG-1 interaction is a determinant for Hsp70-mediated apoptosis regulation is still elusive. An interesting correlation between the expression of Hsp70 and susceptibility to in vitro apoptosis has been found in acute myeloid leukemia cells14 but not with the expression of Hsp90.
In the present study, we investigated the mechanism by which Bcl-2 functions in mast cells as an antiapoptotic factor in relation to its association with proteins not belonging to the Bcl-2 family. We show that Hsp90β is associated with Bcl-2 and that this interaction is a prerequisite for optimal Bcl-2 antiapoptotic function. We also present evidence that the disruption of the Bcl-2-Hsp90β interaction inhibits the antiapoptotic activity of Bcl-2 and initiates cytochrome c mitochondrial release, caspase activation, and cell death.
Materials and methods
Cell culture
Rat basophilic leukemia (RBL-2H3) cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, antibiotic mix (100 U/mL penicillin, 100 μg/mL streptomycin), 2 mM nonessential amino acids (Biological Industries, Beit Haemek, Israel), and 50 μM β-mercaptoethanol (Fisher Science, Medford, MA).
Femoral bone marrow cells derived from C57BL/6 mice were cultured in IL-3–containing medium for 3 weeks to generate bone marrow–derived mast cells (BMMCs), as previously described.15 All the cells were grown in a humidified incubator at 37°C with 5% CO2.
In vitro protein interaction assay
Protease-deficient Escherichia coli BL21 expressing different GST recombinant proteins (Hsp90 full length, N-, M-, and C-domains of Hsp90; all plasmids kindly provided by F. U. Hartl, Max-Planck Institute, Martinsreid, Germany) were lysed in BugBuster buffer (Novagen, Madison, WI) supplemented with bacterial protease inhibitor cocktail (Sigma, St Louis, MO) at a dilution of 1:100 and clarified by centrifugation before purification of recombinant proteins using glutathione–Sepharose 4B resins (Amersham Biosciences, Uppsala, Sweden). Bcl-2 was produced, and 35[S]-methionine was labeled in a coupled transcription/translation system using the rabbit reticulocyte lysate (Promega, Madison, WI) from the plasmid pcDNA-Bcl-2, kindly provided by S. Korsmayer (Dana Farber Cancer Institute [DFCI], Boston, MA).
Pull-down assays were performed by incubating equal amounts of the different recombinant GST-Hsp90 (full-length, N-, M-, and C-domains, and GST alone for control) immobilized onto glutathione–Sepharose beads, and preincubated for 1 hour at 4°C in 1 mL binding buffer (100 mM KCl, 20 mM HEPES,1 mM dithiothreitol,1 mM EDTA, 5% glycerol, 0.1% Nonidet P-40). One microliter to 10 μL 35[S]-labeled protein Bcl-2 was added to each preincubation mix, and the binding reaction was carried out overnight at 4°C with gentle agitation. Proteins retained on the Sepharose beads were washed 4 times in 1 mL phosphate-buffered saline (PBS)/290 mM NaCl, boiled for 7 minutes in sample buffer, and resolved using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the gels were treated with staining solution (GelCode; Pierce, Rockford, IL), dried, and subjected to autoradiography to detect binding between GST-Hsp90 (full length, N-, M-, and C-domains) and Bcl-2.
Antibodies
Antibodies against Bcl-2, Bcl-2-S70, Bcl-2-S87, BclXL, Mcl-1, A1, Bax, cytochrome c, anti–cytochrome c oxidase subunit IV, actin, and tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and monoclonal anti-Hsp90 was purchased from Transduction Laboratories (Lexington, KY).
Immunoprecipitation assays
RBL or BMMCs were lysed by the addition of 250 μL cold lysis buffer (0.01 M Tris-HCl, pH 7.4, 1% deoxycholate, 1% Triton X-100, 0.1% SDS, and 0.15 M NaCl) and 10 μL protease inhibitor cocktail (Sigma). After homogenization, the lysates were incubated for 1 hour at 4°C with the desired antibody and then for another 2 hours with 10 μL Ultralink Protein A/G Sepharose beads (Pierce). After 3 washes with cold lysis buffer (1:2), recovered immunocomplexes were solubilized in Laemmli sample buffer containing 0.5% SDS and were separated on 10% or 12% SDS-PAGE. The protein was visualized by Western blotting using a specific antibody.
LC MS/MS analysis of Bcl-2–associated proteins
After Bcl-2 immunoprecipitation from RBL whole-cell lysate, the protein complexes were run on 12% SDS-PAGE and stained with staining solution (GelCode; Pierce). After they were cut from the gel, Bcl-2–associated proteins were dissolved in 8 M urea and 100 mM Tris-HCI (pH 8.0), treated with DTT and iodoacetamide, digested with endoproteinase Lys-C, and further digested with trypsin after dilution. Resultant peptides were loaded onto a 75-μm ID column packed with 3-μm C18 reverse-phase particles (Vydac HPLC Columns; Bucher Biotec AG, Basel, Switzerland), and eluted into a Q-tof-2 mass spectrometer (MicroMass UK, Manchester, United Kingdom) with capillary V 1200, cone V 35, and collision Energy 25-40 for ms/ms spectra. Fragmentation spectra were recorded using information-dependent acquisition and duty-cycle enhancement. Proteins were identified in the National Center for Biotechnology Information nonredundant database using the Mascot program (Matrix Science, Boston, MA).
Subcellular fractionation and mitochondria isolation
Cytosolic and membrane fractions were prepared by selective centrifugation. Briefly, control and experimental RBL cells were washed once in PBS and resuspended in isotonic buffer A (20 mM mannitol, 7 mM sucrose, 1 mM EGTA, 10 mM HEPES, pH 7.5), supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 10 μg/mL pepstatin A, 10 μg/mL soybean trypsin inhibitor, and 10 μg/mL aprotinin). The supernatants (cytosol) were collected after centrifugation at 15 000g for 10 minutes. After centrifugation, the pellet was further extracted with ice-cold detergent (1% CHAPS) in buffer A containing protease inhibitors for 60 minutes at 4°C to release membrane- and organelle-bound proteins, including mitochondrial cytochrome c. Detergent-soluble (membrane) and insoluble fractions were collected by low-speed (15 000g) and high-speed (500 000g) centrifugation, respectively. The purity of each fraction was verified by Western blotting using the specific markers antitubulin and anti–cytochrome c oxidase subunit IV for cytosol for mitochondria, respectively.
RNAi assay
The primer gaagccctgccctctgtat was designed for inhibition of Hsp90β expression. These 19 nucleotides derived from the HSP90B target (GenBank NM_008302, residues 2283-2301) were separated by a short spacer from the complement of the same 19-nucleotide sequence and cloned under the control of the polymerase-III H1-RNA promoter, as described previously.16 The cassette of the H1-RNA promoter, together with the Hsp90β primer, was further cloned in modified-HIV-1–based SIN18 vector that harbors the reporter gene–enhanced green fluorescence protein (EGFP) under the control of the human phosphoglycerate kinase (hPGK) promoter.17 As control, the cassette of the H1-RNA promoter alone cloned in the SIN18 vector was used. All the constructs were verified by sequencing. Viruses were produced by transient cotransfection of 3 plasmids into 293T cells, as described earlier.17 Briefly, 1.5 × 106 293T cells were transfected using the TransIT-LT1 Transfection Reagent (Mirus Bio, Wisconsin, IL) with a total of 20 μg plasmid DNA/3.5 μg envelope plasmid pMD.G harboring the gene encoding VSV-G, 6.5 μg packaging plasmid pCMVAR8.91, and 10 μg transfer vector. The medium containing the viral particles was collected 48 and 72 hours after transfection and filtered through 0.45-μm filters (Sartorius, Göttingen, Germany). Virus was concentrated by ultracentrifugation at 50 000g at 4°C for 2 hours. Viral titers (TU/mL) were determined by transduction of 293T cells with serial dilutions of the viral supernatant and Fluorescence-activated cell sorting (FACS) analysis of the percentage of EGFP-expressing cells. For transduction, BMMCs were incubated directly with the viral particles (RNAi and control) in 5 μg/mL Polybrene (Sigma), at 37°C for 2 hours, and then in fresh IL-3–enriched medium.
Caspase assays
The activity of caspase 3 was measured using the colorimetric CaspACE 3 kit (Promega), according to the manufacturer's instructions. The activity of caspase 3/7 was measured using a fluorometric assay by Apo-ONE Homogenous Caspase-3/7 Assay (Promega) according to the manufacturer's instructions.
RNA isolation and RT-PCR
RNA from control and RNAi-treated cells was extracted with the Ultraspec RNA Isolation System (Biotecx Laboratories, Houston, TX) according to the manufacturer's instructions. Hsp90α and Hsp90β genes from control and transduced cells were identified through reverse transcription–polymerase chain reaction (RT-PCR). Hsp90α was amplified with specific primers (forward, 5′-ccacttggcagtcaagcactt; reverse, 5′-aacacacggcggacatacaat). Hsp90β was amplified with specific primers (forward, 5′-tggcagtcaagcacttctctgt; reverse, 5′-tgatgaacacacggcgga). Annealing was performed at 52.5°C for Hsp90β primers and at 50°C for Hsp90α primers, with total elongation of 25 cycles.
Cell viability
RBL cells were grown in 150 μL complete medium on 96-well plates and were exposed to growing concentrations of geldanamycin (GA; Sigma; 0, 0.25, 0.5, 0.75, and 1 μM). The CellTiter-Blue reagent (Promega) was added directly to each well, and the fluorescence signal was measured at 560/590 nm.
Apoptosis detection
Annexin V–fluorescein isothiocyanate/propidium iodide (FITC/PI) double-stain assay was used to examine the induced apoptosis after lentiviral transduction of mast cells with RNAi. Briefly, BMMCs transduced with specific or control RNAi were washed in cold PBS and resuspended in binding buffer (HEPES-buffered PBS supplemented with 2.5 mM CaCl2) before the addition of FITC-labeled annexin V for 10 minutes at room temperature. PI was added at a final concentration of 2 μg/mL 5 minutes before a final wash in PBS and immediate analysis of the cells on the flow cytometer. Flow cytometry was performed on mast cells gated on the basis of their forward and side light scatter with any cell debris excluded from analysis. Apoptotic cells were defined as FITC+/PI- cells. Gated mast cells were then plotted for annexin V–FITC and PI in a 2-way dot plot to assess the percentage of apoptotic mast cells.
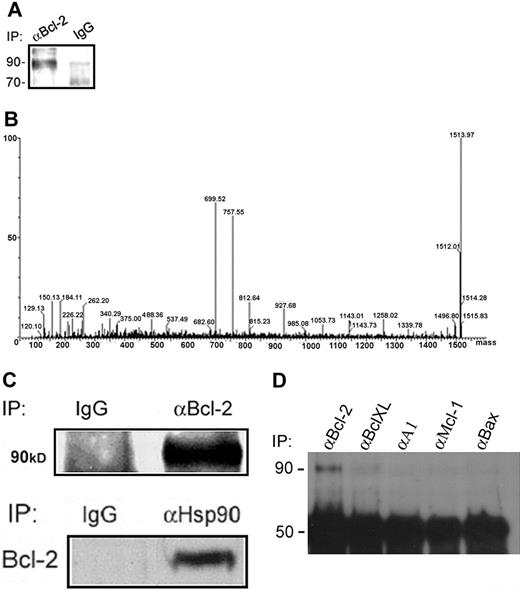
Identification of Hsp90β as a Bcl-2–interacting protein in RBL cells. (A) Bcl-2–interacting proteins were isolated by immunoprecipitation of Bcl-2 from RBL-cell lysates using an anti–Bcl-2 polyclonal antibody. The samples were electrophoresed on a 10% SDS polyacrylamide gel and then stained with a staining solution. (B) The bands at approximately 90 kDa were excised, digested with trypsin, and analyzed by mass spectrometry, as shown by the deconvoluted spectrum of the unknown protein. (C) Verification of the Hsp90β interaction with Bcl-2 in RBL cells by coimmunoprecipitation assays. Bcl-2 was immunoprecipitated from RBL-cell lysates, and Hsp90β was detected by Western blotting using a monoclonal anti-Hsp90 antibody. Results of 1 of 3 representative experiments are shown. Reciprocal coimmunoprecipitation: Hsp90 was immunoprecipitated from RBL-cell lysates, and Bcl-2 in complex was detected by a specific antibody. Results of 1 of 3 representative experiments are shown. (D) Hsp90 specifically interacts with Bcl-2 but not with other Bcl-2 family members. After immunoprecipitation of Bcl-2, BclXL, A1, Mcl-1, and Bax from RBL cells, the interaction of Hsp90β with these proteins was assessed by Western blotting using a monoclonal anti-Hsp90 antibody. Results of 1 of 3 representative experiments are shown.
Identification of Hsp90β as a Bcl-2–interacting protein in RBL cells. (A) Bcl-2–interacting proteins were isolated by immunoprecipitation of Bcl-2 from RBL-cell lysates using an anti–Bcl-2 polyclonal antibody. The samples were electrophoresed on a 10% SDS polyacrylamide gel and then stained with a staining solution. (B) The bands at approximately 90 kDa were excised, digested with trypsin, and analyzed by mass spectrometry, as shown by the deconvoluted spectrum of the unknown protein. (C) Verification of the Hsp90β interaction with Bcl-2 in RBL cells by coimmunoprecipitation assays. Bcl-2 was immunoprecipitated from RBL-cell lysates, and Hsp90β was detected by Western blotting using a monoclonal anti-Hsp90 antibody. Results of 1 of 3 representative experiments are shown. Reciprocal coimmunoprecipitation: Hsp90 was immunoprecipitated from RBL-cell lysates, and Bcl-2 in complex was detected by a specific antibody. Results of 1 of 3 representative experiments are shown. (D) Hsp90 specifically interacts with Bcl-2 but not with other Bcl-2 family members. After immunoprecipitation of Bcl-2, BclXL, A1, Mcl-1, and Bax from RBL cells, the interaction of Hsp90β with these proteins was assessed by Western blotting using a monoclonal anti-Hsp90 antibody. Results of 1 of 3 representative experiments are shown.
Cell-cycle analysis
Cellular DNA content of BMMCs was determined by cell staining with PI. BMMCs were grown in suspension and washed 3 times with PBS containing 2% FCS. After fixation with cold absolute ethanol for 1 hour at 4°C, cells were washed 3 times with cold PBS and resuspended in PI staining solution (3.4 mM sodium citrate, 0.5 mg/mL boiled RNaseA, 0.5 mg/mL PI). Then the cells were filtrated through silk paper and analyzed with the use of a Coulter flow cytometer (Beckman Coulter, Fullerton, CA) and Multicycle software.
Results
Hsp90β binds Bcl-2 in vivo
To screen for proteins that might associate with Bcl-2 in mast cells, an antibody against Bcl-2 was used to immunopurify the Bcl-2 complex from extracts of resting RBL cells.
Several bands were detected on the gel, with the most prominent being a protein with a relative molecular mass of 90 000 (Mr 90 000) (Figure 1A). This band was analyzed by mass spectrometry, and the deconvoluted spectrum of its peptides showed several peaks of interests (Figure 1B). The deduced sequences of the peptides were blasted against the nonredundant bank of proteins and identified as part of the Hsp90β. Verification of this finding was carried out by coimmunoprecipitation of this Hsp90β with Bcl-2 in resting RBL cells. Figure 1C (left panel) shows that Hsp90 is indeed associated with Bcl-2 in RBL cells. This association also occurred in mouse bone marrow–derived mast cells (data not shown). The reciprocal experiment is shown in Figure 1C (right panel) in which Bcl-2 was bound to Hsp90 in RBL cells. To deny a nonspecific association of Hsp90, we performed coimmunoprecipitation assays with several members of Bcl-2 family (BclXL, A1, Mcl-1 and Bax). The band identified in Figure 1D shows that Hsp90β interacts only with Bcl-2 and not with the other Bcl-2 family members.
Identification of the domain in Hsp90β responsible for its interaction with Bcl-2
Functional analysis has revealed that Hsp90β is composed of well-conserved amino- and carboxyl-terminal domains separated by a charged domain.18 The schematic representation of Hsp90β structure is shown in Figure 2A. To identify the domain or domains of Hsp90β that is or are responsible for the interaction with Bcl-2, an in vitro protein–protein pull-down assay was performed. Using this approach, we determined the binding capability of 35[S]-Met-Bcl-2 with GST fusion proteins spanning the N-, M-, and C-domains of Hsp90β and of the full-length Hsp90β (Figure 2B). Bcl-2 was found to interact with the M-domain of Hsp90β (Figure 2B), but it was also able to interact with the N-domain of Hsp90β (Figure 2B). No interaction was observed between Bcl-2 and the C-domain of Hsp90β (Figure 2B).
Domains of interaction between Bcl-2 and Hsp90β and the effect of GA on this interaction. (A) Schematic structure of Hsp90. (B) Hsp90β and its domains, N, M, and C, were expressed as GST-recombinant proteins in BL21 bacteria and incubated with radiolabeled 35[S]-Met-Bcl-2, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (C) RBL cells were exposed to various concentrations of GA (0-0.5 μM) for 12 hours at 37°C. Bcl-2 was then immunoprecipitated from the cell lysates using anti–Bcl-2 antibody. The interaction of Bcl-2 with Hsp90β was determined by Western blotting using anti-Hsp90, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (D) In vitro dissociation between Hsp90 and Bcl-2. The pull-down assay between GST recombinant proteins (Hsp90 full-length and Hsp90 N-terminus) and 35[S]-Bcl-2 was assessed in the presence of different concentrations of GA (0, 1, 10 μM). Results of 1 of 3 representative experiments are shown.
Domains of interaction between Bcl-2 and Hsp90β and the effect of GA on this interaction. (A) Schematic structure of Hsp90. (B) Hsp90β and its domains, N, M, and C, were expressed as GST-recombinant proteins in BL21 bacteria and incubated with radiolabeled 35[S]-Met-Bcl-2, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (C) RBL cells were exposed to various concentrations of GA (0-0.5 μM) for 12 hours at 37°C. Bcl-2 was then immunoprecipitated from the cell lysates using anti–Bcl-2 antibody. The interaction of Bcl-2 with Hsp90β was determined by Western blotting using anti-Hsp90, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (D) In vitro dissociation between Hsp90 and Bcl-2. The pull-down assay between GST recombinant proteins (Hsp90 full-length and Hsp90 N-terminus) and 35[S]-Bcl-2 was assessed in the presence of different concentrations of GA (0, 1, 10 μM). Results of 1 of 3 representative experiments are shown.
Inhibition of Hsp90β–Bcl-2 interaction by Hsp90 inhibitor
X-ray crystallographic and biochemical studies have demonstrated that GA interacts with the amino-terminal site on Hsp90,19 leading to alterations in the conformation and function of the protein. GA specifically binds to the ATP/ADP pocket of Hsp90 interfering with the ATPase cycles and effectively disrupts Hsp90 interaction with a large number of proteins.19 We used the GA inhibitor to test the chaperone function of Hsp90 in its association with Bcl-2. To detect whether GA affects Bcl-2–Hsp90 interaction in vivo, RBL cells were incubated with various concentrations of GA. This treatment was found to effectively block Hsp90β association with Bcl-2, as assessed by Bcl-2 immunoprecipitation (Figure 2C). This decline in protein association was observed in a dose-dependent fashion. Moreover, the effect of GA on the interaction between Hsp90β and Bcl-2 was also determined in vitro. As shown in Figure 2D, 1 μM GA totally disrupted the association between Bcl-2 and the full-length Hsp90 (left panel), whereas the interaction with the N-terminal of Hsp90 was abolished only with 10 μM GA (right panel).
Interaction with Hsp90β is essential for the antiapoptotic activity of Bcl-2
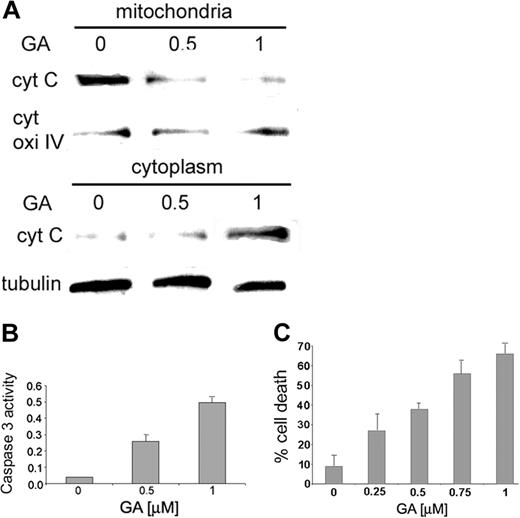
The physiologic function of the Hsp90β–Bcl-2 association was determined by following the concentration of cytochrome c in mitochondria and cytosol of RBL cells treated with increasing doses of GA. As expected, in untreated cells, the concentration of cytochrome c in the mitochondria was found to be much higher than that found in the cytosolic fraction of the untreated cells (Figure 3A, left panel). However, the concentration of cytochrome c increased in the cytosol fraction after the cells were treated with GA (Figure 3A, middle and right panels). Moreover, cells exposed to GA show a concentration-dependent increase in activity of caspase 3 compared with untreated cells (Figure 3B). Low levels of caspase 3 activity were observed in cells treated with the caspase 3 inhibitor zVAD-FMK in addition to GA (data not shown).
After the release of cytochrome c from mitochondria, the effect of GA on cell viability was tested. RBL cells exposed to growing concentrations of GA showed continued increases in the percentage of cell death (more than 60%) compared with untreated cells, as shown in Figure 3C.
In contrast to most other ATP-hydrolyzing proteins, the ATP molecule is bound in a kinked conformation to Hsp90, which is perfectly mimicked by GA.20 Our results demonstrated that there was a decrease in Hsp90β binding to Bcl-2 in RBL cells exposed to increasing concentrations of GA. The dissociation of the complex began at 0.5 μM, whereas disruption of the complex survivin–Hsp90 occurred at 1 μM GA in HeLa cells.21 In addition, partial blocking of the Bcl-2 binding to Hsp90 in HL-60 cells occurred at 0.02 μM, and the apoptosis of these cells was first detected at 10 μM GA.22 Thus, it appears that GA is more effective in our system than what was previously described.
Abolishing Hsp90β expression leads to the initiation of apoptotic cascade
We next examined the role played by endogenous Hsp90β on Bcl-2–dependent activity in mast cells. This was carried out by introducing RNA interference (RNAi) into the cells.
Two known cytosolic isoforms were reported, Hsp90α and Hsp90β, which are encoded by genes located on different chromosomes.23 For this purpose, a sequence of 19 nucleotides derived from the HSP90B target was designed. This oligomer was cloned under the H1-RNA promoter,16 and a new vector was generated for cell transduction.17
Cytochrome c release from the mitochondria and caspase 3 activity on treatment with GA. (A) RBL cells were exposed to increasing concentrations of GA (0, 0.5, 1 μM). Mitochondria were isolated, and cytochrome c was detected in the cytosolic and mitochondrial fractions by anti–cytochrome c antibody, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (B) RBL cells were exposed to increasing concentrations of GA (0, 0.5, 1 μM). Caspase 3 activity was determined by colorimetric product with absorbance at 405 nm. Results of 1 of 3 representative experiments are shown. (C) RBL cells were grown in 96 wells and exposed to increasing concentrations of GA (0, 0.25, 0.5, 0.75, 1 μM) for 15 hours. Cell viability was assessed by cell-viability assay, as described in “Materials and methods.” Data are represented as mean ± SE (n = 4). Results of 1 of 3 representative experiments are shown.
Cytochrome c release from the mitochondria and caspase 3 activity on treatment with GA. (A) RBL cells were exposed to increasing concentrations of GA (0, 0.5, 1 μM). Mitochondria were isolated, and cytochrome c was detected in the cytosolic and mitochondrial fractions by anti–cytochrome c antibody, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (B) RBL cells were exposed to increasing concentrations of GA (0, 0.5, 1 μM). Caspase 3 activity was determined by colorimetric product with absorbance at 405 nm. Results of 1 of 3 representative experiments are shown. (C) RBL cells were grown in 96 wells and exposed to increasing concentrations of GA (0, 0.25, 0.5, 0.75, 1 μM) for 15 hours. Cell viability was assessed by cell-viability assay, as described in “Materials and methods.” Data are represented as mean ± SE (n = 4). Results of 1 of 3 representative experiments are shown.
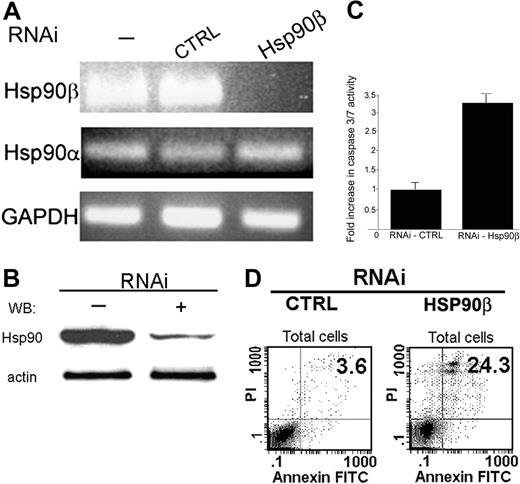
The specificity of RNA interference was verified on the mRNA level. RNA was extracted from the BMMC-transduced cells and then reverse transcribed. The level of mRNA accumulation was determined by PCR. Total elimination in Hsp90β mRNA accumulation was observed (Figure 4A). No changes were observed in the mRNA levels of Hsp90α and GAPDH, which were used as controls (Figure 4A).
The protein level of Hsp90β was significantly decreased 96 hours after transduction in BMMCs treated with the specific Hsp90β RNAi. Untreated cells or cells treated with empty virus showed no change in the protein level of Hsp90β (Figure 4B).
Hsp90β interaction with Bcl-2 is induced by Kit ligand and is cell-cycle dependent
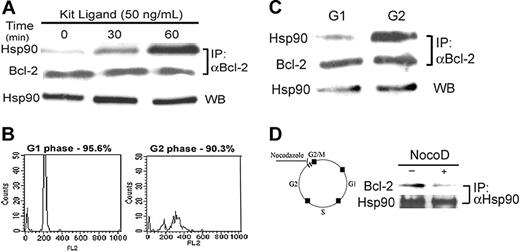
To establish the physiologic function of Bcl-2-Hsp90β association in mast cells, we determined the correlation between growth factor–dependent mast-cell growth and intracellular formation of the complex. Because c-kit is constitutively activated in RBL cells,24 a primary culture of BMMCs was used for this study. In Kit ligand–stimulated BMMCs, complex formation was increased compared with control. The growth factor dependence of the Hsp90β–Bcl-2 association was determined in a primary culture of BMMCs deprived of growth factors for 2 hours, followed by exposure to 50 ng/mL Kit ligand for various periods of time. A time-dependent increase in the Hsp90β-binding to Bcl-2 was observed (Figure 5A). This association reached a peak after 60-minute exposure of the cells to Kit ligand.
Given that Kit ligand is a mitogenic factor for mast cells, the effect of the cell cycle on the interaction between Bcl-2 and Hsp90β was investigated. BMMCs were sorted by FACS and divided into G1 and G2 stages according to the amount of PI in the nucleus, as shown in Figure 5B.
Bcl-2 was then immunoprecipitated from the cell lysates, and Hsp90β was detected by Western blotting. As shown in Figure 5C, Hsp90β binding to Bcl-2 was greater in the G2 phase (Figure 5C, upper right panel) than in the G1 phase (Figure 5C, upper left panel). The level of immunoprecipitated Bcl-2 was equal in the G1 and G2 phases (Figure 5C, middle panel), as was the amount of Hsp90 present in the cells (Figure 5C, bottom panel).
During mitosis, Bcl-2 is phosphorylated and inactivated.25 The microtubule-inhibitor drug nocodazole was found to arrest the cell cycle at G2/M, and this event is associated with Bcl-2 phosphorylation.26 To test the effect of Bcl-2 phosphorylation on the formation of the complex Hsp90β–Bcl-2, BMMCs were treated with 15 μM nocodazole for 16 hours. After immunoprecipitation of Hsp90 from the cell lysates, a decrease in Bcl-2 binding to Hsp90 was only observed in nocodazole-treated cells, as shown in Figure 5D.
Effect of abolishing Hsp90β expression by RNAi on the antiapoptotic activity of Bcl-2. (A) After transduction with RNAi (96 hours), RNA was extracted from BMMCs, and mRNA levels of Hsp90α and Hsp90β were tested by PCR using specific primers, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (B) After transduction with RNAi (96 hours), whole-cell proteins from BMMCs were extracted, and the level of Hsp90 was tested by Western blotting using monoclonal antibody against Hsp90, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (C) After transduction (96 hours), the activity of caspases 3 and 7 was tested in RBL cells using a fluorometric assay, as described in “Materials and methods.” Data are represented as mean ± SE (n = 3). Results of 1 of 3 representative experiments are shown. (D) Effect of RNAi on BMMCs discriminated by annexin V–FITC and PI double stain. Seventy-two hours after lentiviral transduction, BMMCs were double stained with annexin V–FITC and PI and were analyzed by FACS, as described in “Materials and methods.” Representative dot plots of annexin V–FITC and PI staining are shown. The bottom left quadrant in each dot plot contains the vital (double-negative) population. The bottom right quadrant in each dot plot contains the early apoptotic (annexin V+, PI-) population.
Effect of abolishing Hsp90β expression by RNAi on the antiapoptotic activity of Bcl-2. (A) After transduction with RNAi (96 hours), RNA was extracted from BMMCs, and mRNA levels of Hsp90α and Hsp90β were tested by PCR using specific primers, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (B) After transduction with RNAi (96 hours), whole-cell proteins from BMMCs were extracted, and the level of Hsp90 was tested by Western blotting using monoclonal antibody against Hsp90, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (C) After transduction (96 hours), the activity of caspases 3 and 7 was tested in RBL cells using a fluorometric assay, as described in “Materials and methods.” Data are represented as mean ± SE (n = 3). Results of 1 of 3 representative experiments are shown. (D) Effect of RNAi on BMMCs discriminated by annexin V–FITC and PI double stain. Seventy-two hours after lentiviral transduction, BMMCs were double stained with annexin V–FITC and PI and were analyzed by FACS, as described in “Materials and methods.” Representative dot plots of annexin V–FITC and PI staining are shown. The bottom left quadrant in each dot plot contains the vital (double-negative) population. The bottom right quadrant in each dot plot contains the early apoptotic (annexin V+, PI-) population.
Effect of Kit ligand on the interaction between Hsp90 and Bcl-2 in BMMCs. (A) BMMCs underwent growth factor starvation for 2 hours, and then 50 ng/mL Kit ligand was added for various periods of time (0, 30, and 60 minutes). Bcl-2 was immunoprecipitated from the cell lysates by anti–Bcl-2. Its interaction with Hsp90 was determined by Western blotting with anti-Hsp90, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (B) BMMCs were synchronized by deprivation of IL-3 for 4 hours and then grown for more 24 hours in the presence of IL-3. After nuclear staining with PI, the cells were sorted by FACS into G1 and G2 phases, as described in “Materials and methods.” Results of 1 of 2 representative experiments are shown. (C) Bcl-2 was immunoprecipitated from G1 and G2 cell lysates, and the presence of Hsp90 was tested by Western blotting. Results of 1 of 2 representative experiments are shown. (D) BMMCs were treated with 15 μM nocodazole for G2/M arrest, and then Hsp90 was immunoprecipitated. The presence of Bcl-2 in the complex was tested using anti–Bcl-2 antibody. Results of 1 of 3 representative experiments are shown.
Effect of Kit ligand on the interaction between Hsp90 and Bcl-2 in BMMCs. (A) BMMCs underwent growth factor starvation for 2 hours, and then 50 ng/mL Kit ligand was added for various periods of time (0, 30, and 60 minutes). Bcl-2 was immunoprecipitated from the cell lysates by anti–Bcl-2. Its interaction with Hsp90 was determined by Western blotting with anti-Hsp90, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (B) BMMCs were synchronized by deprivation of IL-3 for 4 hours and then grown for more 24 hours in the presence of IL-3. After nuclear staining with PI, the cells were sorted by FACS into G1 and G2 phases, as described in “Materials and methods.” Results of 1 of 2 representative experiments are shown. (C) Bcl-2 was immunoprecipitated from G1 and G2 cell lysates, and the presence of Hsp90 was tested by Western blotting. Results of 1 of 2 representative experiments are shown. (D) BMMCs were treated with 15 μM nocodazole for G2/M arrest, and then Hsp90 was immunoprecipitated. The presence of Bcl-2 in the complex was tested using anti–Bcl-2 antibody. Results of 1 of 3 representative experiments are shown.
Discussion
The hallmarks of the apoptosis pathway are mitochondrial involvement and the formation of apoptosomes. Cell-death signals induce the release of cytochrome c from the mitochondria, which then binds to the apoptosis protease factor-1 (Apaf-1), inducing oligomerization and eventual recruitment of procaspase-9.
Because of its location in the outer mitochondrial membrane, Bcl-2 plays the role of guardian of cytochrome c, keeping it inside the mitochondrion.27 Until now, the role of Bcl-2 in preventing apoptosis was mainly characterized in the context of its interaction with other Bcl-2 family members. At present, however, little is known about the mechanisms involved in the regulation of the antiapoptotic function of Bcl-2 by proteins not related to members of the Bcl-2 family. Here we present a novel regulation of Bcl-2 antiapoptotic activity by a network of chaperones.
We have previously demonstrated the importance of Bcl-2 in mast-cell survival.4 Here we determined whether the antiapoptotic activity of Bcl-2 in mast cells is uniquely modulated. Using a mass spectrometry approach, we found that Hsp90β interacts with Bcl-2 in vivo. The association between Hsp90 and Bcl-2 was previously described in VEGF-stimulated leukemia cells, where it had a survival-promoting effect.22 Disruption of this interaction resulted in a dramatic decrease in Bcl-2 antiapoptotic activity. In mast cells, our data indicate that GA leads to the dissociation of Hsp90 from Bcl-2, and a large percentage of cells died after this treatment (Figure 3C). However, our results imply that the close interaction of Bcl-2 with the specific β isoform of Hsp90 is essential for its antiapoptotic function. This is clearly demonstrated by the RNAi assays in which down-regulation of the Hsp90β level leads to activation of the Bcl-2–governed apoptotic pathway. Elimination of Hsp90β expression in the primary culture of BMMCs caused a significant decrease in the antiapoptotic function of Bcl-2—that is, the release of cytochrome c from mitochondria into cytosol, the activation of caspases 3 and 7, and cell apoptosis. Remarkably, BMMCs were maintained in IL-3–enriched medium, which might affect the expression of Bcl-2; however, its antiapoptotic function is modulated by Hsp90β.
Hsp90 is known to control the balance between folding/maturation and proteosomal destruction of a restricted number of client proteins that are typically involved in signal transduction and cell proliferation.21 This protein can form homodimers,28 which are essential for its function in various biochemical events. Whether Hsp90β forms a complex with Bcl-2 in its single or dimer form has to be further explored.
We have also shown that the interaction of full-length of Hsp90β with Bcl-2 occurs in vitro, but it is abolished in the presence of 1 μM GA. Our results show that Bcl-2 is able to interact with Hsp90β at the N-terminus and the middle (M) domain. The interaction with the N-terminus of Hsp90β, containing the ATP-binding site, was diminished in vitro only at high concentrations of GA (10 μM), suggesting a role for the competitive binding at the same domain of GA and Bcl-2 in vitro. Domain mapping studies have revealed that the M-domain of Hsp90β serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase.28 Thus, we cannot exclude the possibility that an overlap between the N- and M-domains of Hsp90β is responsible for the interaction with Bcl-2. Another explanation relies on the homodimerization capability of Bcl-2. After homodimerization, Bcl-2 might associate with the M- and N-domains of Hsp90β, and this might enhance its antiapoptotic activity. Additional investigation is needed to define the precise site of Hsp90β that binds Bcl-2.
The increase in Hsp90β binding to Bcl-2 after cells were exposed to Kit ligand suggests that this association plays an important role in mast-cell growth. Recently, it was reported that in human mastocytosis, 17-AAG, an analog of GA, is effective in down-regulating cell proliferation.29 Based on our data, such down-regulation in cell proliferation could be interpreted as a direct effect of the drug on the Hsp90β–Bcl-2 complex. Interestingly, an important property of Hsp90 inhibitors (GA and 17-AAG) is their ability to cause simultaneous, combinatorial blockade of multiple cancer-causing pathways by promoting the degradation of many oncogenic client proteins.30 However, the reason for therapeutic selectivity in cancer cells compared with normal cells is unclear. New research now shows that Hsp90 exists in cancer cells in a heightened, activated state that is highly susceptible to inhibition by 17-AAG.31
The role of Hsp90 in stress response regulation is still not as clear as that of Hsp27 or Hsp70. However, Hsp90 is necessary for the folding and activation competence of a large number of kinases.32 Dissociation of the Hsp90-Raf1 complex results in apoptosis in mast cells33 and in B-lymphocytes,34 whereas the disruption of the mitogen-activated protein kinase cascade is accompanied by the activation of JNK in the dexamethasone-induced mast-cell apoptosis model.33 The down-regulation of Raf can also be induced by the sequestration of its activator, BAG-1, by Hsp70 after various forms of stress.35 Cell apoptosis might also be a consequence of the dissociation of Hsp90β from Bcl-2, as our results indicated.
A considerable shift in Hsp90β association to Bcl-2 with the progress of the cell cycle of the primary BMMC culture was observed. This is in accordance with the sequence of molecular events that occur during the cell cycle, in which Bcl-2 is activated until the point of entry to mitosis. During G1, Hsp90β is bound to Bcl-2, keeping it active. During G2, there is a significant shift to Hsp90 binding to Bcl-2 until mitosis. This association might be tight or might sequester domains in Bcl-2, preventing its interaction with proapoptotic factors or even its degradation. In support of this idea, we found a sharp reduction in the Hsp90β–Bcl-2 interaction in G2/M-arrested BMMCs with nocodazole. This might suggest a pathway for recycling Bcl-2 molecules inside the cells. Thus, phosphorylation inactivates Bcl-2 at G2/M, acting as a physiologic means of regulating apoptosis susceptibility during the cell cycle.
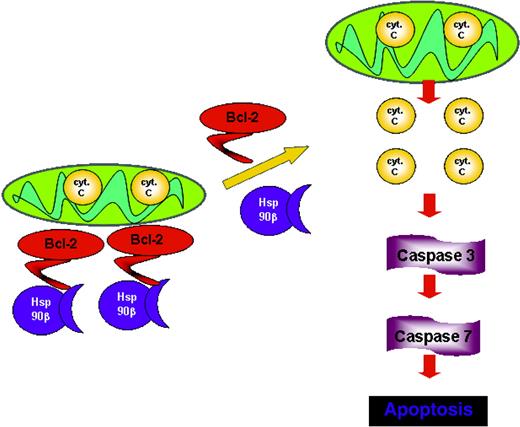
With this in mind, a working model is suggested in Figure 6 in which the dynamic association of Hsp90β to Bcl-2 renders Bcl-2 active throughout the cell cycle. On dissociation between these 2 proteins, Bcl-2 no longer acts as an antiapoptotic factor, leading to the initiation of the apoptotic cascade by releasing cytochrome c, the activation of the effector caspases, and ultimately cell death. The interaction of Bcl-2 with Hsp90β at the N- and M-domains might protect Bcl-2 from posttranslational modifications, such as phosphorylation or interaction with other proteins. It is still to be explored whether a change in cell fate will happen if Bcl-2 lacks the Hsp90β domain.
Proposed model for Bcl-2 function in mast cells. The association with Hsp90β promotes the antiapoptotic activity of Bcl-2, whereas the dissociation from Hsp90β leads to loss of Bcl-2 function.
Proposed model for Bcl-2 function in mast cells. The association with Hsp90β promotes the antiapoptotic activity of Bcl-2, whereas the dissociation from Hsp90β leads to loss of Bcl-2 function.
Although the precise mechanism remains to be determined, our data show that Hsp90β recognizes Bcl-2 and modulates its antiapoptotic activity. This is a unique contribution because the antiapoptotic activity of Bcl-2 has been reported to be predominantly modulated by its interaction with proteins belonging to the Bcl-2 family. Moreover, Hsp90 is most recognized as responsible for the maturation and activity of a variety of key client proteins involved in cell growth and proliferation. Thus, Hsp90β may be an early, crucial player in the transduction of structural cues into survival signals. The data presented in our study might be useful for the future treatment of mastocytosis or Bcl-2–associated diseases.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-07-2648.
Supported by the United States Binational Science Foundation (grant 2003009) (E.R.), the Israeli Academy of Science (grant 144/04) (E.R.), and the German-Israeli Foundation for Scientific Research and Development (grant I-726-10.2/2002) (E.R.).
C.C.-S. and E.R. conceived and designed the experiments. C.C.-S., I.C., and A.K. performed the experiments and analyzed the data. C.C.-S. and E.R. wrote the paper, with comments from I.C.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ofra Moshel for mass spectrometry analysis and Dr Gillian Kay for comments and manuscript preparation

![Figure 2. Domains of interaction between Bcl-2 and Hsp90β and the effect of GA on this interaction. (A) Schematic structure of Hsp90. (B) Hsp90β and its domains, N, M, and C, were expressed as GST-recombinant proteins in BL21 bacteria and incubated with radiolabeled 35[S]-Met-Bcl-2, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (C) RBL cells were exposed to various concentrations of GA (0-0.5 μM) for 12 hours at 37°C. Bcl-2 was then immunoprecipitated from the cell lysates using anti–Bcl-2 antibody. The interaction of Bcl-2 with Hsp90β was determined by Western blotting using anti-Hsp90, as described in “Materials and methods.” Results of 1 of 3 representative experiments are shown. (D) In vitro dissociation between Hsp90 and Bcl-2. The pull-down assay between GST recombinant proteins (Hsp90 full-length and Hsp90 N-terminus) and 35[S]-Bcl-2 was assessed in the presence of different concentrations of GA (0, 1, 10 μM). Results of 1 of 3 representative experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/4/10.1182_blood-2005-07-2648/2/m_zh80040690850002.jpeg?Expires=1769369108&Signature=o772vEsoGmRreqDM1tk9t1nsmjU6acEYkwtLj9V50B3bgzFYNJuxDk~V1OsJ9p7gxOH6gRHlk8qkBRWOs37nRgXxFds8Jt2bgGbMIM4re2X19dOTLDCd9mZt6n9SpVUMlRqaDNv5oy9BJ0E2uGy9VK4ZoxUjIk5ZMqfF6VzrhDZcVQzeW5hqT5P4xLrg7~c7Ky3F-5qdKBXIK-eoIU0bu-v81VuaMgm27J1nXWJ3XGfPynoXlB~e61~YB2YYxVE~z1lZWhlei~uOFjzo0No374aZEmd2AqA3f9nUUTGSZJZ17XUDDHbEiRLnr2eFcDnpMZpQ4snRe0Ajee~sEO8lSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)