The recruitment of memory T cells from blood into tissues is a central element of immune surveillance and adaptive immune responses and a key feature of chronic cutaneous inflammatory diseases such as psoriasis and atopic dermatitis. Human memory T cells that infiltrate skin express the carbohydrate epitope cutaneous lymphocyte-associated antigen (CLA). Expression of the CLA epitope on T cells has been described on P-selectin glycoprotein ligand-1 (PSGL-1) and associated with the acquisition of both E-selectin and P-selectin ligand functions. In this report, we show that CD43, a sialomucin expressed constitutively on T cells, can also be decorated with the CLA epitope and serve as an E-selectin ligand. CLA expressed on CD43 was found exclusively on the high-molecular-weight (125 kDa) glycoform bearing core-2-branched O-linked glycans. CLA+ CD43 purified from human T cells supported tethering and rolling in shear flow via E-selectin but did not support binding of P-selectin. The identification and characterization of CD43 as a T-cell E-selectin ligand distinct from PSGL-1 expands the role of CD43 in the regulation of T-cell trafficking and provides new targets for the modulation of immune functions in skin.
Introduction
Human T cells exhibit tropism for specific tissues mediated by cell-surface receptors that interact with counter ligands differentially expressed on the vascular endothelium.1 The complement of receptors expressed by subsets of T cells, in turn, determines the tissues those cells can enter. Naive T cells, for example, emigrate from blood into peripheral lymph nodes (PLNs) via the sequential interaction of the leukocyte receptors L-selectin (CD62L), CCR7, and leukocyte function antigen 1 (LFA-1) with their ligands peripheral node addressin (PNAd), secondary lymphoid tissue cytokine (SLC) (CCL21), and ICAM-1, respectively, presented on high endothelial venules (HEVs). T cells that lack one or more of the receptors for the ligands expressed at a particular site will not be able to complete the adhesion/emigration process and return to the blood flow. Memory T cells, for example, that lack L-selectin or CCR7 cannot enter PLNs.2 In a similar fashion, patterns of receptor/counter-receptor expression regulate the recruitment of T cells to other secondary lymphoid tissues as well as to peripheral tissues.
T-cell recruitment to skin is a central feature of many acute and chronic cutaneous inflammatory conditions, including eczema, psoriasis, vitiligo, and alopecia areata.3-5 The subgroup of human effector/memory T cells that participate in immune responses in skin can be identified by expression of the cutaneous lymphocyte-associated antigen (CLA). CLA, a carbohydrate epitope, functions as a T-cell receptor for E-selectin expressed on postcapillary venules in skin. Although not an exclusive homing receptor, CLA is expressed at high levels on the T cells in inflammatory lesions in skin and is generally absent on T cells infiltrating other tissues.6,7 P-selectin glycoprotein ligand-1 (PSGL-1) on CLA+ T cells has been shown to bear CLA carbohydrate epitope and is the only E-selectin and P-selectin ligand previously described on these cells.8,9 In this study, we demonstrate that sialomucin CD43 (leukosialin) on T cells can also be decorated with CLA epitope and, in contrast to PSGL-1, that CLA+ CD43 serves as an E-selectin ligand but does not have P-selectin binding activity.
Materials and methods
Cells
Peripheral blood mononuclear cells (PBMCs) were prepared by density gradient separation (Ficoll-Histopaque 1.077; Sigma-Aldrich, St Louis, MO) of peripheral blood from healthy donors. All blood samples for these studies were collected under protocols approved by the Brigham and Women's Hospital institutional review board. Informed consent, where required, was provided according to the Declaration of Helsinki. The generation of CLA+-cultured T cells using XVIVO15 medium (BioWhittaker, Walkersville, MD) and CLA--cultured T cells using RPMI medium (Mediatech, Herndon, VA) was performed as described.9 CLA+ peripheral blood T cells were obtained from fresh PBMCs by depletion of non-T cells using a Pan T cell kit (Miltenyi Biotec, Auburn, CA) followed by positive selection of CLA+ T cells with anti-CLA monoclonal antibody (mAb) HECA-452 and mouse anti-rat kappa magnetic beads (Miltenyi Biotec), following the manufacturer's instructions.
Western blots
The preparation of cell lysates using 1% N-octyl glucoside (Roche, Indianapolis, IN), reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting techniques were all performed as described.9 The nonreducing SDS-PAGE loading buffer used in these studies was composed of 100 mM Tris-Cl, 4% SDS, 0.2% or less bromophenol blue, and 20% glycerol. Molecular weight markers were purchased from Amersham Pharmacia Biotech (Piscataway, NJ): high range (220, 97, 66, 45, 30, 20.1, and 14.3 kDa) and full range (250, 160, 105, 75, 50, 35, 30, 25, 15, and 10 kDa). All gels except immunoprecipitation (IP) studies were loaded with 50 μg total protein per lane.
Antibodies
Anti-CLA mAb HECA-452, anti-CD43 mAb 1G10 and mAb L60, anti-CD62E mAb 68-5H11, anti-CD62L mAb DREG-56, and anti-CD62P mAb AK-4 antibodies were purchased from BD Pharmingen (San Diego, CA). Anti-PSGL-1 CD162 mAbs PL-1 and PL-2 were purchased from Beckman Coulter (Miami, FL) and KPL-1 was purchased from BD Pharmingen. Anti-PSGL-1 mAb 4H10 was generously provided by Genetics Institute (Cambridge, MA). Anti-CD43 mAb MT-1, rat IgM, and goat anti-mouse IgG alkaline phosphatase were obtained from Zymed (South San Francisco, CA). Mouse IgG1, mouse IgG2a, rat IgG2a, goat anti-mouse Ig FITC, goat anti-rat IgM alkaline phosphatase, goat anti-rat IgM FITC, rabbit anti-rat IgG alkaline phosphatase, and Neutralite Streptavidin alkaline phosphatase were all purchased from Southern Biotechnology (Birmingham, AL). Anti-CD43 mAb 1D4 was purchased from Medical and Biological Laboratories (Watertown, MA). Anti-CD43 mAb DF-T1 was obtained from Neomarkers (Fremont, CA). Anti-CD43-biotin mAb DF-T1 and mouse IgG1-biotin were obtained from Ancell (Bayport, MN). Anti-CD44 mAb Hermes-1 was purchased from Pierce Biotechnology (Rockford, IL).
Immunoprecipitations
Immunoprecipitation protocols were performed as previously described.9 IP reactions used either anti-PSGL-1 mAb 4H10 or anti-CD43 mAb 1G10, as indicated.
Blot-rolling assay
The blot-rolling assay allows for real-time observation of selectin-mediated interactions with cellular glycoproteins segregated by molecular weight and immobilized on Western blots. The parallel plate flow chamber system, maintenance and processing of transfected Chinese hamster ovary (CHO) cells, and use of the blot-rolling assay have been described in detail.9-12 Briefly, lysates of CLA+ T cells were subjected to SDS-PAGE under reducing or nonreducing conditions, blotted onto PVDF membranes, and immunostained using standard methods. Equilibration of the stained membranes in media with 10% glycerol alters the opacity of the membrane sufficiently to allow transmission of light and the direct visualization of cells interacting with the surface of the blot by standard light microscopy. A parallel plate laminar flow chamber, used routinely for observing real-time interactions under controlled shear conditions of cells with substrates immobilized on glass or plastic, can be mounted directly on the blots via a low-pressure vacuum seal, with the entire apparatus placed on the stage of an inverted microscope. CHO cells expressing defined levels of E- or P-selectin, as well as control mock-transfected CHO cells, are then introduced into the chamber under controlled shear conditions and monitored for interaction with the blot surface by direct observation. At areas of blots bearing known or unknown selectin ligands, cells can be observed in real-time tethering to the upstream edge of the stained area, rolling across the band, and releasing from the downstream edge. Although this assay is nonphysiologic by design, employing vascular selectins on nonadherent cells and immobilizing the leukocyte glycoproteins, the rolling behavior of bound cells is qualitatively similar to that observed in traditional binding assays employing leukocyte binding to glass- or plastic-immobilized selectin. All experiments are videotaped for offline analysis. Results shown in Figures 1, 4, and 6 were obtained at a wall shear stress value of 0.53 dyne/cm2. Although blot-bound ligands can support rolling in the physiologic shear range if present in sufficient quantity, the surface of the membranes is irregular on the cellular scale and causes turbulence that limits observations at higher shear levels. These experiments are routinely performed at low shear to enhance sensitivity while allowing easy differentiation of cells rolling on the substrate (selectin-mediated behavior) from unbound and firmly attached (nonspecific) cells. Video analysis was performed manually or using Cell Motion software (Ed Marcus Laboratories, Newton, MA).
Antigen capture assay
Substrate spots for the antigen capture assay were prepared as follows. First, 0.4 μg purified anti-CD43 mAb 1G10 or mouse IgG1 (20 μL at 20 μg/mL in PBS) was applied to plastic Petri dishes and incubated at 37°C for 2 hours. Next, the bulk of the antibody solution was removed and 100 μg human serum albumin (HSA; 50 μL at 2 mg/mL in phosphate-buffered saline [PBS]) was added and incubated at 4°C overnight. The HSA solution was then removed, cloning chambers were applied to the plastic dishes using silicone vacuum grease, and 12.5 μg CLA+- or CLA--cultured T-cell lysates (50 μL at 250 μg/mL in 1% N-octyl glucoside lysis buffer) or 1% N-octyl glucoside lysis buffer alone was applied and incubated overnight at 4°C. All incubations were conducted inside a “humidity chamber” consisting of a large sealed dish containing a moist paper towel to prevent drying of the spots. Prior to use, the cloning chambers were removed, silicone grease was wiped away with a cotton swab, and the plates were rinsed and filled with Ca2+-free and Mg2+-free Hanks balanced salt solution (HBSS) supplemented with 10 mM Hepes (H/H). H/H buffer supplemented with 2 mM CaCl2 (H/H/Ca2+) or with 5 mM EDTA (H/H/EDTA) was used during the flow assays. CHO cells were prepared and loaded into the parallel plate flow chamber in the same fashion as described for the blot-rolling assay. The results shown in Figure 5 were obtained at a wall shear stress value of 0.56 dyne/cm2.
Results
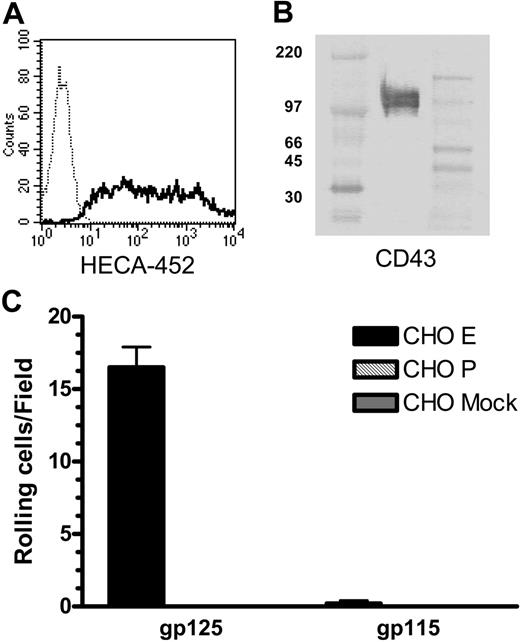
As we have shown previously, CLA appears as a single band of approximately 140 kDa on Western blots of CLA+ T-cell lysates separated by reducing SDS-PAGE (Figure 1A Original).8 IP of CLA+ T-cell lysate with antibodies directed to the protein core of PSGL-1 yields a 140-kDa HECA-452-reactive protein (Figure 1A Pellet 1) that has strong E-selectin (Figure 1B Pellet 1) and P-selectin ligand (not shown) activity assessed by the blot-rolling assay.9 However, the presence of a strong HECA-452-reactive band (Figure 1A Supn 1) and E-selectin ligand activity (Figure 1B Supn 1) remaining in the supernatant of this IP reaction suggested that the removal of PSGL-1 was incomplete. Exhaustive IP of PSGL-1, documented after 3 rounds by the absence of a PSGL-1-reactive band in the supernatant (not shown) as well as the absence of a HECA-452-reactive band or selectin ligand activity in the PSGL-1 immunoprecipitated pellet (Figure 1A-B Pellet 3), did not eliminate HECA-452-reactive material or E-selectin ligand activity from the residual supernatant (Figure 1A-B Supn 3). This result indicated the presence of another HECA-452-decorated glycoprotein in the lysates of CLA+ T cells that comigrates with PSGL-1 in reducing SDS-PAGE. Consistent with this hypothesis, Western blots of CLA+ T-cell lysates separated by nonreducing SDS-PAGE showed 3 distinct HECA-452-reactive bands (Figure 2) migrating at apparent molecular weights of approximately 240 kDa, 140 kDa, and 125 kDa. The 140-kDa and 240-kDa bands correspond to the predicted molecular weights of monomer and dimer PSGL-1, respectively, and were identified by immunoblotting with anti-PSGL-1 mAb (Figure 3). Identical Western blots stained with anti-L-selectin and anti-CD44 mAb showed that the 125-kDa band did not correspond to any of the reported ligands for E-selectin on human leukocytes or hematopoietic progenitor cells. Staining for CD43, a surface sialomucin similar in structure to PSGL-1, showed 2 bands, one of approximately 125 kDa that comigrates with the HECA-452-reactive band and an additional band at 115 kDa that did not stain with HECA-452. These bands are known to represent differential glycosylation and decoration of CD43 with core-2-branched carbohydrates in response to T-cell activation and maturation.13 This modified form of CD43 can be identified on human cells by reactivity with monoclonal antibody 1D4.14 As seen in Figure 3, staining with this antibody identified the 125-kDa CD43 band that comigrates with the HECA-452-reactive band on nonreducing SDS-PAGE.
Immunoprecipitation with anti-PSGL-1 mAb does not clear T-cell lysates of CLA or E-selectin ligand activity. (A) Lysates of cultured human peripheral-blood T cells expressing high levels of CLA were immunoprecipitated with anti-PSGL-1 mAb 4H10 and the products subjected to reducing SDS-PAGE. Representative Western blots stained for CLA (mAb HECA-452) are shown in panel A. All blots include high-range molecular weight markers run in the flanking lanes. Unmanipulated lysate (Original) shows a 140-kDa CLA+ band as previously described. IP of PSGL-1 results in recovery of 140-kDa CLA+ PSGL-1 (Pellet 1 is product of first IP) with complete clearance of PSGL-1 from the lysate by 3 rounds of IP (Pellet 3). The band of CLA+ material at 140 kDa observed in the residual soluble material after PSGL-1 IP (Supn 1 and Supn 3) indicates the presence of an additional CLA+ protein distinct from PSGL-1. Blots from panel A were assayed for functional E-selectin ligand activity by blot-rolling analysis (B). E-selectin ligand activity was observed on both immunoprecipitated 140-kDa PSGL-1 (Pellet 1) and the residual unknown CLA+ protein (Supn 3). Results shown are mean and range of 2 to 4 separate determinations on each blot and are representative of 3 similar immunoprecipitation experiments.
Immunoprecipitation with anti-PSGL-1 mAb does not clear T-cell lysates of CLA or E-selectin ligand activity. (A) Lysates of cultured human peripheral-blood T cells expressing high levels of CLA were immunoprecipitated with anti-PSGL-1 mAb 4H10 and the products subjected to reducing SDS-PAGE. Representative Western blots stained for CLA (mAb HECA-452) are shown in panel A. All blots include high-range molecular weight markers run in the flanking lanes. Unmanipulated lysate (Original) shows a 140-kDa CLA+ band as previously described. IP of PSGL-1 results in recovery of 140-kDa CLA+ PSGL-1 (Pellet 1 is product of first IP) with complete clearance of PSGL-1 from the lysate by 3 rounds of IP (Pellet 3). The band of CLA+ material at 140 kDa observed in the residual soluble material after PSGL-1 IP (Supn 1 and Supn 3) indicates the presence of an additional CLA+ protein distinct from PSGL-1. Blots from panel A were assayed for functional E-selectin ligand activity by blot-rolling analysis (B). E-selectin ligand activity was observed on both immunoprecipitated 140-kDa PSGL-1 (Pellet 1) and the residual unknown CLA+ protein (Supn 3). Results shown are mean and range of 2 to 4 separate determinations on each blot and are representative of 3 similar immunoprecipitation experiments.
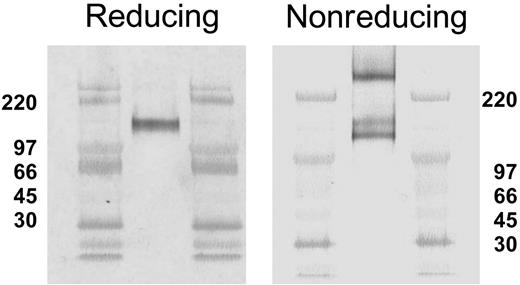
Reducing and nonreducing SDS-PAGE conditions result in different CLA band patterns on Western blots. Aliquots of CLA+-cultured T-cell lysate were subjected to SDS-PAGE under reducing (left panel) and nonreducing (right panel) conditions, transferred to PVDF membranes, and immunostained for CLA (mAb HECA-452). Three CLA+ bands (240 kDa, 140 kDa, and 125 kDa) are observed on Western blots following nonreducing SDS-PAGE, as opposed to one band (140 kDa) after reducing SDS-PAGE. High-range molecular weight markers are run in the flanking lanes as shown. Molecular weight values in kDa are shown beside each blot.
Reducing and nonreducing SDS-PAGE conditions result in different CLA band patterns on Western blots. Aliquots of CLA+-cultured T-cell lysate were subjected to SDS-PAGE under reducing (left panel) and nonreducing (right panel) conditions, transferred to PVDF membranes, and immunostained for CLA (mAb HECA-452). Three CLA+ bands (240 kDa, 140 kDa, and 125 kDa) are observed on Western blots following nonreducing SDS-PAGE, as opposed to one band (140 kDa) after reducing SDS-PAGE. High-range molecular weight markers are run in the flanking lanes as shown. Molecular weight values in kDa are shown beside each blot.
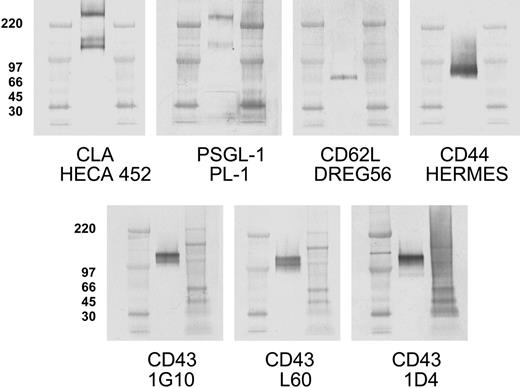
One hundred twenty-five kilodalton CLA+ glycoprotein comigrates with core-2-decorated CD43, not known selectin ligands. Aliquots of CLA+-cultured T-cell lysate were subjected to SDS-PAGE under nonreducing conditions, transferred to PVDF membranes, and immunostained for CLA (mAb HECA-452), PSGL-1 (mAb PL-1), CD62L (mAb DREG56), CD44 (mAb HERMES), CD43 core protein (mAb 1G10), CD43 sialic acid-dependent epitope (mAb L60), and a CD43 core-2-branched O-glycan-dependent epitope (mAb 1D4). PSGL-1 migrates in nonreducing SDS-PAGE as both a 240-kDa dimer band and a 140-kDa monomer band. The HECA-425-reactive bands at 240 kDa and 140 kDa correspond to CLA-decorated PSGL-1 dimer and monomer, respectively. The HECA-452-reactive band at 125 kDa comigrates with high-molecular-weight CD43 and not PSGL-1, CD62L, or CD44. Blots for CD43 L60 epitope and 1D4 epitope indicate that 125-kDa CD43 is decorated with both sialic acid and core-2-branched carbohydrate. Anti-PSGL-1-specific mAbs PL-2, KPL-1, and PSL-275 showed reactivity identical to PL-1 (not shown). The 125-kDa band and 115-kDa band seen with mAb 1G10 are also observed with additional CD43 antibodies to the core protein (MT-1 and DF-T1; not shown). Isotype control blots show no bands (not shown). High-range and full-range molecular weight markers are run in flanking lanes as shown. Molecular weights in kDa for the high-range markers are shown to the left of each blot row.
One hundred twenty-five kilodalton CLA+ glycoprotein comigrates with core-2-decorated CD43, not known selectin ligands. Aliquots of CLA+-cultured T-cell lysate were subjected to SDS-PAGE under nonreducing conditions, transferred to PVDF membranes, and immunostained for CLA (mAb HECA-452), PSGL-1 (mAb PL-1), CD62L (mAb DREG56), CD44 (mAb HERMES), CD43 core protein (mAb 1G10), CD43 sialic acid-dependent epitope (mAb L60), and a CD43 core-2-branched O-glycan-dependent epitope (mAb 1D4). PSGL-1 migrates in nonreducing SDS-PAGE as both a 240-kDa dimer band and a 140-kDa monomer band. The HECA-425-reactive bands at 240 kDa and 140 kDa correspond to CLA-decorated PSGL-1 dimer and monomer, respectively. The HECA-452-reactive band at 125 kDa comigrates with high-molecular-weight CD43 and not PSGL-1, CD62L, or CD44. Blots for CD43 L60 epitope and 1D4 epitope indicate that 125-kDa CD43 is decorated with both sialic acid and core-2-branched carbohydrate. Anti-PSGL-1-specific mAbs PL-2, KPL-1, and PSL-275 showed reactivity identical to PL-1 (not shown). The 125-kDa band and 115-kDa band seen with mAb 1G10 are also observed with additional CD43 antibodies to the core protein (MT-1 and DF-T1; not shown). Isotype control blots show no bands (not shown). High-range and full-range molecular weight markers are run in flanking lanes as shown. Molecular weights in kDa for the high-range markers are shown to the left of each blot row.
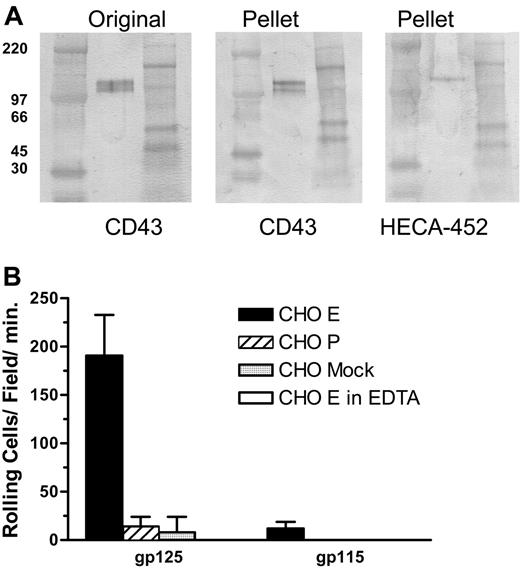
To confirm that CD43 on human T cells is decorated with HECA-452-reactive carbohydrates and can serve as an E-selectin ligand, we immunoprecipitated CD43 from lysates of CLA+ T cells and analyzed the product by Western blot and blot-rolling assay. CD43 immunopurified from CLA+ T-cell lysate migrates in nonreducing SDS-PAGE as 2 bands of apparent molecular weights of 115 and 125 kDa (Figure 4A Pellet, CD43 stain). An identical blot stained with HECA-452 (Figure 4A Pellet, HECA-452 stain) showed that only the 125-kDa form is decorated with the CLA epitope. Blot-rolling analysis showed that the 125-kDa CLA+ glycoform of CD43 has E-selectin ligand activity but not P-selectin ligand activity, in contrast to CLA+ PSGL-1, which binds both P- and E-selectin.9 The 115-kDa CLA- glycoform did not support either E-selectin- or P-selectin-mediated rolling (Figure 4B). While published studies document isoforms of PSGL-1 that bind P-selectin or P-selectin and E-selectin,8,9,15 this is the first report of a T-cell ligand that selectively binds E-selectin and not P-selectin.
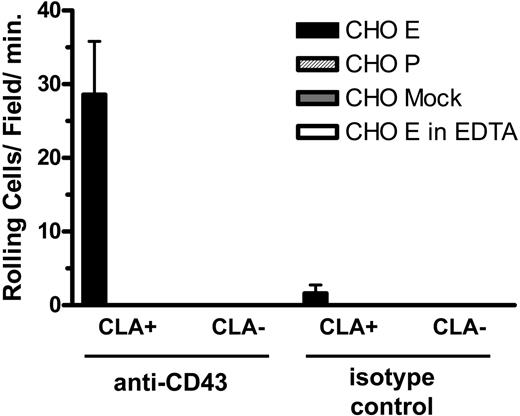
Since the blot-rolling technique involves denaturation of the core protein, we designed an assay of nondenatured material to confirm these findings. For this assay, anti-CD43 and control antibodies were adsorbed to a plastic plate and used to capture antigen from lysates of CLA+ and CLA- T cells. These substrates were then incorporated into the parallel plate flow chamber and assayed for the ability to support binding and rolling of selectin-expressing cells in shear flow. CD43 immunoadsorbed from CLA+ T-cell lysates, but not from CLA- T-cell lysates, supported E-selectin- but not P-selectin-mediated attachment and rolling functions (Figure 5). Control substrates and cells showed no selectin ligand activity. These results confirm the observations seen by blot rolling and support the conclusion that CD43 on CLA+ T cells is an E-selectin ligand.
CLA+ CD43 displays E-selectin ligand activity in a blot-rolling assay. (A) Lysates of cultured CLA+ T cells were immunoprecipitated with anti-CD43 mAb 1G10 and the products were subjected to nonreducing SDS-PAGE. Representative Western blots stained for CD43 (mAb DF-T1) and CLA (mAb HECA-452). High-range (left) and full-range (right) molecular weight markers are included in the flanking lanes of each blot. Unmanipulated lysate (Original) shows 125- and 115-kDa CD43+ bands as seen in Figure 3. IP of CD43 results in recovery of both 125-kDa and 115-kDa CD43 (Pellet, CD43 stain). Staining of an identical blot for CLA (Pellet, HECA-452 stain) shows the 125-kDa isomer of CD43 bears CLA whereas the 115-kDa form does not. Blots from panel A were assayed for functional E-selectin ligand activity by blot-rolling analysis (B). E-selectin ligand activity was observed on immunoprecipitated 125-kDa CD43, but not immunoprecipitated 115-kDa CD43 (▪), was calcium dependent (5 mM EDTA, □). Neither form supported P-selectin ( ) or control cell binding (
) or control cell binding ( ) in shear flow. Results shown are mean and SEM of 3 to 6 separate determinations on each band collected in 2 independent experiments.
) in shear flow. Results shown are mean and SEM of 3 to 6 separate determinations on each band collected in 2 independent experiments.
CLA+ CD43 displays E-selectin ligand activity in a blot-rolling assay. (A) Lysates of cultured CLA+ T cells were immunoprecipitated with anti-CD43 mAb 1G10 and the products were subjected to nonreducing SDS-PAGE. Representative Western blots stained for CD43 (mAb DF-T1) and CLA (mAb HECA-452). High-range (left) and full-range (right) molecular weight markers are included in the flanking lanes of each blot. Unmanipulated lysate (Original) shows 125- and 115-kDa CD43+ bands as seen in Figure 3. IP of CD43 results in recovery of both 125-kDa and 115-kDa CD43 (Pellet, CD43 stain). Staining of an identical blot for CLA (Pellet, HECA-452 stain) shows the 125-kDa isomer of CD43 bears CLA whereas the 115-kDa form does not. Blots from panel A were assayed for functional E-selectin ligand activity by blot-rolling analysis (B). E-selectin ligand activity was observed on immunoprecipitated 125-kDa CD43, but not immunoprecipitated 115-kDa CD43 (▪), was calcium dependent (5 mM EDTA, □). Neither form supported P-selectin ( ) or control cell binding (
) or control cell binding ( ) in shear flow. Results shown are mean and SEM of 3 to 6 separate determinations on each band collected in 2 independent experiments.
) in shear flow. Results shown are mean and SEM of 3 to 6 separate determinations on each band collected in 2 independent experiments.
As cultured T cells may produce altered glycosylation products, we also isolated CLA+ T cells from fresh peripheral-blood CD3+ cells using mAb HECA-452 and magnetic beads (Figure 6A). Western blots of CD43 immunoprecipitated from lysates of unstimulated circulating CLA+ T cells show bands of 125 kDa and 115 kDa apparent molecular weight (Figure 6B) corresponding to core-2-modified and non-core-2-modified forms of CD43 (data not shown) as seen in cultured T cells. Blot-rolling analysis confirmed that 125-kDa CD43 from fresh, resting human CLA+ T cells can support E-selectin- but not P-selectin-mediated binding and rolling in shear flow (Figure 6C).
CLA+ CD43 displays E-selectin ligand activity in an antigen capture flow assay. Anti-CD43 mAb 1G10 (left) and isotype control Ab (right) bound to plastic were overlayed with lysates of cultured T cells. CHO-E cells (▪), but not CHO-P ( ) or mock-transfected cells (
) or mock-transfected cells ( ), tethered and rolled on CD43 adsorbed from lysates of CLA+ T cells. No significant adhesion was observed on CD43 adsorbed from lysates of CLA- T cells or on isotype control substrates. CHO-E binding to CLA+ CD43 was abrogated in the presence of EDTA (□). Results shown are mean and SEM of 3 to 6 separate determinations on each substrate collected in 2 independent experiments.
), tethered and rolled on CD43 adsorbed from lysates of CLA+ T cells. No significant adhesion was observed on CD43 adsorbed from lysates of CLA- T cells or on isotype control substrates. CHO-E binding to CLA+ CD43 was abrogated in the presence of EDTA (□). Results shown are mean and SEM of 3 to 6 separate determinations on each substrate collected in 2 independent experiments.
CLA+ CD43 displays E-selectin ligand activity in an antigen capture flow assay. Anti-CD43 mAb 1G10 (left) and isotype control Ab (right) bound to plastic were overlayed with lysates of cultured T cells. CHO-E cells (▪), but not CHO-P ( ) or mock-transfected cells (
) or mock-transfected cells ( ), tethered and rolled on CD43 adsorbed from lysates of CLA+ T cells. No significant adhesion was observed on CD43 adsorbed from lysates of CLA- T cells or on isotype control substrates. CHO-E binding to CLA+ CD43 was abrogated in the presence of EDTA (□). Results shown are mean and SEM of 3 to 6 separate determinations on each substrate collected in 2 independent experiments.
), tethered and rolled on CD43 adsorbed from lysates of CLA+ T cells. No significant adhesion was observed on CD43 adsorbed from lysates of CLA- T cells or on isotype control substrates. CHO-E binding to CLA+ CD43 was abrogated in the presence of EDTA (□). Results shown are mean and SEM of 3 to 6 separate determinations on each substrate collected in 2 independent experiments.
CD43 from fresh, resting human peripheral-blood T cells is decorated with CLA and binds E-selectin. (A) Purification of CLA+ T cells from peripheral blood was assessed by staining with anti-CLA mAb HECA-452 (solid line) versus unstained control cells (dotted line). Lysates of CLA+ peripheral-blood T cells were immunoprecipitated with anti-CD43 mAb 1G10 and subjected to nonreducing SDS-PAGE. (B) Western blots stained with anti-CD43 mAb DF-T1-biotin show 2 bands of 125 kDa and 115 kDa, similar to those seen with cultured T cells. High-range and full-range molecular weight markers were run in the left and right flanking lanes, respectively. (C) Blot-rolling analysis showed that CHO-E cells (▪), but not CHO-P ( ) or mock-transfected CHO (
) or mock-transfected CHO ( ) cells, tether and roll on the 125-kDa isoform, but not the 115-kDa isoform, of CD43 from peripheral-blood T cells. Separation of CD43 from PSGL-1 in this IP was confirmed by the lack of CHO-E or CHO-P binding unstimulated or CLA+ stain in the 140-kDa region of these Western blots (not shown). CHO-E binding to 125-kDa CD43 was abrogated in the presence of 5 mM EDTA (not shown). Results shown are mean and SEM of 3 or more observations.
) cells, tether and roll on the 125-kDa isoform, but not the 115-kDa isoform, of CD43 from peripheral-blood T cells. Separation of CD43 from PSGL-1 in this IP was confirmed by the lack of CHO-E or CHO-P binding unstimulated or CLA+ stain in the 140-kDa region of these Western blots (not shown). CHO-E binding to 125-kDa CD43 was abrogated in the presence of 5 mM EDTA (not shown). Results shown are mean and SEM of 3 or more observations.
CD43 from fresh, resting human peripheral-blood T cells is decorated with CLA and binds E-selectin. (A) Purification of CLA+ T cells from peripheral blood was assessed by staining with anti-CLA mAb HECA-452 (solid line) versus unstained control cells (dotted line). Lysates of CLA+ peripheral-blood T cells were immunoprecipitated with anti-CD43 mAb 1G10 and subjected to nonreducing SDS-PAGE. (B) Western blots stained with anti-CD43 mAb DF-T1-biotin show 2 bands of 125 kDa and 115 kDa, similar to those seen with cultured T cells. High-range and full-range molecular weight markers were run in the left and right flanking lanes, respectively. (C) Blot-rolling analysis showed that CHO-E cells (▪), but not CHO-P ( ) or mock-transfected CHO (
) or mock-transfected CHO ( ) cells, tether and roll on the 125-kDa isoform, but not the 115-kDa isoform, of CD43 from peripheral-blood T cells. Separation of CD43 from PSGL-1 in this IP was confirmed by the lack of CHO-E or CHO-P binding unstimulated or CLA+ stain in the 140-kDa region of these Western blots (not shown). CHO-E binding to 125-kDa CD43 was abrogated in the presence of 5 mM EDTA (not shown). Results shown are mean and SEM of 3 or more observations.
) cells, tether and roll on the 125-kDa isoform, but not the 115-kDa isoform, of CD43 from peripheral-blood T cells. Separation of CD43 from PSGL-1 in this IP was confirmed by the lack of CHO-E or CHO-P binding unstimulated or CLA+ stain in the 140-kDa region of these Western blots (not shown). CHO-E binding to 125-kDa CD43 was abrogated in the presence of 5 mM EDTA (not shown). Results shown are mean and SEM of 3 or more observations.
Discussion
In this report we show that CD43 on CLA+ T cells can be decorated with the CLA epitope and serves as an E-selectin, but not a P-selectin, ligand on these cells. This is the first demonstration of a selectin ligand function for CD43 on T cells and the first functional description of a T-cell E-selectin ligand other than PSGL-1. The existence of at least 2 distinct selectin ligands on T cells, and the evidence for differential glycosylation of both CD43 and PSGL-1 protein scaffolds, suggests that these separate structures serve different functions and may be implicated in distinct inflammatory conditions.
The presence of a neutrophil E-selectin ligand distinct from PSGL-1 has been suggested in studies using PSGL-1-deficient (PSGL-1-/-) mice,16,17 although the identity of this residual activity is not known. A recent report has indicated that CD44 may have E-selectin ligand function on murine and human neutrophils.18 The absence of CLA epitope and E-selectin binding activity in the region of CD44 on the blots examined in this report suggests that CD44 is not a major selectin ligand on human CLA+ T-cell populations. The absence of selectin ligand functions in mice deficient in the fucosyltransferase IV and VII, in contrast, indicates that the biosynthetic pathway for producing the appropriate glycans on CD43 is similar, if not identical, to that used to decorate PSGL-1.19
Although CD43 (leukosialin) is known as one of the most abundant T-cell surface proteins, its function(s) has not been clearly defined. CD43 is expressed exclusively on hematopoietic cells, where it is the major sialic acid-bearing surface glycoprotein.20-22 Similar to other sialomucins, extensive O-linked glycosylation holds CD43 in an extended rodlike structure that extends up to 45 nm above the plasma membrane.23 These features make CD43 an ideal candidate for interaction with other cells and support its function as a regulator of immune responses. CD43 is known to be decorated with a diverse set of carbohydrate epitopes, some of which are expressed continuously, whereas others vary during leukocyte development or in response to activation, suggesting that they may be involved in T-cell function.24,25
CD43 has been reported to be involved in cell migration,26-28 thymocyte maturation,29 signal transduction,30-32 regulation of T-cell receptor (TCR) activation,33 and inhibition of TCR-CD3-mediated apoptosis.34 Interestingly, CD43 has been proposed both as a leukocyte adhesion molecule and as an antiadhesive molecule.26,27,35-37 Inhibition of cellular interactions by CD43 has been suggested to be due to steric hindrance26 or to the net negative surface charge resulting from the abundant sialic acid present on CD43.38,39 CD43 is also shed by proteolytic cleavage after cellular activation, hypothetically reducing these antiadhesive properties and promoting T-cell immune functions.38,40 Recent reports suggest that CD43 may regulate cell adhesion by modulating the formation of the immune synapse and regulating the interactions of integrins with their ligands.33,41-43
In contrast, treatment of T cells with mAb to CD43 has been shown to induce activation, cytokine production, and proliferation44-46 and may enhance costimulatory signaling.47 Anti-CD43 mAbs have been shown to reduce T-cell binding to PLNs and Peyer Patch HEVs, inhibit emigration into LNs, and reduce leukocyte recruitment to tissues in a number of inflammatory models, suggesting that CD43 is a positive promoter of leukocyte adhesion in these systems.27,28,48 Siglec-1 (CD169), a sialic acid-binding lectin expressed on macrophages in peripheral lymphoid tissues,49 has been shown to bind CD43 on murine T cells (both core-1- and core-2-modified glycoforms) and is thought to be important in promoting T-cell contact with macrophages in lymph nodes.50 CD43 has also been reported to bind ICAM-1,51 although this finding has not been supported in other studies.27,52,53
Studies using CD43-deficient mice (CD43-/-) also present somewhat conflicting results. In vitro studies using leukocytes from CD43-/- mice have shown increased tethering and rolling on both L-selectin26 and E-selectin,54 consistent with an antiadhesive role. In vivo migration studies, however, have shown both increased emigration of CD43-/- leukocytes into PLNs26 and decreased emigration into the peritoneum and brain.54-56 Reports of response to viral infection are also mixed, with reports showing both increased proliferation and enhanced cytotoxic T lymphocyte (CTL) responses to vaccinia infection36 and reduced T-cell expansion to murine lymphocytic choriomeningitis virus (LCMV) and allogeneic skin transplantation.55 The authors of this latter study have suggested that the role of CD43 may be different in activated versus nonactivated T cells and proposed that differential glycosylation may result in opposing adhesive effects in different stages of CD8 T-cell responses.
As binding to vascular selectins is required for T-cell emigration to skin, altered binding of T cells to E-selectin may have implications for the development of inflammatory skin disorders. It is interesting to note that Wiskott-Aldrich syndrome (WAS), a disorder characterized by T-cell immune dysfunction and extensive eczema, is associated with increased expression of a high-molecular-weight (130 kDa) core-2-decorated isoform of CD43 on T cells.57-60 While CD43 does not appear to be directly implicated in the functional defects seen in T cells from WAS patients,61,62 cross-linking of CD43 can modulate signaling cascades that target the WAS protein.32,63,64 Expression of CD43 and proliferation of T cells in response to CD43 stimulation are also increased in atopic dermatitis.65 It is interesting to speculate that expression of excess E-selectin ligand activity or increased activation of skin-homing T cells via CD43 may result in the prominent eczema seen in WAS patients.
In summary, we have shown that CD43 on CLA+ T cells is decorated with CLA epitope and can function as an E-selectin ligand. We propose that CLA+ CD43 may play a key role in inflammatory skin diseases in which differential glycosylation of CD43 may regulate a balance between antiadhesive and proadhesive functions of CD43 and contribute to the regulation of both physiologic and pathologic skin inflammation.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-05-2112.
Supported by National Institutes of Health (NIH) grants R01 AI056084 and R01 AI-41707.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.