Recently, we published the existence of 2 populations of anti-β2-glycoprotein I (β2-GPI) IgG antibodies. Type A antibodies recognize epitope G40-R43 in domain I of β2-GPI and are strongly associated with thrombosis. Type B antibodies recognize other parts of β2-GPI and are not associated with thrombosis. In this study we demonstrate that type A antibodies only recognize plasma-purified β2-GPI when coated onto a negatively charged surface and not when coated onto a neutrally charged surface. The affinity of type B antibodies toward plasma-purified β2-GPI was independent of the charge of the surface to which β2-GPI was coated. Type A antibodies did not recognize plasma-purified β2-GPI in solution, whereas they did recognize recombinant β2-GPI both in solution and coated onto a neutrally charged plate. When the carbohydrate chains were removed from plasma-purified β2-GPI, we found that type A antibodies did recognize the protein in solution. This supports the hypothesis that the difference in recognition of plasma-purified and recombinant β2-GPI is caused by the difference in glycosylation and that epitope G40-R43 of plasma-purified β2-GPI is covered by a carbohydrate chain. Type A anti-β2-GPI antibodies can only recognize this epitope when this carbohydrate chain is displaced as a result of a conformational change. This finding has major implications both for the detection of pathogenic anti-β2-GPI antibodies and the comprehension of the pathophysiology of the antiphospholipid syndrome.
Introduction
The antiphospholipid syndrome is a systemic autoimmune disease that is characterized serologically by the presence of antiphospholipid antibodies in plasma of patients and clinically by vascular thrombosis and/or recurrent pregnancy morbidity.1-3 In 1990 it was shown that these so-called antiphospholipid antibodies do not recognize phospholipids directly but a phospholipid-binding protein: β2-glycoprotein I (β2-GPI).4 Ever since, antiphospholipid antibodies with affinity for a large number of other phospholipid-binding proteins have been described. Only antiphospholipid antibodies with affinity for β2-GPI are thought to be clinically relevant.5 Several assays are available to detect anti-β2-GPI antibodies. The most common one is the anticardiolipin enzyme-linked immunosorbent assay (ELISA). In this assay the antibodies recognize β2-GPI bound to immobilized cardiolipin.6 A second assay is the lupus anticoagulant assay which detects anti-β2-GPI antibodies with capacity to prolong phospholipid-dependent coagulation assays (LAC).7 A third method is an ELISA-based method in which antibodies recognize β2-GPI coated directly onto ELISA plates.3
β2-GPI (formerly known as apolipoprotein H) is a protein of 44 kDa with a plasma concentration of approximately 150 μg/mL. β2-GPI is highly glycosylated because it contains 4 N-linked carbohydrate side chains. Together these carbohydrate side chains account for approximately 15% (wt/wt) of the total molecular mass of β2-GPI. The protein β2-GPI consists of 326 amino acids organized in 5 complement control protein domains.8,9 Each domain consists of 60 amino acids, except for domain V. Domain V consists of 82 amino acids, caused by a C-terminal extension of 19 amino acids and an insertion of 6 amino acids, forming a hydrophobic loop. This specific structure of domain V is responsible for the binding properties of β2-GPI to anionic phospholipids.8,9
Although the 3-dimensional structure of β2-GPI was known for more than 5 years, the mechanism by which anti-β2-GPI antibodies recognize β2-GPI is unclear.9,10 Two main theories have been proposed to explain the binding of antiphospholipid antibodies to β2-GPI. The first and most accepted hypothesis is known as the “dimerization theory”; one antibody must bind 2 β2-GPI molecules to obtain considerable avidity.11-14 To achieve this, a high density of β2-GPI on an ELISA plate is essential. It has been reported that the coated amount of β2-GPI must exceed a certain threshold before antiphospholipid antibodies are able to bind.15 The studies in favor of the dimerization theory seem rather convincing, but several observations remain unexplained and are in favor of a second hypothesis. This hypothesis is based on the recognition of a cryptic epitope by antiphospholipid antibodies. This epitope is only exposed after binding of β2-GPI to a negatively charged surface.16-18 Supporting this latter hypothesis is the fact that the structure of β2-GPI in solution differs from that of crystallized β2-GPI.9,10,19 Moreover, no β2-GPI-antibody complexes can be detected in the circulation of patients with the antiphospholipid syndrome (B.d.L., unpublished observations, May 2004). Furthermore, it was shown by circular dichroism measurements that cardiolipin can induce a conformational change in β2-GPI.16
We have recently published that the population of anti-β2-GPI antibodies recognizing epitope G40-R43 (type A antibodies) cause LAC and strongly correlate with thrombosis (odds ratio, 18.9; 95% confidence interval, 53.2-6.8). The other group of anti-β2-GPI antibodies (type B antibodies) recognized other parts of β2-GPI and did not correlate with thrombosis (1.1; 95% confidence interval, 2.8-0.4). In this study we have further investigated the epitope(s) on β2-GPI recognized by type A and type B anti-β2-GPI antibodies.
Patients, materials, and methods
Patients
Fifty-two patient plasma samples positive for ant-β2-GPI IgG antibodies were included in this study; 36 patients with systemic lupus erythematosus (SLE), 11 patients with lupuslike disease (LLD), and 5 patients with primary antiphospholipid syndrome. Patients with SLE meet at least 4 ACR (American College of Rheumatology) criteria for the classification of SLE, and patients with LLD met 1 to 3 of these criteria.20 Patients with primary antiphospholipid syndrome have antiphospholipid antibodies (LAC and/or anticardiolipin antibodies) and a history of thrombosis in the absence of any sign of a systemic autoimmune disease. By chart review the number of objectively verified thromboembolic events was recorded for each patient.5 For the diagnosis of thrombosis of intracerebral vessels, computed tomographic scanning or magnetic resonance imaging was used. Myocardial infarction was diagnosed by typical electrocardiographic features and an elevated fraction creatine kinase myoglobin (CK-MB). Peripheral arterial thrombosis and thrombosis of the distal aorta were diagnosed by arteriography. Retinal thrombosis was documented by funduscopy and fluorescence angiography. Deep vein thrombosis (DVT) was diagnosed by ultrasonography or venography; pulmonary embolism was diagnosed by radionuclide lung scanning. Portal vein thrombosis was diagnosed by angiography. A patient was diagnosed with superficial thrombophlebitis if the manifestation was diagnosed clinically. Blood was drawn from these patients at an arbitrary visit at the lupus clinic of the University Medical Center Utrecht, Utrecht, the Netherlands. The Institutional Review Board of the University Medical Center Utrecht approved this study, and informed consent was obtained from all patients.
Serologic assays
Anti-β2-GPI IgG antibody ELISA. Antibodies against β2-GPI were measured in an ELISA as described before (interassay variability, 11.5%).21 In short, plasma-purified β2-GPI (10 μg/mL diluted in a Tris based solution [TBS]: 50 mM Tris in 100 mM NaCl) was incubated onto hydrophilic ELISA plates (catalog no. 9102; Costar, New York, NY). After 1 hour the plates were incubated with 4% bovine serum albumin (BSA) in TBS for 1 hour. This was followed by incubation with patient plasma (1:50 diluted in blocking solution) for 1 hour. Then the plates were incubated with a goat anti-human IgG alkaline-phosphatase-labeled antibody (diluted 1:1000; Biosource, Camarillo, CA) followed by staining with para-nitrophenyl phosphatase (PnPP; 0.6 mg/mL diluted in diethynolamine [DEA] buffer; Sigma, St Louis, MO). Plasma samples were regarded positive when the absorbed value exceeded the cut-off value (mean + 3 SD of 40 healthy volunteers) and corrected for the absorption of standard positive plasma.
LAC. To determine LAC activity, an activated partial thromboplastin time (APTT) and a dilute Russell viper venom time (DRVVT) were performed.5 For the APTT (PTT-LA, interassay variability 2.0%; Diagnostica Stago, Gennevilliers, France), 50 μL patient plasma was diluted 1:1 with normal pool plasma of 40 healthy volunteers and incubated with 50 μL APTT reagent. Coagulation was initiated by the addition of 50 μL CaCl2 (25 mM). As a control, samples were tested in an actin-FS-based APTT (APTT-FS; Dade Behring, Marburg, Germany) which is a LAC-insensitive assay. Patients were considered positive when the ratio PTT-LA/APTT-FS was greater than 1.20.10 The DRVVT was performed according to the instructions of the manufacturer (interassay variability, 3.2%; Gradipore Ltd, North Ryde, Australia) and considered positive when LAC screen/LAC confirmation was greater than 1.20. A patient was considered LAC positive if 1 of the 2 LAC assays was positive.
Anticardiolipin antibody ELISA. Anticardiolipin antibodies were measured in an ELISA as described before (interassay variability, 6.9%).22 Nine IgG/IgM calibrators were used to report anticardiolipin antibody levels as GPL or MPL units. Levels above 10 GPL or GPM units were considered positive.
Anti-domain I IgG ELISA. We recently described a method in which we were able to discriminate between anti-β2-GPI IgG antibodies with reactivity toward epitope G40-R43 in domain I (type A antibodies) and anti-β2-GPI IgG antibodies with other reactivity (type B antibodies).23 In short, hydrophilic plates (catalog no. 9102; Costar) and hydrophobic plates (catalog no. 2595; Costar) were coated with domain I of β2-GPI (5 μg/mL diluted in TBS) for 1 hour at 37°C. After every incubation step the plates were washed 3 times with 0.1% Tween/TBS. The plates were blocked with 4% BSA/0.1% Tween/TBS and subsequently incubated with patient plasma (diluted 1:100 in blocking solution) containing anti-β2-GPI IgG antibodies. The bound IgG antibodies were detected by a goat anti-human IgG alkaline-phosphatase-labeled antibody (diluted 1:1000; Biosource). This was followed by staining with PnPP (0.6 mg/mL in DEA solution). Anti-β2-GPI IgG antibodies were regarded as type A antibodies when these showed decreased affinity for domain I coated onto hydrophilic plates compared with that with domain I coated onto hydrophobic plates. Anti-β2-GPI IgG antibodies were regarded as type B antibodies when they showed equal affinity for domain I on both plates. A ratio of greater than 2 between the OD measured with the hydrophobic plate and the OD with the hydrophilic plate discriminates between type A and type B anti-β2-GPI IgG antibodies. This ratio is an indication for the relative amount of anti-β2-GPI IgG antibodies that recognize the positive charge (epitope G40-R43) on domain I (interassay variability, 3.8%).
Purification of β2-GPI from plasma
Human plasma was dialyzed against a solution containing 0.04 M Tris, 0.01 M succinate, 0.005% polybrene, 1 mM EDTA, 1 mM benzamidin, 43 mM NaCl, and 0.02% NaN3. Then, the plasma was added to DEAE-Sephadex column, and the flow-through was collected, pooled, and added to a protein-G-Sepharose column. Then the effluent pool was added to a mono-S Sepharose column. The bound β2-GPI was eluted by a linear salt-gradient (138-550 mM NaCl), and checked for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).24 The purified β2-GPI was analyzed with gel filtration and showed a single peak at the expected molecular weight.
Recombinant β2-GPI and domain deleted mutants of β2-GPI
Recombinant full-length β2-GPI (DI-V) and 8 domain deletion mutants of β2-GPI (comprising domain I [DI]; domains I and II [DI-II]; domains I, II, and III [DI-III]; domains I, II, III, and IV [DI-IV]; domains II, III, IV, and V [DII-V]; domains III, IV, and V [III-V]; domains IV and V [DIV-V]; and domain V [DV]) were obtained by a generous gift from Dr M. Iverson of La Jolla Pharmaceutical Company.25 The recombinant full-length β2-GPI was analyzed with gel filtration and showed a single peak at the expected molecular weight. On SDS-PAGE electrophoresis the molecular weight of recombinant β2-GPI was slightly lower than the molecular weight of plasma-purified β2-GPI.
Purification of immunoglobulin G (IgG) antibodies from plasma samples
Blood samples were collected by venipuncture using plastic tubes containing 3.8% trisodium citrate (0.129 M) as the anticoagulant (9:1, vol/vol). To obtain platelet-poor plasma the samples were centrifuged twice at 2000g for 10 minutes, and subsequently stored at -50°C until further use. The IgG fraction was purified by the use of a protein G-Sepharose column. By adding the IgG fraction to a column coated with β2-GPI, anti-β2-GPI antibodies were purified. The purity of the (affinity-) purified IgG fractions was checked by using SDS-PAGE.
Binding studies with β2-GPI coated onto hydrophobic plates or hydrophilic plates
Hydrophilic plates (catalog no. 9102; Costar) and hydrophobic plates (catalog no. 2595; Costar) were incubated with 50 μL plasma-purified β2-GPI or recombinant β2-GPI (10 μg/mL in TBS) for 1 hour at 37°C. Subsequently, the plates were washed 3 times with washing solution (TBS/0.1% Tween) and blocked with 150 μL blocking solution (4% BSA)/TBS/0.1% Tween) for1 hour at 37°C. The plates were washed 3 times and incubated with either patient plasma (diluted 1:50), patient IgG antibodies (type A and type B), monoclonal antibody 4F3, monoclonal antibody 2B2, or monoclonal antibody 27G7 in various concentrations (diluted in blocking buffer), for 1 hour at 37°C.26,27 Monoclonal antibodies 2B2 and 27G7 were kindly provided by Dr A. Tincani and Dr J. Arnout, respectively. To detect the bound monoclonal antibodies, the plates were washed and incubated with 50 μL rabbit anti-mouse peroxidase-labeled antibody (diluted 1:1000; Dako, Glostrup, Denmark), followed by staining with an ortho-phenylene diamine solution (OPD solution; 4 mg/mL OPD diluted in 0.1 M NaH2PO4.2H2O/0.1 M Na2HPO4.H2O). The coloring reaction was stopped with the addition of 1 M H2SO4, and absorbance was measured at 490 nm. To detect the bound patient IgG antibodies, the plates were incubated with an alkaline-phosphatase-labeled goat anti-human IgG antibody (diluted 1:1000; Biosource). PnPP was used as coloring substance. The reaction was stopped by the addition of 2.4 M NaOH, and absorbance was measured at 405 nm.
Binding of patient-derived IgG antibodies to recombinant and plasma-purified β2-GPI in solution
Hydrophilic ELISA plates (catalog no. 9102; Costar) were coated with 10 μg/mL plasma-purified β2-GPI for 1 hour at 37°C. The plates were washed 3 times with washing solution (TBS/0.1% Tween) and blocked with 150 μL blocking solution (4% BSA/TBS/0.1% Tween) for 1 hour at 37°C. Then, the patient-derived IgG type A antibodies (total IgG fraction) were incubated to the plate in the presence of various concentrations of plasma-purified β2-GPI and recombinant β2-GPI. The patient-derived IgG antibodies bound to the plate were detected by using an alkaline-phosphatase-labeled goat anti-human IgG antibody (diluted 1:1000; Biosource). PnPP (0.6 mg/mL diluted in DEA buffer) was used as coloring substance. The reaction was stopped by the addition of 2.4 M NaOH, and absorbance was measured at 405 nm.
Recognition of plasma-purified β2-GPI in solution in the presence of cardiolipin vesicles and phosphatidylserine/phosphatidylcholine (PS/PC) vesicles
Affinity-purified patient IgG type A antibodies (20 μg/mL) of patient 1 and patient 2 were diluted in 150 mM Na2CO3.10 H2O/350 mM NaHCO3 (pH 9.6) to reach a final concentration of 20 μg/mL and were coated onto hydrophobic ELISA plates (catalog no. 2595; Costar) for 12 hours at 4°C. The plates were washed 3 times with TBS/0.1% Tween and subsequently blocked with 4% BSA/TBS/0.1% at 37°C. After 1 hour the plates were washed 3 times and incubated with plasma-purified β2-GPI (10 μg/mL), plasma-purified β2-GPI (10 μg/mL) in the presence of cardiolipin vesicles (20 μM), or plasma β2-GPI (10 μg/mL) in the presence of PS/PC vesicles (20%/80%, 20 μM), all diluted in blocking solution. The cardiolipin and PS/PC vesicles were prepared as described before.7,28 Then the plates were washed and incubated with a polyclonal goat anti-human anti-β2-GPI antibody (10 μg/mL). After washing, the plates were incubated with a rabbit anti-goat peroxidase-labeled antibody (diluted 1:1000; Biosource). Coloring was performed by using OPD. The coloring reaction was stopped by the addition of 1 M H2SO4, and absorbance was measured at 490 nm.
Influence of aggregating plasma-purified β2-GPI on the recognition by type A antibodies
To aggregate plasma-purified β2-GPI, the protein was incubated with 1% glutaraldehyde for 15 minutes at room temperature. Then, the solution was dialyzed against TBS and analyzed by using SDS-PAGE.
The aggregated β2-GPI, plasma-purified β2-GPI, and recombinant β2-GPI were coated onto a hydrophobic ELISA plate (catalog no. 2595; Costar), at a concentration of 10 μg/mL for 1 hour at 37°C. Then, the plates were blocked with 4% BSA/TBS/0.1% Tween. After washing the plates, they were incubated with 2 type A plasma samples (1:100 diluted in blocking solution) for 1 hour at 37°C. The bound IgG antibodies were detected with an alkaline-phosphatase-labeled goat anti-human IgG antibody (diluted 1:1000; Biosource), and coloring was performed by using PnPP. The coloring reaction was stopped by the addition of 2.4 M NaOH, and absorbance was measured at 405 nm.
For the inhibition ELISA we coated 10 μg/mL plasma-purified β2-GPI to a hydrophilic ELISA plate (catalog no. 2595; Costar). The plates were washed 3 times with TBS/0.1% Tween and subsequently blocked with 4% BSA/TBS/0.1% at 37°C. After 1 hour the plates were washed 3 times and incubated with 10 μg/mL anti-β2-GPI antibodies of patient 1 (type A) in the presence of either 100 μg/mL plasma-purified β2-GPI, 100 μg/mL recombinant β2-GPI, or 100 μg/mL glutaraldehyde-treated β2-GPI. The antibodies that bound the coated β2-GPI were detected with an alkaline-phosphatase-labeled goat anti-human IgG antibody (diluted 1:1000; Biosource), and coloring was performed by using PnPP. The coloring reaction was stopped by the addition of 2.4 M NaOH, and absorbance was measured at 405 nm.
Removal of the carbohydrate side chains of plasma-purified β2-GPI
β2-GPI was deglycosylated by incubating 50 μL sample (β2-GPI, 1.1 mg/mL diluted in TBS) with 5 units N-Glycosidase F (Roche, Mannheim, Germany) for 24 hours at 37°C. The extent of deglycosylated β2-GPI was analyzed by using SDS-PAGE. The deglycosylated β2-GPI was analyzed with gel filtration and showed a single peak at the expected molecular weight.
Production of F(ab) fragments of monoclonal antibody 4F3
First, monoclonal antibody 4F3 was dialyzed against 100 mM sodium acetate (pH 6.2). Then cysteine, EDTA, and sodiumazide were added to the antibody solution to obtain a final concentration of subsequently 50 mM, 1 mM, and 0.05%. This was followed by the addition of papain (10 μg papain/1 mg antibody) (Sigma; P3125). After incubating the solution for 8 hours at 37°C, iodoacetamide was added (final concentration, 75 mM). By using a protein G-coupled Sepharose column the Fc parts of the antibodies were removed from the solution, and the purity of the F(ab) fragments was analyzed by using SDS-PAGE.
Binding of patient IgG to plasma-purified β2-GPI, deglycosylated β2-GPI, and recombinant β2-GPI in solution
Affinity-purified patient IgG type A antibodies (20 μg/mL) of patient 1 and patient 2, monoclonal antibody 4F3 (3 μg/mL), F(ab) fragments of monoclonal antibody 4F3 (15 μg/mL), and monoclonal antibody 2B2 (3 μg/mL) were diluted in 150 mM Na2CO3.10 H2O/350 mM NaHCO3 (pH 9.6) and coated onto hydrophobic ELISA plates (catalog no. 2595; Costar), for 12 hours at 4°C. The plates were washed 3 times with TBS/0.1% Tween and subsequently blocked with 4% BSA/TBS/0.1% at 37°C. After 1 hour the plates were washed 3 times and incubated with different concentrations of plasma-purified β2-GPI, deglycosylated plasma-purified β2-GPI, or recombinant β2-GPI, all diluted in blocking solution. Then the plates were washed and incubated with a polyclonal goat anti-human anti-β2-GPI antibody. After washing, the plates were incubated with a rabbit anti-goat peroxidase-labeled antibody (diluted 1:1000). Coloring was performed by using OPD. The coloring reaction was stopped by the addition of 1 M H2SO4, and absorbance was measured at 490 nm.
Domain specificity of patient IgG antibodies and monoclonal antibodies 4F3 and 2B2
The reactivity of antibodies against β2-GPI domain deletion mutants was tested by coating 96-well hydrophobic ELISA plates (catalog no. 2595; Costar) with deletion mutants in a concentration of 10 μg/mL for 1 hour at 37°C. The plates were washed 4 times with TBS/0.1% Tween and subsequently blocked with a 4% BSA/TBS solution. Patient IgG fractions (10 μg/mL) and monoclonal antibodies 4F3 and 2B2 (both 3 μg/mL) were diluted in blocking solution and added to the wells (50 μL/well, 1 hour at 37°C). The plates were washed 4 times (0.1% Tween/TBS) and incubated with alkaline-phosphatase-labeled goat anti-human IgG antibodies (diluted 1/1000; Biosource) to detect the bound patient IgG antibodies (1 hour at 37°C). Staining was performed with PnPP (Sigma) at a concentration of 0.6 mg/mL diluted in DEA buffer. The coloring reaction was stopped with 2.4 M NaOH, and absorbance was measured at 405 nm. The bound monoclonal antibodies were detected by peroxidase-labeled rabbit antimouse antibodies, and coloring was performed with an OPD solution. The coloring reaction was stopped by 1 M H2SO4, and absorbance was measured at 490 nm.
Influence of increasing NaCl concentration on the recognition of β2-GPI by anti-β2-GPI IgG antibodies
Hydrophilic and hydrophobic plates (catalog nos. 9102 and 2595; Costar) were incubated with 10 μg/mL plasma-purified β2-GPI or recombinant β2-GPI for 1 hour at 37°C. After washing, the plates were blocked with 4% BSA/TBS/0.1%Tween at 37°C for 1 hour. Subsequently the plates were washed and incubated with patient plasma diluted (1:100) in blocking solution containing either 150 mM NaCl or 5 M NaCl. After washing, the plates were incubated with a alkaline-phosphatase-labeled goat anti-human IgG antibody (diluted 1:1000). Coloring was performed by using PnPP. The coloring reaction was stopped by the addition of 2.4 M NaOH, and absorbance was measured at 405 nm.
Results
Serologic and clinical characterization of the patient population
Thirty (58%) of 52 plasma samples with anti-β2-GPI IgG antibodies tested positive in the anti-domain I IgG ELISA (type A antibodies). Twenty-two (42%) plasma samples tested negative in the anti-domain I IgG ELISA (type B antibodies). All 52 (100%) plasma samples tested positive for anticardiolipin antibodies. Thirty-four (65%) plasma samples showed LAC activity. Of these, 28 (82%) were positive for type A and 6 (18%) for type B antibodies.
Twenty-five (83%) of 30 patients with type A antibodies had a history of thrombosis. Of these, 16 (53%) patients had a history of venous thrombosis and 12 (40%) of arterial thrombosis. Seven (32%) patients with type B antibodies had a history of thrombosis. Of these, 5 (23%) had a history of venous thrombosis and 4 (18%) a history of arterial thrombosis.
Recognition of β2-GPI when coated onto different types of plates
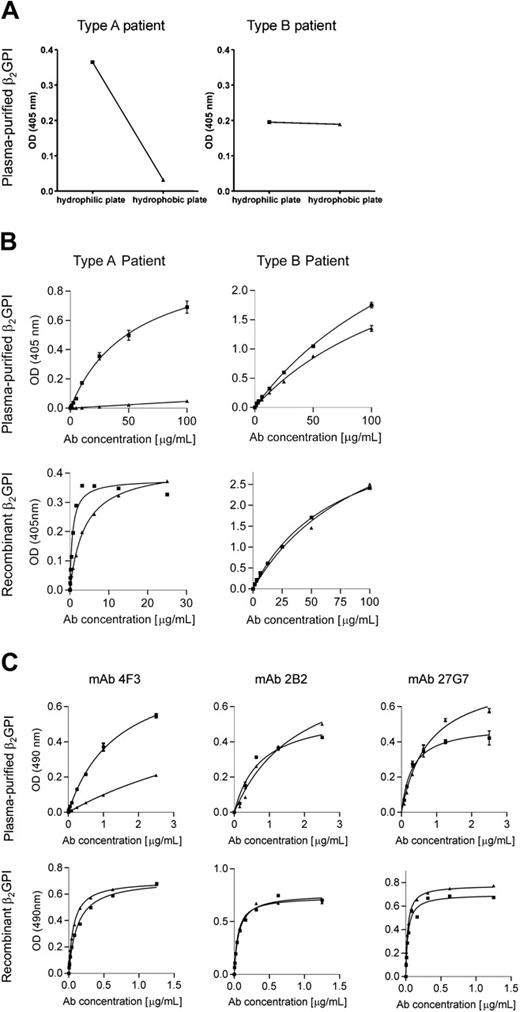
To investigate whether different coating conditions influence the recognition of β2-GPI by type A and type B IgG antibodies, plasma-purified β2-GPI was coated onto hydrophilic and hydrophobic ELISA plates. Type A patient plasma samples (n = 30) recognized plasma-purified β2-GPI coated onto a hydrophilic plate much better than when coated onto a hydrophobic plate (median OD of 0.262 versus median OD of 0.010, P = .001). This is a reduction of 96% (OD, 0.252). For type B plasma samples (n = 22) we did not observe a difference in recognition when plasma-purified β2-GPI coated onto either a hydrophilic plate or a hydrophobic plate (median OD of 0.091 versus median OD of 0.076, no significant difference). This is a reduction of 16% (OD, 0.015). For both type A and type B plasma a typical example is shown in Figure 1A.
For total IgG, we performed this type of experiment with 3 total IgG samples of each type of plasma. All patients with type A we tested had venous thrombosis in the past, and 1 of the 3 patients with type B had venous thrombosis. We observed the same pattern as with the plasma samples; Type A IgG antibodies showed a high affinity for plasma-purified β2-GPI coated onto hydrophilic plates but showed negligible affinity for plasma-purified β2-GPI coated onto hydrophobic plates. Type B IgG antibodies showed equal affinity for plasma-purified β2-GPI coated onto hydrophilic or hydrophobic plates. Both type A and type B IgG antibodies had similar affinity for recombinant β2-GPI coated onto hydrophilic or hydrophobic plates. For both type A and type B IgG a typical example is shown in Figure 1B.
Patient-derived antibodies (and monoclonal antibody 4F3) do not recognize plasma-purified β2-GPI coated onto a hydrophobic plate. Plasma-purified β2-GPI and recombinant β2-GPI were coated onto hydrophilic (▪) and hydrophobic (▴) plates. Then, the plates were incubated with either patient plasma (A), patient IgG antibodies (B), monoclonal antibody 4F3, monoclonal antibody 2B2, or monoclonal antibody 27G7 (C). The bound mAbs were detected by a rabbit anti-mouse peroxidase-labeled antibody, and coloring was performed by OPD. To detect the bound patient IgG antibodies, the plates were incubated with an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. The obtained optical density (OD) was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (panel A hydrophilic plate: OD, 0.087; hydrophobic plate: OD, 0.130). Error bars represent mean ± SEM of duplicate points. Ab indicates antibody.
Patient-derived antibodies (and monoclonal antibody 4F3) do not recognize plasma-purified β2-GPI coated onto a hydrophobic plate. Plasma-purified β2-GPI and recombinant β2-GPI were coated onto hydrophilic (▪) and hydrophobic (▴) plates. Then, the plates were incubated with either patient plasma (A), patient IgG antibodies (B), monoclonal antibody 4F3, monoclonal antibody 2B2, or monoclonal antibody 27G7 (C). The bound mAbs were detected by a rabbit anti-mouse peroxidase-labeled antibody, and coloring was performed by OPD. To detect the bound patient IgG antibodies, the plates were incubated with an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. The obtained optical density (OD) was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (panel A hydrophilic plate: OD, 0.087; hydrophobic plate: OD, 0.130). Error bars represent mean ± SEM of duplicate points. Ab indicates antibody.
Monoclonal antibody 4F3 showed the same recognition pattern as type A IgG antibodies (Figure 1C). For 2 other monoclonal antibodies, 2B2 and 27G7, the recognition of β2-GPI was independent of the type of plate (Figure 1C). This indicates that both types of ELISA plates bound comparable amounts of plasma-purified β2-GPI and recombinant β2-GPI.
Recognition of β2-GPI in solution
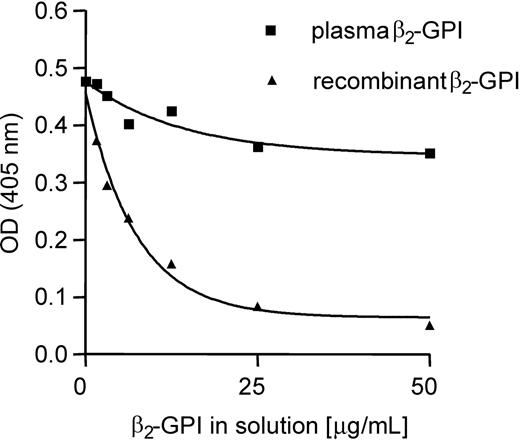
To further investigate the differences in recognition of plasma-purified β2-GPI and recombinant β2-GPI by type A IgG antibodies, we performed several experiments with β2-GPI in solution. We coated plasma-purified β2-GPI to a hydrophilic ELISA plate and studied whether plasma-purified β2-GPI and recombinant β2-GPI were able to inhibit the binding of patient type A IgG antibodies to the coated plasma-purified β2-GPI on the plate. Both patients with type A tested in this experiment had venous thrombosis in the past. Figure 2 shows that the binding to the coated β2-GPI could be completely inhibited when patient type A IgG antibodies were preincubated with recombinant β2-GPI. In contrast, the binding of patient type A IgG antibodies to coated β2-GPI could only be inhibited for approximately 25% by plasma-purified β2-GPI.
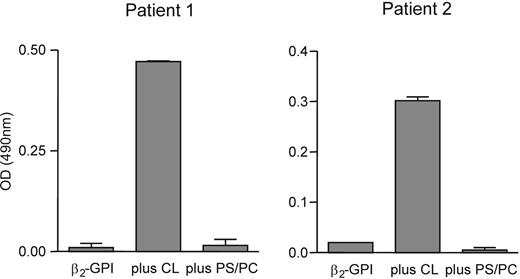
To expand this observation, we coated affinity-purified patient type A IgG antibodies to a hydrophobic ELISA plate. Subsequently, the plate was incubated with plasma-purified β2-GPI, plasma-purified β2-GPI in the presence of cardiolipin vesicles, and plasma-purified β2-GPI in the presence of PS/PC vesicles. We found that the patient type A IgG antibodies recognized plasma-purified β2-GPI only in the presence of cardiolipin vesicles (Figure 3).
Influence of aggregating of β2-GPI on the recognition of β2-GPI by type A antibodies
Galazka et al29 described that modifying β2-GPI with glutaraldehyde induces the exposure of a cryptic epitope that is recognized by anti-β2-GPI antibodies. We investigated whether this modification influences the recognition of plasma-purified β2-GPI by type A antibodies. We coated plasma-purified β2-GPI, recombinant β2-GPI, and plasma β2-GPI modified by glutaraldehyde onto a hydrophobic ELISA plate. Two randomly chosen plasma samples positive for type A antibodies (with a history of venous thrombosis) were incubated to the plate, and the bound IgG antibodies were detected. As with purified IgG antibodies in Figure 1B, we found that both plasma samples recognized recombinant β2-GPI (Figure 4). Plasma-purified β2-GPI was hardly recognized by both samples, but treatment with glutaraldehyde induced binding of both samples to plasma-purified β2-GPI, to an extent that was comparable to recombinant β2-GPI.
Binding of type A IgG antibodies to β2-GPI in solution. Hydrophilic ELISA plates were coated with 10 μg/mL plasma-purified β2-GPI, and patient type A IgG antibodies were incubated to the plate in the presence of various concentrations of plasma-purified β2-GPI and recombinant β2-GPI. The patient type A IgG antibodies bound to the plate were detected by using an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. The reaction was stopped by the addition of 2.4 M NaOH, and absorbance was measured at 405 nm. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (OD, 0.168).
Binding of type A IgG antibodies to β2-GPI in solution. Hydrophilic ELISA plates were coated with 10 μg/mL plasma-purified β2-GPI, and patient type A IgG antibodies were incubated to the plate in the presence of various concentrations of plasma-purified β2-GPI and recombinant β2-GPI. The patient type A IgG antibodies bound to the plate were detected by using an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. The reaction was stopped by the addition of 2.4 M NaOH, and absorbance was measured at 405 nm. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (OD, 0.168).
Type A IgG antibodies recognize plasma-purified β2-GPI in solution only in the presence of cardiolipin. Affinity-purified type A IgG antibodies (20 μg/mL) of patient 1 and patient 2 were coated onto hydrophobic ELISA plates and incubated with plasma-purified β2-GPI, plasma-purified β2-GPI in the presence of cardiolipin vesicles, or plasma β2-GPI in the presence of PS/PC vesicles (20%/80%). Then, the plates were incubated with a polyclonal goat anti-human anti-β2-GPI antibody. Subsequently, the plates were incubated with a rabbit anti-goat peroxidase-labeled antibody. Coloring was performed by using OPD. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (incubation with plasma β2-GPI: OD, 0.086; incubation with PS/PC: OD, 0.087; incubation with CL: OD, 0.088).CL indicates cardiolipin vesicles. Error bars represent mean ± SEM of triplicate points.
Type A IgG antibodies recognize plasma-purified β2-GPI in solution only in the presence of cardiolipin. Affinity-purified type A IgG antibodies (20 μg/mL) of patient 1 and patient 2 were coated onto hydrophobic ELISA plates and incubated with plasma-purified β2-GPI, plasma-purified β2-GPI in the presence of cardiolipin vesicles, or plasma β2-GPI in the presence of PS/PC vesicles (20%/80%). Then, the plates were incubated with a polyclonal goat anti-human anti-β2-GPI antibody. Subsequently, the plates were incubated with a rabbit anti-goat peroxidase-labeled antibody. Coloring was performed by using OPD. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (incubation with plasma β2-GPI: OD, 0.086; incubation with PS/PC: OD, 0.087; incubation with CL: OD, 0.088).CL indicates cardiolipin vesicles. Error bars represent mean ± SEM of triplicate points.
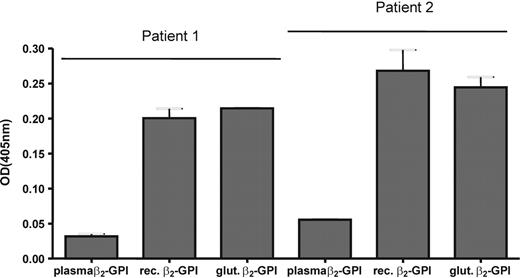
We performed an inhibition experiment with the anti-β2-GPI antibodies of patient 1. Plasma-purified β2-GPI was coated to a hydrophilic plate, and antibodies of patient 1 were incubated to the plate in the presence of plasma-purified β2-GPI, recombinant β2-GPI, or glutaraldehyde-treated plasma-purified β2-GPI. We observed a maximal decrease in signal of 15.2% when the antibodies were incubated with plasma-purified β2-GPI. For glutaraldehyde-treated plasma-purified β2-GPI we observed a decrease of 66.7%, which was comparable to recombinant β2-GPI (63.0%).
Type A IgG antibodies recognize plasma-purified β2-GPI after treatment with glutaraldehyde. A hydrophobic ELISA plate was coated with plasma-purified β2-GPI (10 μg/mL), recombinant β2-GPI (10 μg/mL), and plasma-purified β2-GPI treated with glutaraldehyde (10 μg/mL). After blocking the plates, plasma of 2 patients with type A antibodies was added to the wells (1:100 dilution in blocking solution). The bound patient IgG antibodies were detected with an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (OD, 0.126). Error bars represent mean ± SEM of quadruple points.
Type A IgG antibodies recognize plasma-purified β2-GPI after treatment with glutaraldehyde. A hydrophobic ELISA plate was coated with plasma-purified β2-GPI (10 μg/mL), recombinant β2-GPI (10 μg/mL), and plasma-purified β2-GPI treated with glutaraldehyde (10 μg/mL). After blocking the plates, plasma of 2 patients with type A antibodies was added to the wells (1:100 dilution in blocking solution). The bound patient IgG antibodies were detected with an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (OD, 0.126). Error bars represent mean ± SEM of quadruple points.
Importance of carbohydrate side chains for the recognition of β2-GPI in solution
There is no difference in amino acid composition between plasma-purified β2-GPI and recombinant β2-GPI (data not shown); therefore, the difference in recognition by monoclonal antibody 4F3 and patient type A IgG antibodies might be due to a difference in glycosylation. By using N-Glycosidase F we removed the carbohydrate side chains from plasma-purified β2-GPI (Figure 5A). Then, affinity-purified patient type A IgG antibodies (both with a history of venous thrombosis) were coated onto a hydrophobic ELISA plate and incubated with recombinant β2-GPI, plasma-purified β2-GPI, or deglycosylated plasma-purified β2-GPI. Figure 5B shows that the coated patient type A IgG antibodies did recognize recombinant β2-GPI and plasma-purified β2-GPI without the carbohydrate chains, in contrast to plasma β2-GPI (with carbohydrate chains). We obtained comparable results with monoclonal antibody 4F3 as with the patient type A IgG antibodies (Figure 5C). We found no difference in binding pattern between monoclonal antibody 4F3 and F(ab) fragments of this antibody (Figure 5D). Monoclonal antibody 2B2 recognized all forms of β2-GPI in solution (Figure 5E).
Domain-specificity of the antibodies
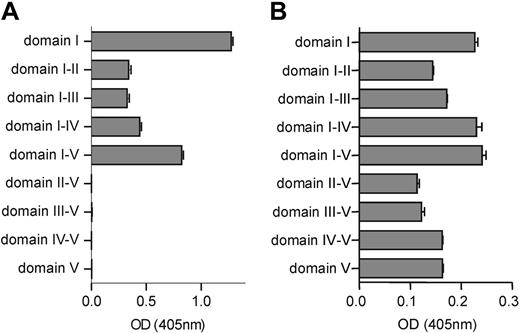
To investigate the specificity of type A IgG antibodies, type B IgG antibodies, monoclonal antibody 4F3, and monoclonal antibody 2B2, we coated domain deletion mutants of β2-GPI to a hydrophobic ELISA plate. All patient type A IgG antibodies (n = 30) showed a high avidity for all domain deletion mutants that contained domain I (Figure 6A). Patient type B antibodies (n = 22) recognized full-length recombinant β2-GPI, domains I and V and also domains I and V extended with additional domains (Figure 6B). Monoclonal antibody 4F3 showed a moderate affinity for domain deletion mutant I-II but showed no reactivity when domain I was not present. This indicates that monoclonal antibody 4F3 recognizes the intersection between domain I and domain II. Monoclonal antibody 2B2 predominantly recognized the domain deletion mutants that contained domain III.
Recognition of recombinant β2-GPI and (deglycosylated) plasma-purified β2-GPI in solution. Type A IgG antibodies of patient 1 and patient 2 (B), monoclonal antibodies 4F3 (3 μg/mL) (C), F(ab) fragments of monoclonal antibody 4F3 (15 μg/mL) (D), and monoclonal antibody 2B2 (3 μg/mL) (D) were coated onto hydrophobic ELISA plates. Then, the plates were incubated with different concentrations of either plasma-purified β2-GPI, deglycosylated plasma-purified β2-GPI (A), or recombinant β2-GPI. Subsequently, the plates were washed and the bound β2-GPI was detected by a polyclonal goat anti-human anti-β2-GPI antibody. After washing, the plates were incubated with a rabbit anti-goat peroxidase-labeled antibody. Coloring was performed with OPD. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (panel B: OD, 0.179; panel D: OD, 0.117). Error bars represent mean ± SEM of quadruplicate points.
Recognition of recombinant β2-GPI and (deglycosylated) plasma-purified β2-GPI in solution. Type A IgG antibodies of patient 1 and patient 2 (B), monoclonal antibodies 4F3 (3 μg/mL) (C), F(ab) fragments of monoclonal antibody 4F3 (15 μg/mL) (D), and monoclonal antibody 2B2 (3 μg/mL) (D) were coated onto hydrophobic ELISA plates. Then, the plates were incubated with different concentrations of either plasma-purified β2-GPI, deglycosylated plasma-purified β2-GPI (A), or recombinant β2-GPI. Subsequently, the plates were washed and the bound β2-GPI was detected by a polyclonal goat anti-human anti-β2-GPI antibody. After washing, the plates were incubated with a rabbit anti-goat peroxidase-labeled antibody. Coloring was performed with OPD. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (panel B: OD, 0.179; panel D: OD, 0.117). Error bars represent mean ± SEM of quadruplicate points.
Influence of NaCl concentration on the recognition of β2-GPI by anti-β2-GPI IgG antibodies
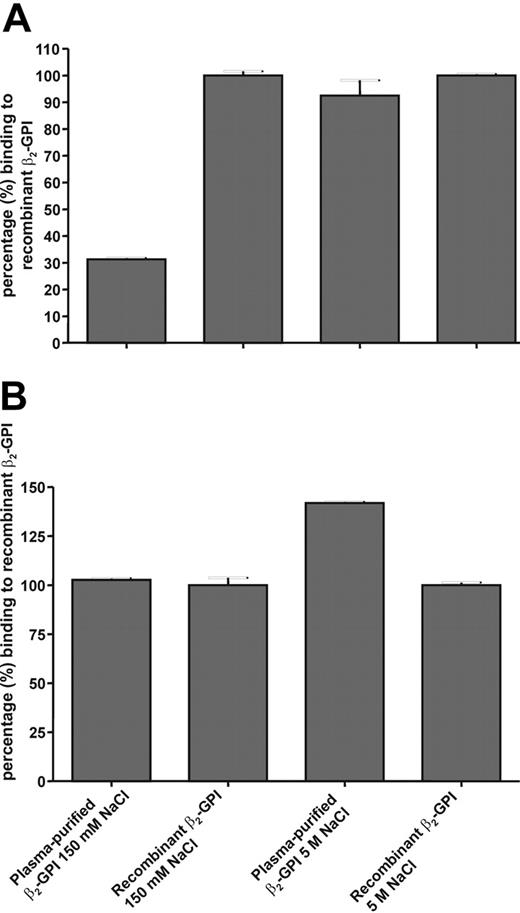
Hammel et al19 showed that a carbohydrate side chain was positioned on domain I of β2-GPI, probably covering epitope G40-R43. Because epitope G40-R43 is positively charged and the carbohydrate side chain is negatively charged, we investigated whether an increase in salt concentration might induce a displacement of this carbohydrate side chain. Figure 7A shows that type A anti-β2-GPI IgG antibodies showed a decreased affinity for plasma β2-GPI compared with recombinant β2-GPI when coated onto a hydrophobic plate. In the presence of 5 M NaCl, plasma β2-GPI and recombinant β2-GPI were equally recognized by type A antibodies. This recognition pattern was not seen when β2-GPI was coated onto hydrophilic plates (Figure 7B). For type B antibodies we observed no residual binding in the presence of 5 M NaCl for either recombinant β2-GPI or plasma β2-GPI (data not shown). For patients with type A and B antibodies we tested 2 randomly chosen patient samples in this experiment. Both type A patient samples had a history of venous thrombosis, both type B patient samples did not have a history of thrombosis.
Reactivity of patient-derived IgG antibodies and monoclonal antibodies 4F3 and 2B2 toward the different domain deletion mutants of β2-GPI. Domain deletion mutants of β2-GPI were coated onto an ELISA plate to test the domain specificity of patient-derived type A IgG fractions (A) and patient-derived type B IgG fractions (B). The patient-derived IgG fractions and monoclonal antibody 4F3 and monoclonal antibody 2B2 were incubated to the plates. Subsequently, the plates were washed and incubated with alkaline-phosphatase-labeled goat anti-human IgG Abs to detect the bound patient IgG antibodies. Staining was performed by using PnPP. The coloring reaction was stopped by 2.4 M NaOH, and absorbance was measured at 405 nm. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (panels A and B: OD, 0.101). Error bars represent mean ± SEM of triplicate points.
Reactivity of patient-derived IgG antibodies and monoclonal antibodies 4F3 and 2B2 toward the different domain deletion mutants of β2-GPI. Domain deletion mutants of β2-GPI were coated onto an ELISA plate to test the domain specificity of patient-derived type A IgG fractions (A) and patient-derived type B IgG fractions (B). The patient-derived IgG fractions and monoclonal antibody 4F3 and monoclonal antibody 2B2 were incubated to the plates. Subsequently, the plates were washed and incubated with alkaline-phosphatase-labeled goat anti-human IgG Abs to detect the bound patient IgG antibodies. Staining was performed by using PnPP. The coloring reaction was stopped by 2.4 M NaOH, and absorbance was measured at 405 nm. The obtained optical density was corrected for the optical density obtained with total IgG isolated from pooled plasma from 40 healthy volunteers (panels A and B: OD, 0.101). Error bars represent mean ± SEM of triplicate points.
Discussion
Whether antiphospholipid antibodies recognize a cryptic epitope on β2-GPI has been the subject of a lengthy debate. On the basis of this study, we conclude that a conformational change in β2-GPI is necessary for type A antibodies to recognize the protein. This conformational change is induced by the binding of β2-GPI to a negatively charged surface via a positive-charged patch in domain Vof β2-GPI.
We recently published that the population of anti-β2-GPI antibodies that binds domain I of β2-GPI at position 40 to 43 highly correlates with thrombosis.23 This area of domain I is probably covered by a carbohydrate chain in solution, as could be concluded from the studies by Hammel et al.19 We hypothesized that this carbohydrate chain impedes antiphospholipid antibodies to bind epitope G40-R43 in solution. An anionic surface may induce a conformational change in β2-GPI which results in the displacement of the carbohydrate side chain. Antiphospholipid antibodies are then able to bind epitope G40-R43. Indeed, the removal of the carbohydrate chains with N-glycosidase F resulted in an increased affinity for plasma-purified β2-GPI in solution by our monoclonal antibody 4F3 and patient type A IgG antibodies. Furthermore, by increasing the concentration of NaCl we were able to induce binding of anti-β2-GPI IgG antibodies to plasma β2-GPI on a hydrophobic plate. This indicates that the carbohydrate side chain no longer covers epitope G40-R43 and is now accessible for antibodies to bind. This hypothesis is further supported by the fact that proteins produced in insect cells have truncated carbohydrate side chains instead of complex carbohydrate side chains (as produced in mammalian cells). Because of these shorter carbohydrate side chains, recombinant β2-GPI exposes epitope G40-R43 constitutively.
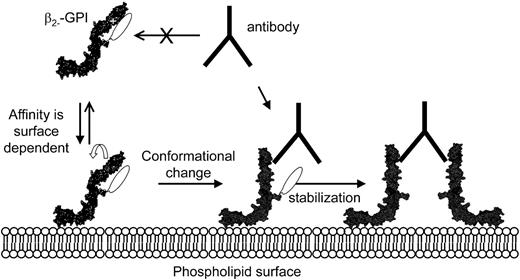
From our studies it is clear that anti-β2-GPI antibodies bind a cryptic epitope that is only exposed on β2-GPI when bound to a negatively charged surface. This by no means excludes a role for dimerization of β2-GPI. Antibody-induced dimerization of β2-GPI seems important for functional activity because LAC activity is not seen when Fab1 fragments of anti-β2-GPI antibodies are used.30 Furthermore, we have also shown that dimerized β2-GPI and not monomeric β2-GPI induces LAC activity.11 We postulate that the recognition of β2-GPI by anti-β2-GPI antibodies occurs as follows (Figure 8): β2-GPI has a relatively low affinity for anionic phospholipids, and a certain concentration of β2-GPI is necessary for binding. After binding of β2-GPI to anionic phospholipids via domain V, the protein undergoes a conformational change. This results in the exposure of several cryptic epitopes, including epitope G40-R43 in domain I, enabling antibodies to bind this epitope.17,23,31 When the amount of β2-GPI bound to the anionic phospholipids reaches a certain density, 1 antibody can bind 2 β2-GPI molecules, thereby increasing the affinity of the interaction with anionic phospholipids and stabilizing the complex.
This model has several consequences for the assays we use to detect anti-β2-GPI antibodies. It is obvious that for the LAC assay, both dimerization and exposure of the cryptic epitope are necessary. Clotting assays use PS as catalytic surface, but the affinity of β2-GPI for PS is low; thus, stabilization of the interaction via dimerization is essential.3,13 In contrast to the LAC assay, in the anticardiolipin antibody ELISA cardiolipin is used as binding surface for β2-GPI. The affinity of β2-GPI for cardiolipin is 30 to 40 times higher than the affinity for PS.13 This relatively high affinity enables β2-GPI of becoming stabilized on the cardiolipin, and there is no need for dimerization via the antibodies to increase the affinity of β2-GPI for the surface. Only the exposure of a cryptic epitope is essential in this assay. In the ELISA in which β2-GPI is directly coated onto the plate, the situation is more complex.32-34 Some brands of ELISA plates are unable to induce the conformational change (because of a difference in structure or charge) and are unsuitable for use in an anti-β2-GPI ELISA.33 Other brands of ELISA plates do induce the conformational change, but their affinity for β2-GPI is rather low. These influences of different ELISA plates on our test results emphasize the need for standardization of the anti-β2-GPI ELISA. In this respect it is interesting to mention that we observed differences in binding of β2-GPI onto plates of different lot numbers of the same brand (unpublished data).
Influence of NaCl concentration on the recognition of β2-GPI by anti-β2-GPI IgG antibodies. Plasma-purified β2-GPI and recombinant β2-GPI were coated to hydrophobic (A) and hydrophilic (B) plates. Subsequently, the plates were blocked with 4% BSA/TBS/Tween and incubated with (type A) patient plasma that was diluted in blocking solution with either 150 mM NaCl or 5 M NaCl. To detect the bound patient IgG antibodies, the plates were incubated with an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. Error bars represent mean ± SEM of quadruplicate points.
Influence of NaCl concentration on the recognition of β2-GPI by anti-β2-GPI IgG antibodies. Plasma-purified β2-GPI and recombinant β2-GPI were coated to hydrophobic (A) and hydrophilic (B) plates. Subsequently, the plates were blocked with 4% BSA/TBS/Tween and incubated with (type A) patient plasma that was diluted in blocking solution with either 150 mM NaCl or 5 M NaCl. To detect the bound patient IgG antibodies, the plates were incubated with an alkaline-phosphatase-labeled goat anti-human IgG antibody. PnPP was used as coloring substance. Error bars represent mean ± SEM of quadruplicate points.
Mechanism describing the binding of antiphospholipid antibodies toβ2-GPI. Our proposed mechanism is based on our findings in this study together with both the crystal structure and the NMR structure of β2-GPI in solution: (1) Antiphos-pholipid antibodies cannot bind β2-GPI in solution because epitope G40-R43 is covered by one of the carbohydrate chains. (2) Binding to a phospholipid membrane induces a conformational change in β2-GPI. (3) As a result, the carbohydrate chain is not able to cover epitope G40-R43 anymore and is now able to bind antiphospholipid antibodies.
Mechanism describing the binding of antiphospholipid antibodies toβ2-GPI. Our proposed mechanism is based on our findings in this study together with both the crystal structure and the NMR structure of β2-GPI in solution: (1) Antiphos-pholipid antibodies cannot bind β2-GPI in solution because epitope G40-R43 is covered by one of the carbohydrate chains. (2) Binding to a phospholipid membrane induces a conformational change in β2-GPI. (3) As a result, the carbohydrate chain is not able to cover epitope G40-R43 anymore and is now able to bind antiphospholipid antibodies.
Another consequence of our results concerns the use of different sources of β2-GPI. We found that patient type A IgG antibodies (and monoclonal antibody 4F3) did show reactivity toward recombinant β2-GPI coated onto hydrophobic plates, in contrast to plasma-purified β2-GPI coated onto hydrophobic plates. We also found that patient type A IgG antibodies (and monoclonal antibody 4F3) did recognize recombinant β2-GPI in solution but showed hardly any reactivity toward plasma-purified β2-GPI. This difference in binding characteristics between plasma-purified β2-GPI and recombinant β2-GPI can be explained by the difference in glycosylation. Furthermore, bovine β2-GPI has an additional glycosylation site at position 73 which can easily influence the binding of epitope G40-R43 by type A anti-β2-GPI antibodies.19 In addition to this, it was already suggested that aggregation of β2-GPI promotes the binding of certain anti-β2-GPI antibodies.35 Galazka et al29 found that a cryptic epitope was exposed on β2-GPI when it was treated with glutaraldehyde. We found that these 2 features also hold true for type A antibodies. The importance of the carbohydrate chains and conformation of β2-GPI has major consequences for the binding of antiphospholipid antibodies. One should check for aggregates of β2-GPI in a (plasma) sample, and one should be careful in using β2-GPI isolated from other sources than human plasma when studying the interaction between β2-GPI and anti-β2-GPI antibodies.
In conclusion, we found that pathogenic antiphospholipid antibodies bind a cryptic epitope on domain I of β2-GPI. This epitope (G40-R43) is accessible for antiphospholipid antibodies only after a conformational change.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-05-1943.
Supported by the Netherlands Organisation for Health Research and Development (ZonMw grant 902-26-290).
B.d.L. performed the research, analyzed the data, and wrote the paper; M.v.L. and M.T.T.P. performed the research and contributed vital reagents; and P.G.d.G. and R.H.W.M.D. designed the research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.