Telomerase is considered a universal tumor-associated antigen (TAA) due to its high rate of expression by cancers (≈90%), and clinical trials are in progress to test the immunotherapeutical efficacy of antitelomerase immunization in patients with cancer. However, the data concerning frequency and functional activity of telomerase-specific cytotoxic T lymphocytes (CTLs) in patients with cancer are few and conflicting, although their knowledge would be mandatory to predict the efficacy of telomerase-specific immunotherapy in selected patients. We performed this study to analyze frequency and cytolytic function of circulating CD8+ T lymphocytes specific for the p540 telomerase peptide in a series of human leukocyte antigen (HLA)–A2+ cancer patients. The results show that most patients with cancer have circulating telomerase-specific CD8+ T lymphocytes, but a high frequency of telomerase-specific CTLs are present only in a fraction of them. Furthermore, CTL lines able to kill telomerase-positive tumor cells, including autologous cancer cells, can be expanded ex vivo from some, but not all, patients with cancer. In conclusion, the results of the study support the development of clinical protocols using telomerase peptides as an immunizing agent. However, they underline the necessity to study single patients immunologically before undergoing vaccination, to select the patients adequately, and to eventually adapt the immunization schedule to the patient's immunologic status.
Introduction
In the past decade great effort has been devoted to the search of tumor-associated antigens (TAAs)1 with the aim to identify molecules selectively expressed on tumor cells that could represent optimal targets for cytolytic immune responses. This could allow the development of vaccines against cancer to be used alone or as complement to conventional anticancer therapies. However, this immunotherapeutic approach has been halted so far by problems related to the specificity, the distribution among histologically different tumors and the frequency of expression, and the immunogenicity of TAA.2,3
Telomerase, the reverse transcriptase of eukaryotic cells deputed to synthesis of the telomeric regions of chromosomes, allows the maintenance of chromosomal length and stability.4-6 Telomerase, present in embryonic but not in adult somatic cells with few exceptions,7,8 is essential for cell immortalization: this explains why it is expressed by more than 85% of tumors (independently on the histologic type).9-11 Indeed, telomerase allows cancer cells to indefinitely proliferate without undergoing aging and apoptosis.11 For this reason, telomerase is now considered a potential “universal” TAA.12,13 This view is further supported by the finding that cytotoxic T lymphocytes (CTLs) able to kill cancer cells through the specific recognition of peptides derived from the catalytic subunit of the human telomerase reverse transcriptase (hTERT) have been identified in the peripheral blood of both healthy subjects and cancer patients.14,15 Thus, telomerase-specific T lymphocytes seem to escape thymic deletion, although telomerase is an endogenous molecule. These results suggest the possibility of using telomerase as an immunogenic antigen for the induction of antitumor immune responses.16-18 However, the efficacy of an immune response depends on both frequency and activation status of precursors of antigen-specific T-cell clones.2,19 Few and conflicting data currently exist in the literature relative to frequency and functional activity of telomerase-specific CTLs in cancer patients.20-22 Thus, we planned a study aimed to analyze frequency and cytolytic function of circulating antitelomerase-specific CD8+ T lymphocytes in a series of cancer patients. We approached this issue by phenotypic and functional methodologies: flow cytometry using human leukocyte antigen (HLA)–A2+ tetramers loaded with the p540 peptide (p540+ tetramers) encompassing residues 540 to 548 of the catalytic subunit of hTERT,13-15 limiting dilution assay (LDA) for the identification and enumeration of hTERT-specific CTL precursors (CTLp's), and generation of p540 peptide–specific CTL lines. This strategy allowed us to obtain data on both the total number of hTERT-reactive CD8+ T cells and the number of functionally active hTERT-specific cytotoxic cells. These data can provide insights on the possibility and opportunity to develop immunotherapeutic protocols adopting telomerase as immunogenic antigen for the treatment of cancer.
Patients, materials, and methods
Patients
Twenty-seven patients with HLA-A2+ cancer were enrolled in the study affected with the following diseases: prostate cancer (18 patients), breast cancer (2 patients), lung cancer (2 patients), gastric cancer (1 patient), non-Hodgkin lymphomas (NHL) (3 patients), and hepatocarcinoma (1 patient). Four HLA-A2- cancer patients, 2 affected with prostate cancer (with disease stage pT2c/N0/Mx and pT3b/N1/G2, respectively) and 2 with breast cancer (both with disease stage T4N1M0) were also analyzed as negative controls. Prostate cancer patients were diagnosed, selected, and submitted to surgical treatment (radical retropubic prostatectomy) at the Department of Urology of the University of Genoa. Patients affected with breast, gastric, and lung cancer as well as non-Hodgkin lymphoma were diagnosed at the Division of Internal Medicine and Immunology, Department of Internal Medicine, at the University of Genoa. All patients were studied before undergoing surgery and/or chemotherapy. The study was approved by the Ethical Committee of the Department of Internal Medicine at the University of Genoa, and each patient gave his informed consent to the research. The list of patients, including disease type, staging at diagnosis, therapy administered, and disease evolution at the present, is shown in Table 1. Analysis of HLA-A2 expression was performed both by immunofluorescence using the anti–HLA-A2 BB7.2 monoclonal antibody23 (mAb) and by polymerase chain reaction (PCR) amplification using a commercially available kit (Texas Biosystem, Dallas, TX). Six HLA-A2+ and 4 HLA-A2- healthy donors were also studied as further controls.
Peptide
p540 peptide encompassing residues 540 to 548 of hTERT13-15 was manually synthesized using the standard method of solid-phase peptide synthesis which follows the 9-fluorenylmethoxycarbonyl (Fmoc) strategy with minor modifications.24
All the synthesized compounds were purified by reverse-phase high-performance liquid chromatography (HPLC) and the molecular weights finally confirmed by electrospray iontrap mass spectrometry. The purification of individual compounds was obtained on a Shimadzu LC-9A preparative HPLC (Shimadzu, Kyoto, Japan) equipped with a Waters C18 μBondapack column (19 × 300 mm; Waters, Milford, MA).
Cells
Peripheral-blood mononuclear cells (PBMCs) were isolated by centrifugation on Ficoll gradient. As target for cytotoxic assay the following HLA-A2+ neoplastic cell lines were used: transporter associated with antigen processing (TAP)–deficient T2 cells25 (a kind gift from Prof R. S. Accolla, University of Insubria, Varese, Italy), Burkitt lymphoma–derived BJAB cells,26 LNCaP prostate cancer cells (American Type Culture Collection [ATCC], Manassas, VA), and, in the case of 4 patients, autologous primary prostate cancer cell lines generated as specified in “Generation of primary cell lines from prostate cancer specimens.” In some experiments HLA-A2- telomerase-positive Burkitt lymphoma–derived RAJI cells27 were used as negative control.
Generation of primary cell lines from prostate cancer specimens
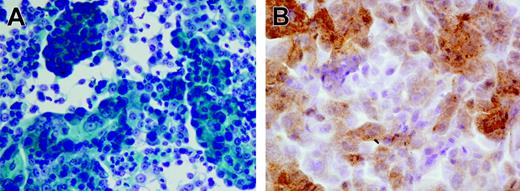
Tissue fragments were carefully minced into 2- to 3-mm cubes in a small volume of growth medium, and the resultant slurry of tissue and cells was dispensed into 6-well plates. All cultures were initiated in a volume of 1 mL/well and incubated at 37°C in the presence of 5% CO2. After 2 to 3 days, which were necessary to allow viable cells and tissue chunks to settle and attach to the plate, the unattached debris were carefully aspirated and wells were re-fed with 3 to 5 mL fresh medium. Culture medium was routinely replaced every 2 to 4 days, and proliferating adherent cells were transferred in new wells after detachment with trypsin. Established growing cultures were maintained in tissue culture flasks. Growth medium for the generation of prostate cell lines consisted of Keratinocyte Serum Free Medium (Keratinocyte SFM Life Technologies, Grand Island, NY) containing 25 μg/mL bovine pituitary extract, 5 ng/mL epidermal growth factor, 2 mM l-glutamine, 10 mM HEPES buffer, antibiotics, and 5% heat-inactivated fetal bovine serum (FBS). For the initiation of epithelial cultures from fresh tissue specimens, the concentration of FBS was reduced to 1%, and cholera toxin was added at 20 ng/mL to avoid outgrowth of contaminating fibroblasts.28 The successful establishment of a primary prostate cancer cell line was confirmed by morphologic analysis using Papanicolau staining and by immunocytochemistry using a kit (Ventana Medical System, Tucson, AZ) evaluating prostate-specific antigen (PSA) production by the cells. Figure 1A and 1B show 1 representative prostate cell line originated from patient no. 18.
Evaluation of telomerase expression by tumor cells
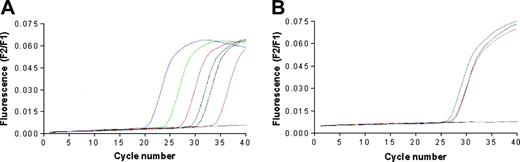
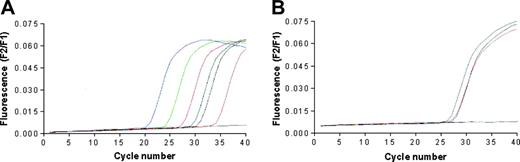
The quantitative detection of mRNA encoding for human telomerase catalytic subunit hTERT was performed using the LightCycler TeloTAGGG hTERT Quantification Kit (Roche Diagnostics, Milan, Italy) and the LightCycler thermocycler instrument. LNCaP, BJAB, and RAJI cells, but not T2 cells, were found positive for the expression of hTERT (Figure 2). Primary prostate cancer cell lines generated in our laboratory were not tested due to the insufficient number of available cells.
Tetramers
p540+ tetramers were provided initially by the National Institutes of Health (NIH) Tetramer Facility (Atlanta, GA) and subsequently by Proimmune (Oxford, United Kingdom). NIH and Proimmune tetramers were used for the analyses of patients 1, 2, 3, 6, 7, 8, 9, 19, 21, and of patients 6, 7, 8, 9, 11, 12, 13, 14, 15, 17, 18, 20, 24, 25, 26, and 27, respectively. Patients 6, 7, 8, and 9 were analyzed by both tetramer preparations to verify their functional equivalence; consistent results were obtained in all 4 cases.
Pathologic analysis of the prostate cell line generated from patient no. 18. (A) Highly differentiated adenocarcinoma cells; Papanicolau staining. (B) Anti-PSA staining. Images were acquired with a BX51 microscope (Olympus, Tokyo, Japan) with a 600×/0.80 NA objective, a DP70 digital camera (Olympus), and Olympus DP70 Windows XP–compatible acquisition software.
Pathologic analysis of the prostate cell line generated from patient no. 18. (A) Highly differentiated adenocarcinoma cells; Papanicolau staining. (B) Anti-PSA staining. Images were acquired with a BX51 microscope (Olympus, Tokyo, Japan) with a 600×/0.80 NA objective, a DP70 digital camera (Olympus), and Olympus DP70 Windows XP–compatible acquisition software.
Telomerase expression by the cancer cell lines used in the study. The analysis was performed by real-time PCR performing 40 cycles of DNA amplifications; data are expressed as fluorescence intensity. (A) The blue, green, red, black, and pink lines correspond to the positive standards constituted by 1 × 106, 1 × 105, 1 × 104, 1 × 103, and 1 × 102 copies of hTERT RNA, respectively. The blue line corresponds to the negative control; the dark green line corresponds to the positive control provided by the kit manufacturer. (B) The gray, purple, orange, and blue lines correspond to the curves of hTERT cDNA amplification starting from RNA extracted from LnCap, Raji, Bjab, and T2 cell lines, respectively.
Telomerase expression by the cancer cell lines used in the study. The analysis was performed by real-time PCR performing 40 cycles of DNA amplifications; data are expressed as fluorescence intensity. (A) The blue, green, red, black, and pink lines correspond to the positive standards constituted by 1 × 106, 1 × 105, 1 × 104, 1 × 103, and 1 × 102 copies of hTERT RNA, respectively. The blue line corresponds to the negative control; the dark green line corresponds to the positive control provided by the kit manufacturer. (B) The gray, purple, orange, and blue lines correspond to the curves of hTERT cDNA amplification starting from RNA extracted from LnCap, Raji, Bjab, and T2 cell lines, respectively.
Immunophenotypic analysis with tetramers
The study of the frequency of circulating CD8+ T lymphocytes recognizing p540 peptide in the context of HLA-A2 molecules was performed on PBMCs from HLA-A2+ or HLA-A2- cancer patients and from HLA-A2+ and HLA-A2- healthy subjects. Samples were analyzed immediately after PBMC isolation (without freezing); each PBMC from cancer patients was analyzed comparatively with freshly isolated PBMCs from 1 HLA-A2+ and 1 HLA-A2- healthy subjects. Cell viability, analyzed before every test, was always 97% or greater. PBMCs were incubated for 30 minutes at 4°C with a FITC-conjugated anti-CD8 mAb, washed, and incubated for 45 minutes at 4°C with 5 μL of tetramer preparation. After washing, double-positive cells were quantified by flow cytometry using an Epics XL Cytometer (Beckman-Coulter, Marseille, France). This procedure was chosen after preliminary experiments in which different concentrations of tetramers as well as various times and temperatures of incubations had been tested.
LDA
PBMCs, resuspended in culture medium (RPMI 1640 supplemented with 10% FBS, 2% glutamine, and 100 U/mL penicillin–0.1 mg/mL streptomycin), were seeded in U-bottomed 96-well microculture plates at concentrations ranging from 10 000 cells/well to 10 cells/well using a dilution factor of 1:2. A constant number (10 000 cells/well) of irradiated (50 Gy [5000 rad]), p540 peptide–pulsed T2 cells was also added to each well together with interleukin 2 (IL2; 50 U/mL) in a total volume of 200 μL. At each lymphocyte dilution 24 identical replicates were set up. Culture medium was partially replaced every 3 days and cultures were restimulated weekly with same number of irradiated, p540 peptide–pulsed T2 cells. After 3 weeks each replicate was split into 2 identical aliquots that were used for comparative cytotoxic assays. To this end, the 2 aliquots were separately cultured in the presence of either p540 peptide–pulsed or –nonpulsed Na251CrO4–labeled T2 cells (1 × 103 cells/well). Lytic activity in each well was considered significant when it exceeded the value obtaining by adding 3 standard deviations (SDs) to the mean of spontaneous release by the targets. Wells showing lytic activity against both pulsed and nonpulsed T2 cells were not considered for the calculation of CTL precursor frequency. Regression analysis of the responder-cell number per well against log percentage of negative wells was then performed using GraphPad Prism version 3.0 software (San Diego, CA). On the basis of the single-hit Poisson model, the frequency of antigen-specific CTLp's was estimated from the initial responder-cell number at which 37% of the wells were negative for cytotoxicity.29 In patients 17, 18, and 26, LDA analysis was performed on both PBMC-depleted and nondepleted from tetramer-positive cells.
PBMC depletion from tetramer-positive cells
PBMCs (5 × 106 cells) were resuspended in 100 μL buffer solution (phosphate-buffered saline [PBS] + 0.5% BSA + 2 mM EDTA) and incubated with 20 μL of PE-stained p540+ tetramers for 20 minutes at 4 °C in the dark; after washings, cells were resuspended in 80 μL of the same buffer solution and 20 μL of anti-PE MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were added. After 30 minutes of incubation at 4 °C in the dark, 2 mL buffer solution was added and the cells were centrifuged at 354g for 10 minutes. Then, cells were resuspended in 500 μL buffer solution and sorted on an LS column (Miltenyi Biotec). Two samples were collected, one containing PBMCs depleted from tetramer-positive cells, and one containing the sorted tetramer-positive lymphocytes. The first cell sample did not show presence of tetramer-positive cells at flow cytometry, confirming the efficiency of negative selection. Cells contained in the second sample were collected and cultured for 3 weeks in the presence of IL2 (50 U) and T2 cells (1 × 103 cells) pulsed with the p540 peptide. Then, they were tested for cytotoxicity against T2 cells pulsed or nonpulsed with the p540 peptide.
Generation of p540-specific CTL lines
p540-specific CTL were generated as described.15 Briefly, PBMCs from HLA-A2+ cancer patients (1.5 × 106 cells/mL) were incubated in 24-well plates in culture medium containing human AB serum and 10 μg/mL p540 peptide. After 2 days, 12 U/mL IL2 was added to each well. Five days later, cells were collected, resuspended at 5 × 105 cells/mL, and seeded in the presence of autologous, irradiated (50 Gy [5000 rad]) PBMCs pulsed with the p540 peptide at a responder-stimulator cell ratio of 1:5. On the day after, 12 U/mL IL2 and 30 U/mL IL7 were added to the wells. After 1 to 2 weeks, cells were collected, resuspended at 5 × 105 cells/mL in medium containing 30 U/mL IL7 and plated in the presence of autologous, irradiated antigen-presenting cells (APCs) prepared as follows. Autologous, irradiated PBMCs were resuspended at 4 × 106 cells/mL in medium containing both 5 μg/mL β2-microglobulin and 10 μg/mL p540 peptide, and distributed in 24-well plates at 1 mL/well at 37°C. After 2 hours, nonadherent cells were removed and adherent cells were used as APCs. The latter cycle of stimulation was repeated weekly for 5 times before analysis of CTL cytotoxicity.
Cytotoxic assay
Pelleted target cells (T2, BJAB, LNCaP, RAJI, or autologous primary prostate cancer cell lines; 5 × 105 cells) were labeled using 150 μL (0.037 MBq/μL [1 μCi/μL]) Na251CrO4 for 90 minutes at 37°C. During the labeling T2 cells, but not the other cell lines, were also incubated (or not) with 10 μg of p540 peptide. After washings, 5 × 103/well target cells were incubated in flat-bottomed 96-well plates with CTLs at a CTL–target-cell ratio of 40:1. In the cytotoxic assays performed with sorted tetramer-positive cells a 10:1 effector-to-target cell ratio was employed using 1 × 103 T2 target cells/well. Target cells incubated with medium alone or with Triton-X100 diluted 1:100 were used to calculate spontaneous and maximum 51Cr release, respectively. After 6 hours of incubation supernatants were collected and the radioactivity was detected by a gamma-counter (Wallac, Turku, Finland). The percentage of cytotoxicity was calculated as follows: percentage of lysis = sample counts per minute (cpm) - spontaneous release cpm/maximum release cpm - spontaneous release cpm × 100. In some experiments the anti–HLA class I W6/32 mAb (100 μg/mL)30 or an unrelated isotype-matched (IgG2a) control mAb (Sigma-Aldrich, Milan, Italy) were used as blocking antibody.
Statistical analyses
The existence of statistically significant correlations between the frequency of tetramer-binding CD8+ T lymphocytes and that of p540-specific CTL precursors, as well as between the clinical stage of disease and the frequency of tetramer-binding CD8+ T lymphocytes or of p540-specific CTLp in cancer patients was analyzed by the Spearman test for nonparametric data. The search for statistically significant differences between mean percentages of circulating tetramer-positive CD8+ T lymphocytes in cancer patients with disease stage of 2 or lower and higher than 2 was performed by Mann-Whitney t test for nonparametric values. Calculations were performed using GraphPad Prism version 3.0 software.
Results
Telomerase-specific circulating CD8+ T lymphocytes are present in cancer patients but not in healthy subjects
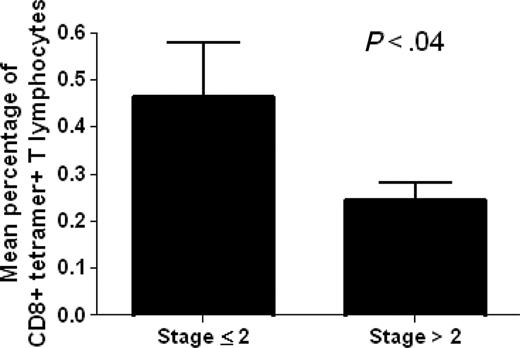
The analysis of ex vivo binding of circulating CD8+ T lymphocytes with the p540 telomerase peptide in the context of HLA-A2 molecules was performed in 22 out of the total 27 HLA-A2+ cancer patients enrolled in the study using p540+ tetramers. HLA-A2- cancer patients as well as HLA-A2+ and HLA-A2- healthy subjects were considered as negative controls. The analyzed HLA-A2+ cancer patients were affected with different types of cancer, including prostate, breast, lung, liver, and gastric cancers as well as non-Hodgkin lymphomas, and were at different stages of disease. This allowed us to obtain data concerning a spectrum of neoplasms and disease severity (Table 1). Ex vivo–positive staining of circulating CD8+ T cells by p540+ tetramers was detected in 20 (90%) of the 22 examined HLA-A2+ cancer patients (Table 2). The percentages of CD8+ tetramer-positive cells ranged between 0.1% to 1.4% (with a median of 0.33%) and were inversely related to disease staging as demonstrated by Spearman correlation test (P = .008; Spearman r = -0.5478) and by t test comparing the mean percentages of CD8+ tetramer-positive cells between patients with stage 2 or lower and those with higher than stage 2 (Figure 3). Taking into consideration exclusively the group of patients with prostate cancer, no statistically significant correlation was found between the percentages of circulating tetramer-positive CD8+ T cells and PSA levels (P = .9) (Table 2). Tetramer-binding cells were not detected in control groups constituted by HLA-A2- cancer patients, as well as by both HLA-A2+ and HLA-A2- healthy subjects (Table 3), as supported by the fact that all the results (ranging between 0.01% to 0.04%) were below the limit of sensitivity of the technique.
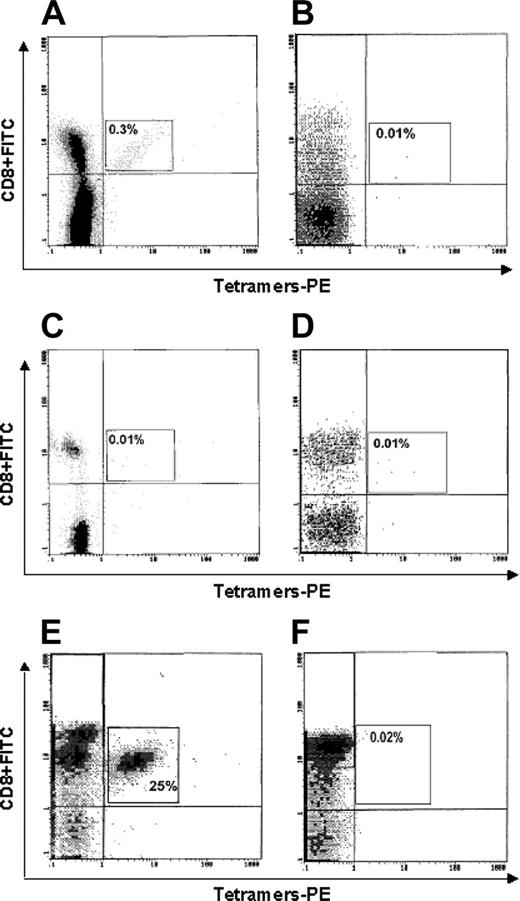
Figure 4 shows the representative cytometric analyses of tetramer binding to cells of one HLA-A2+ cancer patient (no. 1; Figure 4A), one HLA-A2- cancer patient (no. 25; Figure 4B), one HLA-A2+ healthy subject (Figure 4C), one HLA-A2- healthy subject (Figure 4D), one p540-specific CTL line (generated from patient no. 16 and used as positive control; Figure 4E), and one Epstein-Barr virus (EBV)–specific CTL line (used as further negative control; Figure 4F). A significant tetramer binding is observed only in Figure 4A and 4E.
Analysis of the existence of a statistically significant difference between mean percentages of circulating tetramer-positive CD8+ T lymphocytes in cancer patients with disease stage 2 or lower (no. 11) and higher than 2 (no. 11). The analysis demonstrates the existence of a statistically significant difference between the 2 variables. Data are expressed as mean ± SD.
Analysis of the existence of a statistically significant difference between mean percentages of circulating tetramer-positive CD8+ T lymphocytes in cancer patients with disease stage 2 or lower (no. 11) and higher than 2 (no. 11). The analysis demonstrates the existence of a statistically significant difference between the 2 variables. Data are expressed as mean ± SD.
Telomerase-specific CTLs in the peripheral blood of cancer patients
To verify whether cancer patients have telomerase-specific CTLs in the peripheral blood and to understand the level of expansion of telomerase-specific effector-cell populations in these patients, we took into consideration the frequency of circulating p540 peptide–specific CTLp's and the possibility of generating p540 peptide–specific CTL lines. Based on the availability of blood samples, it was possible to perform 1 or both of these analyses in 23 of the total 27 HLA-A2+ cancer patients enrolled for this study.
Frequency of CTLp's
The analysis by LDA of circulating p540 peptide–specific CTLp's, performed in 17 patients (15 with prostate cancer, 1 with lung cancer, and 1 with NHL), revealed variable frequencies among patients (Table 4 and Figure 5). In particular, 2 groups of patients, one with low frequency or absence (patients 1, 3, 7, 8, 9, 12, and 26) and the other one with high frequency (patients 2, 6, 10, 11, 13, 14, 15, 17, and 18) of p540 peptide–specific CTLp's were identified. In this second group, 3 patients (patients 11, 14, and 15) presented an extremely elevated frequency of p540-specific CTLp's (1:298, 1:540, and 1:354, respectively), thus demonstrating that the p540 peptide of hTERT is highly immunogenic in vivo and that efficient presentation of this peptide followed by the onset of an effective immune response may occur in HLA-A2+ cancer patients. Interestingly, the frequencies of CTLp's were in statistically significant direct correlation with those of cells stained by p540+ tetramers (P = .0002; Spearman r = 0.7956). This observation suggests that circulating lymphocytes specifically recognizing the p540 peptide endowed in HLA-A2 molecules are generally functional cells able to mediate cytotoxicity. Notwithstanding this, very low frequencies of CTLp's were detected in 3 patients (nos. 3, 8, and 9) in spite of relatively high percentages of circulating lymphocytes binding p540+ tetramers. Thus, not all the p540 peptide–specific lymphocytes seem to be effective CTLp's.
In 3 patients (nos. 17, 18, and 26), LDA was performed on both whole PBMCs and on PBMCs depleted from tetramer-positive cells. Frequency of CTLp's was 0 (or undetectable) after cell depletion, while CTLp's were present and functional in the corresponding nondepleted PBMCs (Table 4). Moreover, tetramer-positive cells sorted from each PBMC specifically lysed p540 peptide–pulsed T2 cells (Table 5).
Generation of p540 peptide–specific CTL lines
Flow cytometry analysis of binding of p540+ tetramers to circulating CD8+ T lymphocytes. Lymphocytes were from (A) 1 HLA-A2+ cancer patient (no. 1); (B) 1 HLA-A2- cancer patient (no. 17); (C) 1 HLA-A2+ healthy subject; (D) 1 HLA-A2- healthy subject; (E) 1 p540-specific CTL line (generated from patient no. 16 and used as positive control); and (F) 1 EBV-specific CTL line (used as further negative control).
Flow cytometry analysis of binding of p540+ tetramers to circulating CD8+ T lymphocytes. Lymphocytes were from (A) 1 HLA-A2+ cancer patient (no. 1); (B) 1 HLA-A2- cancer patient (no. 17); (C) 1 HLA-A2+ healthy subject; (D) 1 HLA-A2- healthy subject; (E) 1 p540-specific CTL line (generated from patient no. 16 and used as positive control); and (F) 1 EBV-specific CTL line (used as further negative control).
Generation of p540 peptide–specific CTL lines was tested in 16 of the 27 enrolled cancer patients. Twelve analyzed patients had prostate cancer, 2 had lung cancer, 1 had gastric cancer, and 1 had liver cancer. CTL lines were generated from 11 (68%) of 16 tested cancer patients, and in particular from 8 (nos. 1, 2, 4, 5, 6, 11, 16, and 18) of 12 prostate cancer patients, one (no. 22) of 2 lung cancer patients, and 2 patients affected with gastric (no. 23) and liver (no. 27) cancers, respectively (Table 4). Among the 5 patients negative for telomerase-specific CTL lines, 2 were also negative for tetramer-binding cells and for telomerase-specific CTLp's (nos. 7 and 21); the other 3 (nos. 3, 8, and 9) had a very low frequency of telomerase-specific CTLp's by LDA (< 1:10 000, < 1:10 000, and 1:7123, respectively) in spite of a higher frequency of tetramer-binding lymphocytes. These findings further support the concept that not all the circulating p540 peptide–specific lymphocytes are effector (cytotoxic) cells.
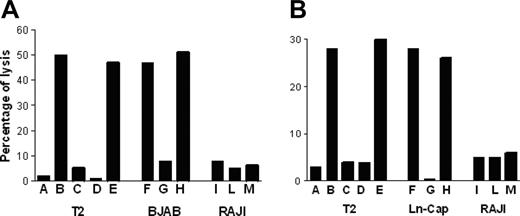
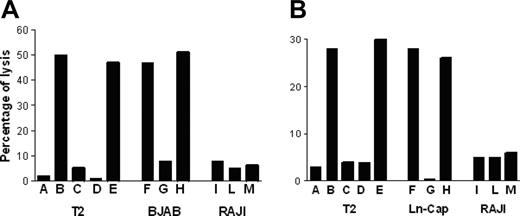
P540-specific CTL lines lysed p540 peptide–pulsed T2 cells but not nonpulsed cells; furthermore, they were cytotoxic against other HLA-A2+ telomerase-positive cancer cell lines, such as BJAB cells and LNCaP cells, while HLA-A2-, telomerase-positive RAJI cells, used as negative control, were not killed. Figure 6 shows the killing activity of 2 representative p540-specific CTL lines generated from patients 11 and 16, respectively. The cytotoxicity was inhibited by the anti–HLA class I W6/32 monoclonal antibody, thus indicating that the cytotoxic function was dependent on HLA class I–mediated presentation of the p540 peptide. In 4 cases, patients 2, 11, 16, and 18, autologous primary prostate cancer cell lines could be generated. These cells were efficiently lysed by autologous CTL lines in an HLA class I–dependent way (Figure 7). Thus, in at least these 4 patients, ex vivo–expanded p540-specific CTLs were able to kill autologous cancer cells.
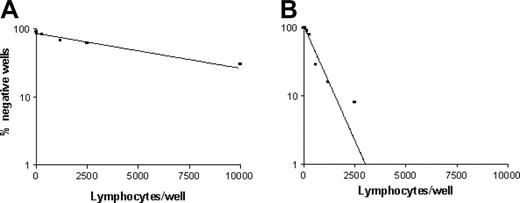
Frequencies of p540 peptide–specific CTLp's in cancer patients. The graphs show the frequency of p540 peptide–specific CTLp's from patients 9 (A) and 14 (B), representative of the 2 groups of cancer patients with low and high frequencies of hTERT-specific CTLp's, respectively.
Frequencies of p540 peptide–specific CTLp's in cancer patients. The graphs show the frequency of p540 peptide–specific CTLp's from patients 9 (A) and 14 (B), representative of the 2 groups of cancer patients with low and high frequencies of hTERT-specific CTLp's, respectively.
Taking into consideration the results originating from both the analysis of frequencies of p540 peptide–specific CTLp's and the generation of p540 peptide–specific CTL lines, presence of telomerase-specific CTLs was demonstrated in 21 (91%) of 23 tested patients by at least one of the 2 procedures (Table 4). Interestingly, both CTLp analysis and CTL line generation did not demonstrate presence of p540-specific cytotoxic lymphocytes in the 2 patients (nos. 7 and 19) in whom the search for p540-binding CD8+ T lymphocytes by tetramers was also negative.
Discussion
The results of our work show that (1) the majority of HLA-A2+ cancer patients have circulating CD8+ T lymphocytes specific for the p540 telomerase peptide; (2) most, but not all, of HLA-A2+ cancer patients have also functionally active CTLs specific for the p540 telomerase peptide; and (3) CTL lines specific for the p540 telomerase peptide expanded ex vivo from cancer patients efficiently kill telomerase-positive tumor cells, including autologous cancer cells.
The development of immunotherapy protocols against cancer based on the expansion/activation of TAA-specific CTLs requires (1) identification of a common TAA whose expression in cancer cells, but not in normal cells, is stable; and (2) the presence in cancer patients of functionally active TAA-specific CTLp's escaping tumor-induced immunosuppression.2 The knowledge collected so far on the generation of telomerase-specific CTLs from cancer patients and healthy subjects suggests that telomerase fulfills point 1.12-15 However, data concerning the frequency of telomerase-specific CTLs in the cancer patient population are scanty and contradictory.20-22 We began our study with the aim to specifically examine this issue. A series of 27 HLA-A2+ cancer patients affected with different neoplasia at various clinical stages were selected. The study was based on a combined procedural approach using tetramers, LDA, and CTL line generation for an internal validation of the results. Such an approach allowed us to achieve information originating from both phenotypic and functional studies, thus avoiding the risk of misinterpreting the results due to the limitation of an incomplete analysis. CD8+ T lymphocytes able to bind p540+ tetramers were found in 90% of studied patients. The percentages of positive cells were quite similar across the cancer population, indicating that histology and staging do not significantly interfere with the presence, frequency, and avidity for antigen of circulating telomerase-specific lymphocytes. These results are in conflict with a previous report from Vonderheide et al21 in a smaller series of cancer patients. It is possible that both the different source of tetramers and the different series of patients could be responsible for the discrepancy. Importantly, the choice of using a panel of negative controls in each test, as suggested in literature,31 could have increased the sensitivity of our analyses. In fact, these kinds of cytometric analyses are greatly affected by the extremely low frequency of the events, and by the consequent slight differences between positive and negative samples. A tetramer constituted by an identical HLA allele and an unrelated peptide was not considered as negative control because of the risk of high unspecific binding to the T-cell receptor (TCR) of the control tetramer that might mask specifically bound events occurring at low frequency.32 Rather, we set the background of each analysis by determining the nonspecific binding of p540+ tetramers to PBMCs of HLA-A2+ and HLA-A2- healthy donors. Interestingly, we did not observe significant tetramer binding to cells derived from any of the 3 control subpopulations; this suggests that the positivity we observed in HLA-A2+ cancer patients is related to the expansion of the reactive, telomerase-specific T-cell subset, a phenomenon specifically occurring in these patients. The fact that we did not detect p540 peptide–specific T cells in HLA-A2+ healthy subjects indicates that telomerase-specific lymphocytes, although likely present, remain unexpanded in the absence of a specific antigenic stimulation mediated by the onset of cancer. The frequency of p540 peptide–specific CD8+ T cells negatively correlated with the staging of disease and positively with the frequency of telomerase-specific CTLp's. These findings may suggest that telomerase-specific cells have a protective role against cancer, limiting disease progression until they are functionally responsive to antigen stimulation in vivo and present at a sufficient concentration. If this were the case, the analysis of p540-specific circulating CD8+ T cells by tetramers could have a prognostic significance and could be adopted in clinics as an easy way to define the immune competence of each patient. A study on a wide series of cancer patients would allow us to pinpoint this topic.
Cytotoxic activity of p540-specific CTL lines. The graphs show the cytotoxic activity of 2 representative p540-specific CTL lines generated from patient nos. 11 (A) and 16 (B), respectively, against HLA-A2+ T2 and BJAB cells as well as HLA-A2- RAJI cells. Effector-target ratio was 40:1. Target cells were as follows: (A) Nonpulsed T2 cells; (B) T2 cells pulsed with the p540 peptide; (C) T2 cells pulsed with the unrelated p13-32 MUC1 peptide (PAHGVTSAPDTRPAPGSTAP); (D) T2 cells pulsed with the p540 peptide plus the anti–HLA class I W6/32 mAb (10 μg/mL); (E) T2 cells pulsed with the p540 peptide plus an unrelated isotype-matched (IgG2a) mAb (10 μg/mL); (F) BJAB cells; (G) BJAB cells plus the anti–HLA class I W6/32 mAb (10μg/mL); (H) BJAB cells plus an unrelated isotype-matched (IgG2a) mAb (10 μg/mL); (I) RAJI cells; (L) RAJI cells plus the anti–HLA class I W6/32 mAb (10 μg/mL); and (M) RAJI cells plus an unrelated isotype-matched (IgG2a) mAb (10 μg/mL).
Cytotoxic activity of p540-specific CTL lines. The graphs show the cytotoxic activity of 2 representative p540-specific CTL lines generated from patient nos. 11 (A) and 16 (B), respectively, against HLA-A2+ T2 and BJAB cells as well as HLA-A2- RAJI cells. Effector-target ratio was 40:1. Target cells were as follows: (A) Nonpulsed T2 cells; (B) T2 cells pulsed with the p540 peptide; (C) T2 cells pulsed with the unrelated p13-32 MUC1 peptide (PAHGVTSAPDTRPAPGSTAP); (D) T2 cells pulsed with the p540 peptide plus the anti–HLA class I W6/32 mAb (10 μg/mL); (E) T2 cells pulsed with the p540 peptide plus an unrelated isotype-matched (IgG2a) mAb (10 μg/mL); (F) BJAB cells; (G) BJAB cells plus the anti–HLA class I W6/32 mAb (10μg/mL); (H) BJAB cells plus an unrelated isotype-matched (IgG2a) mAb (10 μg/mL); (I) RAJI cells; (L) RAJI cells plus the anti–HLA class I W6/32 mAb (10 μg/mL); and (M) RAJI cells plus an unrelated isotype-matched (IgG2a) mAb (10 μg/mL).
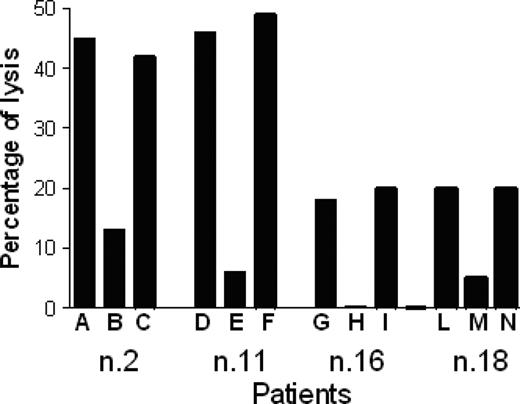
Cytotoxic activity of 4 p540-specific CTL lines generated from patients 2, 11, 16, and 18 against autologous primary prostate cancer cell lines. Percentages of tetramer-positive cells in the different CTL lines were 15%, 18%, 25%, and 20%, respectively. Effector-target ratio was 40:1. The analyses were performed in the absence of mAb (A, D, G, L), and in the presence of either the anti–HLA class I W6/32 mAb (10 μg/mL) (B, E, H, M) or an unrelated isotype-matched control mAb (C, F, I, N).
Cytotoxic activity of 4 p540-specific CTL lines generated from patients 2, 11, 16, and 18 against autologous primary prostate cancer cell lines. Percentages of tetramer-positive cells in the different CTL lines were 15%, 18%, 25%, and 20%, respectively. Effector-target ratio was 40:1. The analyses were performed in the absence of mAb (A, D, G, L), and in the presence of either the anti–HLA class I W6/32 mAb (10 μg/mL) (B, E, H, M) or an unrelated isotype-matched control mAb (C, F, I, N).
Tetramer staining is a useful procedure to measure T lymphocytes able to interact with the specific peptide-HLA complex. However, it does not provide information on the functional status of these cells. To verify the presence of p540 peptide–specific cytotoxic cells in cancer patients we used LDA, for the analysis of CTLp's, and generation of antigen-specific CTL lines. The choice of analyzing the frequency of CTLp's (by LDA) instead of that of activated antigen-specific CTLs (ie, by enzyme-linked immunospot assay [ELISPOT]) rested on the consideration that protective immunologic memory is strictly related to the number of CTLp's.19 Furthermore, it is possible that tumor-dependent mechanisms of tumor immune escape could anergize already activated tumor-specific CTLs, making their identification and quantification difficult.33 This may not be the case of CTLp's due to their preactivation state. Thus, in our opinion, the analysis of the CTLp pool is likely a more reliable indicator of the potential anticancer immune response than the study of the activated CTL pool. Analysis of CTLp frequency showed that in our series cancer patients could be divided in 2 groups with low and high CTLp frequency, respectively. This finding, achieved in a series mainly constituted by patients with prostate cancer, is in agreement with what already observed in patients with melanoma when the frequency of CTLp's specific for a melanoma-associated antigen was studied.34 The low frequency of CTLp's in some patients could be related either with reduced stimulation/expansion of telomerase-specific effector lymphocytes or with their compartmentalization in tissues. It is of interest, however, that in other patients a very high frequency of antitumor CTLp's (patients 11, 14, and 15 in our series) was detected. These levels of TAA-specific CTLp frequency are not unusual in patients with cancer35 and similar to those observed after viral infection.28 This demonstrates that tumors can elicit strong immune responses but also that these responses are not necessarily protective against tumor progression. What determines variability among cancer patients in both intensity and efficacy of anticancer immune response remains to be clarified and could be related to mechanisms of antigen down-regulation by the tumor36-38 and Fas-mediated apoptosis induction in CTLs37,39 as well as the action of regulatory lymphocytes.40
LDA performed with at least 21 days of stimulation time and using antigen-pulsed T2 cells as APCs has overlapping sensitivity with tetramer analysis.34 In fact, comparable frequencies of circulating p540+ tetramer-binding CD8+ T lymphocytes and p540 peptide–specific CTLp's were observed in most patients in whom we had the possibility to perform both analyses. Indeed, the strict relationship existing between tetramer-binding cells and CTLp's is demonstrated by the finding that depletion of tetramer-positive cells from the PBMC population used for LDA analysis in 3 different patients exited in the disappearance of CTLp's. This datum appears relevant since it directly characterizes the peripheral-blood T lymphocytes able to bind tetramers loaded with a universal TAA such as telomerase as CTLp's for the first time. In 4 patients (nos. 3, 8, 9, and 26), a very low CTLp frequency in spite of a relatively high frequency of tetramer-positive cells was observed. This finding could reflect the fact that not all the lymphocytes that are able to bind tetramers are cytotoxic cells (ie, anergized cells [as already demonstrated in patients with melanoma33,41 ] or antigen-specific suppressor lymphocytes).40,42 Indeed, these observations might support the need of an accurate immunologic screening of cancer patients before considering them suitable candidates for immunotherapeutic vaccination protocols against telomerase.
In summary, we studied a series of 23 patients with cancer using a combined approach consisting of tetramer staining, LDA, and CTL line generation. Most (91%) analyzed patients showed presence of p540-specific cytotoxic lymphocytes in their peripheral blood, independently from histologic type and staging. p540-specific CTL lines, generated from 11 patients, were able to lyse not only p540 peptide–pulsed T2 cells but also telomerase-expressing cancer cell lines, including BJAB and LNCap cells as well as, in 4 cases, autologous prostate cancer cells. This is at variance with results reported by Parkhurst et al.22 Differences in both the composition of the respective series of cancer patients and in the methodologies (cytotoxic assay vs ELISPOT, culture conditions affecting HLA expression), as well as possible biologic diversity in patients related to tumor antigen expression and/or tumor-mediated immune-escape mechanisms, may explain the discrepancy between the 2 reports.
In conclusion, our data suggest that the pool of p540-specific cytotoxic lymphocytes is expanded in many (but not all) HLA-A2+ cancer patients, and likely has a role in limiting cancer progression. This view is supported by the observation that p540-specific CTL lines from 4 patients with prostate cancer were able to kill autologous cancer cells. Thus, our findings provide support to the development of clinical protocols using telomerase peptides as an immunizing agent in cancer patients.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-01-0258.
Supported by grants from Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) National Program “Analisi della funzione e della distribuzione dei linfociti T immunoregolatori in pazienti affette da tiroidite autoimmune con dimostrata presenza di microchimerismo” (no. 2002065218_007), from Comitato Interministeriale per la Programmazione Economica (CIPE) (02/07/2004, CBA project), and from Compagnia di San Paolo, Torino, “Analysis of frequency and functional activity of telomerase-specific CD8+ T lymphocytes and CD8+ T suppressor lymphocytes in cancer patients.”
G.F. designed and contributed in performing research (CTL line generation, patient selection and follow up) and wrote the paper; M.F. performed research (limiting dilution assay, CTL line generation); M.S. performed research (tetramer analysis) and contributed in writing the paper; P.T. performed research (selection and clinical follow up, surgery); E.M. performed research (peptide synthesis); D.F. performed research (tetramer analysis and sorting of tetramer-positive cells); S.N. performed research (tetramer analysis); F.F. performed research (real-time PCR); A.R. performed research (selection and clinical follow up of patients); M.B. performed research (patient selection and follow up, tetramer analysis); P.C. performed research (CTL line generation); M.R. performed research (real-time PCR); M.G. participated in designing research; U.B. participated in designing research; G.D. participated in designing research and performing research (peptide synthesis); J.L.R. performed research (pathology analyses on cancer cell lines); G.C. performed research (surgery, clinical follow up); M.Z. designed research and wrote paper; and F.I. designed and coordinated research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.