Allergic, inflammatory, and immune responses carried out by eosinophils are regulated by the cross talk between activatory and inhibitory signals. While much data has been obtained on activatory signals, inhibitory receptors on these cells have received scant attention. Therefore, we screened the surface of human peripheral blood eosinophils for inhibitory receptors using monoclonal antibodies (mAbs) previously generated to recognize receptors on human natural killer cells. Eosinophils from all of the donors examined expressed the inhibitory receptors IRp60, LIR3/ILT5, FcγRIIB, and p75/AIRM but not LIR1/ILT2, p58.1, p58.2, p70, or NKG2A/CD94 (n = 15). Interestingly, 25% of the donors expressed p140. IRp60 cross-linking inhibited eotaxin-dependent transmigration of eosinophils in a calcium-independent fashion. In addition, cross-linking of IRp60 on the eosinophils in the presence of IL-5/GM-CSF inhibited the antiapoptotic effect of these cytokines and blocked the release of TNF-α, IL-1β, IFN-γ, IL-4, and 3T3 fibroblast proliferation. Cross-linking of IRp60 inhibited IL-5-mediated JAK2 phosphorylation as well as eotaxin- and IL-5/GM-CSF-mediated ERK1/2 and p38 phosphorylation. Furthermore, upon cross-linking, IRp60 underwent tyrosine phosphorylation and recruited SHP-1 but not SHP-2. These findings demonstrate a novel pathway for suppressing the activity of human eosinophils, thus indicating IRp60 as a future potential target for the treatment of allergic and eosinophil-associated diseases.
Introduction
Prolonged survival and activation of eosinophils are “hallmark” features of allergic diseases.1,2 Eosinophils are also associated with host defense mechanisms in parasitic infestations, with a variety of idiopathic hypereosinophilic syndromes, and are implicated in the pathogenesis of some immunologic and malignant disorders.3,4 Within cytoplasmic granules eosinophils store 4 types of specific cationic proteins and several cytokines. In addition, upon stimulation they can also synthesize lipid mediators and a number of cytokines and chemokines with proinflammatory, immunoregulatory, and profibrotic properties.5
The activatory signaling pathways regulating the activities of the eosinophils have been extensively studied.6-11 Surprisingly, the role of inhibitory signaling pathways that could restrain eosinophil activation has been scarcely examined. Recently, it has been reported that activation of human eosinophils by siglec-8, even in the presence of IL-5 or GM-CSF, the “eosinophil survival cytokines,” inhibits their survival by inducing apoptosis.12 Furthermore, Mig (CXCL9) by binding to CCR3 has been shown to block murine eosinophil chemotaxis and function.13,14 Notably, the importance of defining new pathways that suppress eosinophil accumulation, survival, and activation has been recently bolstered by the demonstration of a critical role for eosinophils in allergic-airway inflammation in 2 different eosinophil-deficient mice.15,16
Inhibitory receptors have been characterized on cytotoxic T cells and natural killer (NK) cells and recognize diverse ligands such as major histocompatibility complex (MHC) class I molecules, adhesion molecules, or yet undefined ligands.17,18 Two main families of inhibitory receptors exist. The immunoglobulin receptor (IgR) superfamily is characterized by V-type Ig-like domain(s) in their extracellular domain and includes receptors such as KIRs, LIRs/ILTs, siglecs, LAIR-1, gp49B1, and IRp60. The second superfamily of inhibitory receptors consists of the calcium-dependent lectins such as MAFA or CD94/NKG2A.19 Both families can be identified by a consensus amino acid sequence, the immunoreceptor tyrosine-based inhibitory motif (ITIM), which is present in the cytoplasmic domain of these molecules. The archetype ITIM sequence is composed of 6 amino acids (Ile/Val/Leu/Ser)-X-Tyr-X-X-(Leu/Val), where X denotes any amino acid. Upon activation, these inhibitory receptors undergo tyrosine phosphorylation, often by a Src kinase, which provides a docking site for the recruitment of cytoplasmic phosphatases having a Src homology 2 (SH2) domain such as SHP-1, -2 and SHIP-1, -2. Recruitment of SHP-1, -2 and/or SHIP-1, -2 results in upstream inhibition of cell activation before any signaling cascade has been initiated.17-19
IRp60 is a non-MHC-specific inhibitory receptor belonging to the IgR superfamily. It is expressed on many cell types including T cells, NK cells, monocytes, and granulocytes.20 Cross-linking of IRp60 on NK cells results in down-regulation of NK cytolytic activity. In addition, treatment of these cells with sodium pervanadate leads to marked IRp60 tyrosine phosphorylation and association with both SHP-1 and SHP-2.20
In the present work we screened human eosinophils for the expression of various inhibitory receptors and found that human eosinophils express a functional IRp60 inhibitory receptor. To the best of our knowledge this is the first report of a functional inhibitory receptor on human eosinophils that does not actively induce apoptosis12 but rather inhibits activating signals such as eotaxin and IL-5/GM-CSF. Thus, the results of this study reveal a novel pathway regulating human eosinophil functions, implicating a future therapeutic target for modulating allergic and eosinophil-associated diseases.
Patients, materials, and methods
Antibodies
Culture media, reagents, and buffers were purchased from Biological Industries (Beit Haemek, Israel). The following mouse monoclonal antibodies recognizing various inhibitory receptors were produced as described20 : P192 and E59 (anti-IRp60); Z270 (anti-NKG2A); XA185 (anti-CD94); Qa79, Z176, and IT257 (anti-p75/AIRM); 11PB6 (anti-p58.1); GL183 (anti-p58.2); Z27 (anti-p70); Q66 (anti-p140); AZ158 (anti-p70/p140); and F278 (anti-LIR1/ILT2). The antibody recognizing LIR3/ILT5 (15F3) was kindly provided by Dr M. Colonna (Washington University, St Louis, MO). Anti-CD3 (DAKO, Carpentaria, CA) and anti-CD56 (R&D Systems, Minneapolis, MN) were kindly provided by Dr O. Mandelboim (The Hebrew University, Jerusalem, Israel). The following antibodies and reagents were purchased as indicated: mouse IgG1, IgG2a, and PE-conjugated mouse IgG2a were obtained from DAKO (Glostrup, Denmark); FITC-conjugated mouse IgG1 antibodies (Abs) were from Ancell (Bayport, MN). FITC antirabbit, FITC antigoat, Cy5 anti-rabbit goat, anti-mouse F(ab′)2 IgG, and PE anti-mouse F(ab′)2 fragments were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Sheep F(ab′)2 anti-mouse was purchased from ICN Pharmaceuticals (Aurora, OH). Rat anti-human CCR3 was obtained from R&D Systems. Polyclonal rabbit anti-human phosphotyrosine, SHP-1, SHP-2, and IL-5Rβ antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit anti-human phospho JAK2 and JAK2 antibodies were a kind gift from Dr Z. Ben-Sasson (The Hebrew University of Jerusalem, Israel). Calcium Green-1AM was purchased from Molecular Probes (Eugene, OR); phospho-specific antibodies mouse anti-human p-p38 and rabbit anti-human pERK1/2 were obtained from BD-Pharmingen (San Diego, CA) and Biosource (Camarillo, CA), respectively.
Reagents and chemicals
RPMI-1640, MEM-alpha, and DMEM all supplemented with heat-inactivated fetal calf serum (FCS) and penicillin-streptomycin solutions were obtained from Biological Industries. Recombinant human cytokines and chemokines were obtained from Peprotech (Rocky Hill, NJ). All the chemicals and enzymes used in this study were purchased from Sigma (Rehovot, Israel) and were of the best available grade.
Human peripheral blood eosinophil purification
Eosinophils were purified from peripheral blood of untreated mildly atopic individuals (blood eosinophil levels 5%-10%) as previously described.21 Informed consent was obtained from all volunteers according to the guidelines established by the Hadassah-Hebrew University Human Experimentation Helsinki Committee. The cells were purified to greater than 98% (Kimura staining), demonstrated viability of greater than 98% (trypan blue exclusion staining), and were resuspended in RPMI-1640 10% FCS. Importantly, no CD56+/CD3- cells were found in the contaminating fractions as demonstrated by fluorescence-activated cell sorter (FACS) analysis (data not shown).
Human nasal polyp digestion
Cells were isolated from nasal polyposis patients undergoing polypectomy according to the guidelines established by the Hadassah-Hebrew University Human Experimentation Helsinki Committee. Nasal polyps were washed twice in RPMI-2% FCS and the polyps were finely minced to fragments of approximately 1 mm3 and subsequently digested by incubation with an enzymatic cocktail containing collagenase type-I (6 mg/g tissue), hyaluronidase (3 mg/g tissue), and DNase (100 μg/g tissue) for 90 minutes in 37°C. The digested tissue was filtered through a 150-micron nylon mesh, and cells were collected at a viability of greater than 94% (trypan blue exclusion). Eosinophils in the cell suspension were identified as SSChigh and CCR3+ cells using FACS analysis.
Flow cytometry
For FACS analysis, cells (2 × 105) were incubated in 15% human AB serum (to block Fc receptors) in a final volume of 100 μL of Hanks balanced salt solution supplemented with 0.1% bovine serum albumin and 0.02% sodium azide (HBA) for 30 minutes on ice. Eosinophils were cultured with different monoclonal Abs (mAbs) recognizing inhibitory receptor epitopes followed by goat anti-mouse FITC or goat anti-mouse PE Abs (1:500).
For detection of these receptors on human nasal polyp eosinophils, freshly isolated nasal polyp cells were incubated for 30 minutes on ice with PE-conjugated anti-CCR3 and an anti-inhibitory receptor antibody, followed by goat anti-mouse FITC Abs. For intracellular FACS stainings, eosinophils were fixed after the indicated time points in 4% paraformaldehyde in HBA (15 minutes, at room temperature) and then permeabilized in HBA containing saponin (0.1%), BSA (1 mg/mL), and human serum (10%; 30 minutes on ice). Rabbit anti-human phospho ERK1/2, mouse anti-phospho p38, or irrelevant control Abs (1 μg/mL) were added to these fixed, permeabilized cells (30 minutes on ice) and then incubated with FITC antimouse and Cy5 antirabbit Abs (1:500 and 1:800, respectively, 30 minutes on ice).
After staining, the cells were analyzed on a Becton Dickinson FACSCalibur System (Becton Dickinson, San Jose, CA). For each staining at least 10 000 events were collected and data analysis was performed using CellQuest software (BD, Mansfield, MA).
Eosinophil transmigration assay
Eosinophil transmigration was measured using a microwell dual-chamber system (ChemoTx chamber: filter pore size 5 μm, 6-mm diameter wells; Neuro Probe, Gaithersburg, MD). Recombinant human eotaxin (1-100 ng/mL) was first added in triplicates to wells in the bottom chamber and covered with a framed filter. Next, eosinophils (30 000 cells/30 μL) that were previously cross-linked with anti-IRp60 or isotype-matched control or were incubated with anti-CCR3 mAb were placed on the top of the filter over each well and the chamber system was incubated for 90 minutes (37°C, 5% CO2). After incubation, the nonmigrated eosinophil suspension on the top of the filter was removed using tissue paper and the cells in the lower chamber were counted by flow cytometry (FACSCalibur; Becton Dickinson). Relative cell counts were obtained by acquiring events for 60 seconds.
Cell culture and activation
For all IRp60 cross-linking experiments, freshly isolated eosinophils (2 × 105/200 μL) were incubated in 96-well plates (Nunc, Roskilde, Denmark) in medium containing RPMI-1640, 200 U/mL penicillin, 200 μg/mL streptomycin, and 5% vol/vol heat-inactivated FCS. Anti-IRp60 (E59) or matched control antibodies (IgG1 isotype control and anti-p58.2 as an epitope control; unless stated otherwise, referred to in the text as “control antibodies”) were added to the cells (1-5 μg/mL, 30 minutes, 37°, 5% CO2). Afterward the cells were washed twice and divided into 2 groups: one group was cross-linked with sheep anti-mouse F(ab′)2 (25 μg/mL, 30 minutes, 37°C, 5% CO2), whereas the other group was tested with no further cross-linking. Thereafter, the cells were incubated in the presence of recombinant human IL-5 (rhIL-5) or rhGM-CSF (5-100 ng/mL) for the indicated time points. At the end of the incubation, cells were centrifuged (250g, 5 minutes, 4°C) and supernatants were collected, aliquoted, and stored at -80°C until assessed for mediator release. For survival assays, cells were activated as described and annexin V-PI (R&D Systems) staining was performed according to the manufacturer's instructions.
Cytokine release assays
Cytokines were detected using the FlowMultiplex kit (Bender MedSystems, Vienna, Austria), which is designed to identify the following cytokines in the supernatant of cell culture: IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, TNF-α, and TNF-β (detection limits were 12 pg/mL, 6.8 pg/mL, 43.8 pg/mL, 9.6 pg/mL, 6.1 pg/mL, 7.8 pg/mL, 9.6 pg/mL, 12.5 pg/mL, 13.2 pg/mL, and 4.8 pg/mL, respectively). Briefly, 2 sets of microspheres with different sizes are used. Each of the 2 sets consists of 5 bead populations internally dyed with varying intensities of a fluorescent dye. The combination of 2 different bead sizes and 5 varying dye intensities makes it possible to distinguish 10 bead sets in one fluorescence channel using the same principle as enzyme-linked immunosorbent assay (ELISA).
Fibroblast proliferation assay
The mouse 3T3 Swiss albino cell line (CCL-92; American Type Culture Collection, Manassas, VA) was cultured in supplemented DMEM 10% FCS and used between passages 3 and 7. Proliferation of the subconfluent fibroblast monolayer was assessed by [3H]-thymidine incorporation as described.22 Fibroblasts were stimulated with supernatants of eosinophils that were cross-linked with IRp60 or control antibodies in the presence of GM-CSF or IL-5 for 18 to 20 hours. Data are expressed as mean counts per minute (CPM)/well ± SD.
Intracellular Ca2+ mobilization
Eosinophils (3 × 105 cells) that were previously cross-linked by anti-IRp60 or control antibodies followed by sheep anti-mouse F(ab′)2 were washed and transferred into MEM-alpha (FCS, 2% vol/vol). The cells were loaded with Calcium Green-1AM (5 μM, 1 hour, 37°C), washed, and suspended in 400 μL Tyrode gelatin-calcium buffer warmed to 37°C. The cells were allowed to flow freely in the cytometer for 40 seconds, at which time eotaxin (1-100 ng/mL) was added. Changes in FL-1 mean were recorded for a total of 3 minutes.
Immunoprecipitation and Western blot
Cross-linking was performed with anti-IRp60 on freshly isolated human peripheral blood eosinophils (8 × 106) as described in “Cell culture and activation.” The cells were activated with IL-5 (5-50 ng/mL) or eotaxin (10-100 ng/mL) for the indicated time points. Activation was stopped by pelleting the cells through centrifugation (2500g, 3 minutes, 4°C). Subsequently, cell pellets were lysed using a commercial lysis buffer (M-PER; Pierce, Rockford, IL). Cell debris were removed from the lysates by centrifugation (18 000g, 15 minutes, 4°C). The supernatant was precleared and then incubated with anti-IRp60 (E59, 18 hours, 4°C), followed by protein G (Pierce) to precipitate immune complexes. The immunoprecipitates were washed 4 times with PBS, eluted from the Sepharose beads by boiling for 10 minutes in sample buffer, and run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were electrotransferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). Thereafter, membranes were incubated overnight (4°C) with anti-IRp60, antiphosphotyrosine (pY99), and SHP-1 or SHP-2 (1:500), followed by secondary horseradish peroxidase-conjugated anti-mouse or antirabbit antibodies and development by enhanced chemiluminescence (ECL) substrate (Amersham Biosciences, Uppsala, Sweden).
In assays determining phosphorylated forms of ERK or p38, IRp60 was cross-linked on freshly isolated human peripheral blood eosinophils as described. The cells were washed twice (250g, 4°C) and stimulated with IL-5 or eotaxin for the indicated times (37°C, 5% CO2). The cells were subsequently collected, lysed, and analyzed by SDS-PAGE immunoblot using antiphospho-specific antibodies.
Statistical analysis
Statistical significance was determined using parametric analysis (analysis of variance [ANOVA], followed by Tukey-Kramer post hoc test or Student t test). Differences were considered significant at P values less than .05.
Results
Human eosinophils express IRp60
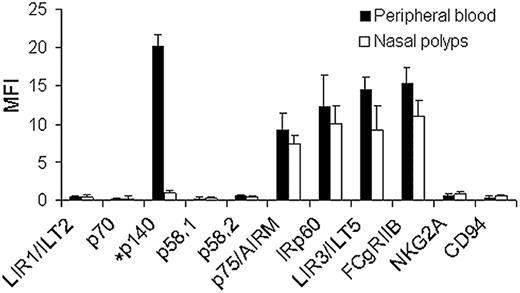
In order to investigate the expression pattern of inhibitory receptors on human eosinophils, the cells were incubated with a large panel of mAbs recognizing various inhibitory receptors previously characterized on NK cells.17,18,20 As shown in Figure 1A, FACS analysis revealed that peripheral blood eosinophils express high levels of IRp60, LIR3/ILT5, FcγRIIB, and p75/AIRM (siglec-7) but not LIR1/ILT2, p58.1, p58.2, p70, NKG2A, or CD94 (n = 15). Interestingly, eosinophils from only 25% of the donors examined expressed p140.
Eosinophil effector functions take place once these cells have penetrated into the inflamed tissue. Therefore, we were interested to determine whether these inhibitory receptors on tissue eosinophils display the same expression pattern as blood eosinophils. As shown in Figure 1B, nasal polyp eosinophils displayed the same inhibitory receptors (n = 10), with the exception of p140, which was not expressed on any of the nasal polyp eosinophils that were examined. To establish whether the differential expression of p140 between blood and tissue eosinophils is not due to the enzymatic digestion process involved in obtaining the nasal polyp cell suspensions, peripheral blood eosinophils were subjected to the same enzymatic digestion and analyzed by FACS for p140 expression. Results show that the expression of p140 was not altered by enzymatic treatment (data not shown). In order to verify whether the expression pattern of the detected inhibitory receptors is antibody dependent, we used different clones recognizing IRp60 (ie, P192 and E59) and p75/AIRM (ie, Qa79, Z176, and IT257) and compared staining patterns by FACS. No differences in expression of IRp60 or p75/AIRM on either blood eosinophils (n = 5) or nasal polyp eosinophils (n = 3) were observed.
Human eosinophils express IRp60. FACS analysis was performed on human peripheral blood eosinophils (▪) and nasal polyp eosinophils (□) using a panel of monoclonal antibodies recognizing NK-cell inhibitory receptors. The eosinophils were incubated with mouse anti-human mAbs, followed by FITC-conjugated antimouse, and analyzed by FACS. Data are expressed as mean fluorescent intensity (MFI ± SD); n = 15 for peripheral blood and n = 10 for nasal polyp eosinophils. *p140 expression was observed in 25% of the examined donors.
Human eosinophils express IRp60. FACS analysis was performed on human peripheral blood eosinophils (▪) and nasal polyp eosinophils (□) using a panel of monoclonal antibodies recognizing NK-cell inhibitory receptors. The eosinophils were incubated with mouse anti-human mAbs, followed by FITC-conjugated antimouse, and analyzed by FACS. Data are expressed as mean fluorescent intensity (MFI ± SD); n = 15 for peripheral blood and n = 10 for nasal polyp eosinophils. *p140 expression was observed in 25% of the examined donors.
IRp60 inhibits eotaxin-induced eosinophil transmigration and IL-5/GM-CSF-induced eosinophil survival and activation
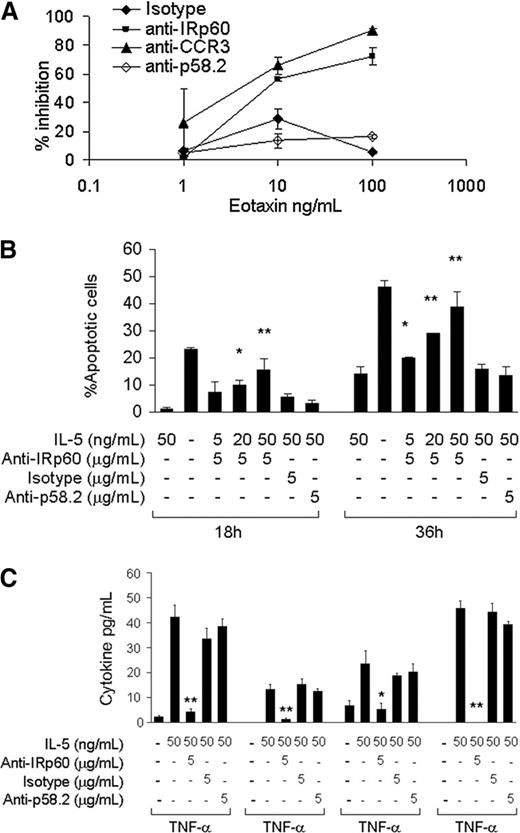
The expression of various inhibitory receptors on the surface of human eosinophils suggests a functional role. Accumulation of eosinophils in the tissues is a tightly regulated process that is dependent mainly on chemokines of the eotaxin family.23,24 In order to investigate whether IRp60 could inhibit this process, IRp60 on freshly isolated eosinophils was cross-linked and their ability to undergo chemotaxis in response to eotaxin-1 was evaluated using a transmigration assay. Engagement of IRp60 significantly decreased the ability of eosinophils to transmigrate in response to eotaxin. For example, at concentrations of 10 ng/mL eotaxin, IRp60 inhibited eosinophil migration by 56% (P < .01). At higher concentrations of eotaxin (100 ng/mL) IRp60 cross-linking resulted in a 72% inhibition (P < .01). This effect was IRp60 specific as transmigration was negligible in cell cultures treated with control antibodies. Furthermore, cross-linking of IRp60 on eosinophils inhibited their chemotactic response toward LTB4 by 35% (10-8M, P < .01; data not shown). Interestingly, chemotactic responses to TNF-α were not affected by IRp60 cross-linking (data not shown).
A prominent feature of eosinophils in inflammatory disorders is their long-lasting survival in the tissue due to the “survival” cytokines IL-5, GM-CSF, and IL-3.25,26 To assess whether IRp60 regulates survival signals delivered to eosinophils by these cytokines, we cross-linked IRp60 on freshly isolated eosinophils and incubated them with IL-5 or GM-CSF. As shown in Figure 2B, this significantly inhibited eosinophil survival measured at both 18 and 36 hours of culture. Interestingly, the effect of IRp60 was stronger with increasing concentrations of GM-CSF. IRp60 cross-linking increased the number of apoptotic cells at 18 hours by 4.4-fold in cells exposed to 20 ng/mL GM-CSF (P < .05) and by 7.5-fold in cells exposed to 50 ng/mL GM-CSF (P < .01). Notably, cross-linking of IRp60 did not actively induce apoptosis (as assessed by annexin V-PI staining and caspase 3 cleavage; data not shown). Similar results were obtained for IL-5-treated eosinophils (data not shown).
IL-5 and GM-CSF are known to prime and activate eosinophils.27,28 Based on the observed inhibitory effect of IRp60 on NK cell activation,20 we hypothesized that IRp60 may also inhibit eosinophil activation. Although the FlowMultiplex cytokine array is able to detect up to 10 cytokines, only TNF-α, IL-1β, IL-4, and IFN-γ were consistently released in response to GM-CSF or IL-5 by the eosinophils from all of the donors. As shown in Figure 2C, IRp60 cross-linking significantly inhibited the release of these cytokines, whereas the control antibodies did not induce any inhibitory effect. Similar results were obtained for GM-CSF-mediated activation of human eosinophils (data not shown). Furthermore, in eosinophils obtained from several but not all the donors, IL-8 release was also observed in response to IL-5/GM-CSF activation. In these cases, cross-linking of IRp60 was also able to inhibit IL-8 release (data not shown).
IRp60 inhibits eosinophil-mediated fibroblast proliferation
In order to establish whether this inhibitory effect may have biologic significance, eosinophils were cross-linked with IRp60 or control antibodies in the presence of GM-CSF for 18 to 20 hours. Supernatants from these cultures were collected and incubated with NIH 3T3 fibroblasts and their effect on fibroblast proliferation was assessed. As shown in Figure 3, supernatants of IRp60 cross-linked cells displayed a significant reduction in their ability to induce fibroblast proliferation (ie, a 16-fold decrease in response to 50 ng/mL GM-CSF, P < .01, n = 3; and a 3.8-fold decrease in response to 100 ng/mL GM-CSF, P < .05, n = 3).
IRp60 inhibits eotaxin-induced eosinophil transmigration and IL-5/GMCSF-induced eosinophil survival and activation. Eosinophils were incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2), followed by sheep anti-mouse F(ab′)2 to cross-link IRp60 receptors. The cells were washed and used in 3 different assays: (A) transmigration, (B) survival, and (C) mediator release. (A) Transmigration was assessed in a microwell dual-chamber system in response to recombinant human eotaxin (1-100 ng/mL) for 90 minutes (37°C, 5% CO2). After incubation, the cells that migrated to the lower chamber were evaluated by FACS analysis obtained by acquiring events for 60 seconds. Data are expressed as percent inhibition of eotaxin-treated cells ± SD; n = 4. (B) Eosinophil survival was evaluated by incubating them with various concentrations of IL-5/GM-CSF (5-50 ng/mL) for the indicated time points. Thereafter, the cells were assessed for apoptosis using annexin V-PI staining (n = 4; *P < .05; **P < .005). (C) Eosinophil mediator release was evaluated by incubating the cells with various concentrations of IL-5/GM-CSF (5-50 ng/mL) for 18 to 20 hours. Cytokine release in the cell supernatants was measured by using the FlowMultiplex array. Data are expressed as pg/mL of each cytokine ± SD (n = 5; *P < .05; **P < .005).
IRp60 inhibits eotaxin-induced eosinophil transmigration and IL-5/GMCSF-induced eosinophil survival and activation. Eosinophils were incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2), followed by sheep anti-mouse F(ab′)2 to cross-link IRp60 receptors. The cells were washed and used in 3 different assays: (A) transmigration, (B) survival, and (C) mediator release. (A) Transmigration was assessed in a microwell dual-chamber system in response to recombinant human eotaxin (1-100 ng/mL) for 90 minutes (37°C, 5% CO2). After incubation, the cells that migrated to the lower chamber were evaluated by FACS analysis obtained by acquiring events for 60 seconds. Data are expressed as percent inhibition of eotaxin-treated cells ± SD; n = 4. (B) Eosinophil survival was evaluated by incubating them with various concentrations of IL-5/GM-CSF (5-50 ng/mL) for the indicated time points. Thereafter, the cells were assessed for apoptosis using annexin V-PI staining (n = 4; *P < .05; **P < .005). (C) Eosinophil mediator release was evaluated by incubating the cells with various concentrations of IL-5/GM-CSF (5-50 ng/mL) for 18 to 20 hours. Cytokine release in the cell supernatants was measured by using the FlowMultiplex array. Data are expressed as pg/mL of each cytokine ± SD (n = 5; *P < .05; **P < .005).
IRp60 does not influence eotaxin-induced calcium influx
Eosinophil activation with chemoattractants and specifically with eotaxin results in increased cytosolic calcium concentration that is due to a combination of intracellular calcium release and an influx of extracellular calcium.29 Therefore, we were interested to determine whether the inhibitory effect of IRp60 was due to an effect on calcium. The effect of IRp60 cross-linking on calcium influx was examined using the calcium sensor Calcium Green-1AM. As shown in Figure 4, within 10 to 20 seconds of eotaxin addition, a marked increase in intracellular calcium was observed. However, this increase was unaffected by IRp60 cross-linking compared with the various control antibodies. Interestingly, in eosinophils from one donor, IRp60 completely abolished the calcium flow (data not shown). Nevertheless, in all eosinophil sources tested, IRp60 suppressed eotaxin-induced transmigration.
IRp60 inhibits eosinophil-mediated fibroblast proliferation. Eosinophils previously incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by the cross-linker sheep anti-mouse F(ab′)2 were washed and incubated with various concentrations of IL-5/GM-CSF (50 ng/mL) for 18 to 20 hours. Cell supernatants were assessed for their ability to induce 3T3 fibroblast proliferation as assessed by [3H]-thymidine incorporation. Data are expressed as mean CPM/well ± SD (n = 3; **P < .005).
IRp60 inhibits eosinophil-mediated fibroblast proliferation. Eosinophils previously incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by the cross-linker sheep anti-mouse F(ab′)2 were washed and incubated with various concentrations of IL-5/GM-CSF (50 ng/mL) for 18 to 20 hours. Cell supernatants were assessed for their ability to induce 3T3 fibroblast proliferation as assessed by [3H]-thymidine incorporation. Data are expressed as mean CPM/well ± SD (n = 3; **P < .005).
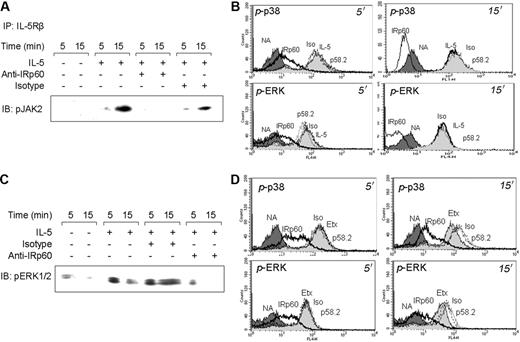
Cross-linking of IRp60 inhibits JAK2 and MAP kinase phosphorylation in response to IL-5 and eotaxin
The importance of JAK2 phosphorylation and consequent secondary messenger recruitment in IL-5R signaling has been well established.9 Therefore, we aimed to examine if IRp60 treatment could inhibit IL-5-induced JAK2 phosphorylation in eosinophils. For this, IL-5Rβ was immunoprecipitated from eosinophils after IRp60 was cross-linked and IL-5 added. IL-5-treated cells alone or with control antibodies displayed enhanced JAK2 phosphorylation at both 5 and 15 minutes. However, IRp60-treated cells showed negligible JAK2 phosphorylation after 5 minutes and no phosphorylation was observed after 15 minutes (Figure 5A).
One of the major mechanisms controlling both eotaxin- and IL-5/GM-CSF-mediated activation of eosinophils is the Ras/Raf pathway.30,31 Hence, we evaluated the ability of IRp60 to inhibit MAP kinase phosphorylation in response to eotaxin/IL-5/GM-CSF-mediated activation of human eosinophils. IL-5 activation resulted in increased p38 and ERK1/2 phosphorylation. Mean fluorescent intensity after 5 minutes was 155.68 and 56.35 IL-5 IRp60-treated versus 6.57 and 6.21 untreated cells for p-p38 and pERK1/2, respectively (P < .001). Cross-linking of IRp60 significantly inhibited the phosphorylation state of p38 and ERK1/2, whereas no inhibitory effect was observed with control antibodies. By 15 minutes, IRp60 cross-linking completely abolished the phosphorylation state of p38 and ERK1/2 (Figure 5B). These observations were also confirmed by immunoblot studies (Figure 5C; data not shown).
Cross-linking of IRp60 also inhibited phosphorylation of both p38 and ERK1/2 in response to eotaxin (Figure 5D; mean fluorescent intensity after 5 minutes: 47.64 and 12.54, pp38 and pERK1/2, respectively, P < .001). The decrease was even more dramatic after 15 minutes (12.21 and 7.85, p-p38 and pERK1/2, respectively, P < .01).
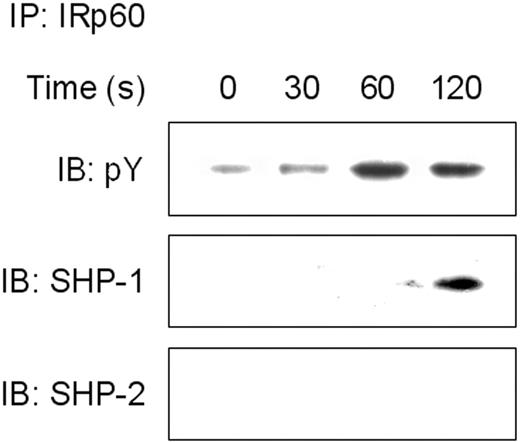
Cross-linking of IRp60 recruits SHP-1 but not SHP-2
Our results suggest that IRp60 recruits tyrosine phosphatases. In NK cells, IRp60 has been shown to mediate its inhibitory effect via recruitment of SHP-1 and SHP-2.20 In further investigation of the inhibitory mechanism of IRp60 on eosinophils, the cells were incubated with IL-5 or eotaxin and cross-linked with anti-IRp60 mAb. IRp60 was immunoprecipitated and the precipitates were examined by immunoblotting blotted for phosphotyrosine, SHP-1, and SHP-2. Although IRp60 displayed a basal phosphorylated state (Figure 6A), it underwent further tyrosine phosphorylation when cross-linked. Phosphorylation of IRp60 peaked at 2 minutes, and IRp60 coprecipitated with SHP-1 but not with SHP-2. Since IRp60 might recruit different tyrosine phosphatases in response to different stimuli, we assessed its ability to recruit SHP-1 or -2 in response to eotaxin activation and observed results similar to those obtained with IL-5 activation (data not shown).
Discussion
A key issue to understanding eosinophil-related diseases is the identification of new inhibitory pathways that will counteract eosinophil functions. Inhibitory receptors are very well characterized on NK and cytotoxic T cells and have been recently identified on the surface of other cell types such as neutrophils, monocytes, and dendritic cells.32,33 Thus, we hypothesized that human eosinophils may express inhibitory receptors as well. Our approach was to screen human peripheral blood and human nasal polyp eosinophils with mAbs recognizing inhibitory receptors. We found that both peripheral blood and nasal polyp eosinophils express the inhibitory receptors IRp60, p75/AIRM, LIR3/ILT5, and FcγRIIB. Tedla et al34 showed that eosinophils express LIR3/ILT5 and that 30% of the donors examined expressed LIR1/ILT2. Although our results support the expression pattern of LIR3/ILT5, we could not detect LIR1/ILT2. This discrepancy may be due to the different sources of antibodies and donor variability (mildly atopic patients in our study and healthy individuals in their work34 ).
While several studies have compared peripheral blood eosinophils with eosinophils recovered from the bronchoalveolar lavage fluid,35,36 this is the first study that directly compares blood and tissue eosinophils, taking into account that eosinophils in the 2 compartments may display surface phenotypes. For example, expression patterns of activatory receptors such as NKp30, NKp44, and NKp46 vary between NK-cell populations in peripheral blood, spleen, and lymph node.37 More relevant to our study, blood eosinophils from healthy individuals exhibit low or undetectable expression of MHC II proteins,38 whereas airway eosinophils of asthmatics or eosinophils from antigen-challenged atopic subjects express evident MHC II.35,36
IRp60 does not influence eotaxin-induced calcium influx. Eosinophils previously incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by the cross-linker sheep anti-mouse F(ab′)2 were washed and loaded with Calcium Green-1AM. The cells were allowed to flow freely in the cytometer until the indicated time point at which eotaxin (1-100 ng/mL) was added (↑). [Ca2+] is expressed as mean fluorescent intensity (MFI). NA indicates not activated; Etx, eotaxin activated (n = 3). Data are expressed as relative cell count ± SD and are representative of 1 of 4 experiments.
IRp60 does not influence eotaxin-induced calcium influx. Eosinophils previously incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by the cross-linker sheep anti-mouse F(ab′)2 were washed and loaded with Calcium Green-1AM. The cells were allowed to flow freely in the cytometer until the indicated time point at which eotaxin (1-100 ng/mL) was added (↑). [Ca2+] is expressed as mean fluorescent intensity (MFI). NA indicates not activated; Etx, eotaxin activated (n = 3). Data are expressed as relative cell count ± SD and are representative of 1 of 4 experiments.
Cross-linking of IRp60 inhibits JAK2 and MAP kinase phosphorylation in response to IL-5 and eotaxin. Eosinophils that were treated with anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by sheep anti-mouse F(ab′)2 were washed and IL-5 (50 ng/mL; A-C) or eotaxin (10-100 ng/mL; D) was added for the indicated time points. In panel A, after the activation step, cells were lysed and precleared. Thereafter, IL-5Rβ chain was immunoprecipitated (IP), analyzed by SDS-PAGE, transferred to a membrane, and blotted with anti-phospho JAK2 (IB). Data shown are representative of 1 of 2 experiments. In panels B and D, after the activation step, the cells were fixed, permeabilized, and stained with mouse anti-phospho p38 (p-p38) or rabbit anti-phospho ERK 1/2 (p-ERK) followed by FITC-labeled goat antimouse and Cy5-labeled goat antirabbit. The intracellular staining was assessed by FACS. Data are representative of 1 of 3 experiments. In panel C, after the activation step, cells were lysed and analyzed by SDS-PAGE, transferred to a membrane, and blotted against phospho ERK 1/2 (IB). NA (dark gray shading) indicates nonactivated; Iso (solid line), isotype-matched control antibody treatment; IRp60 (bold solid line), anti-IRp60 treatment; p58.2 (dashed line), epitope control antibody treatment; IL-5 (light gray shading, B), IL-5 treatment; and Etx (light gray shading, D), eotaxin treatment.
Cross-linking of IRp60 inhibits JAK2 and MAP kinase phosphorylation in response to IL-5 and eotaxin. Eosinophils that were treated with anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by sheep anti-mouse F(ab′)2 were washed and IL-5 (50 ng/mL; A-C) or eotaxin (10-100 ng/mL; D) was added for the indicated time points. In panel A, after the activation step, cells were lysed and precleared. Thereafter, IL-5Rβ chain was immunoprecipitated (IP), analyzed by SDS-PAGE, transferred to a membrane, and blotted with anti-phospho JAK2 (IB). Data shown are representative of 1 of 2 experiments. In panels B and D, after the activation step, the cells were fixed, permeabilized, and stained with mouse anti-phospho p38 (p-p38) or rabbit anti-phospho ERK 1/2 (p-ERK) followed by FITC-labeled goat antimouse and Cy5-labeled goat antirabbit. The intracellular staining was assessed by FACS. Data are representative of 1 of 3 experiments. In panel C, after the activation step, cells were lysed and analyzed by SDS-PAGE, transferred to a membrane, and blotted against phospho ERK 1/2 (IB). NA (dark gray shading) indicates nonactivated; Iso (solid line), isotype-matched control antibody treatment; IRp60 (bold solid line), anti-IRp60 treatment; p58.2 (dashed line), epitope control antibody treatment; IL-5 (light gray shading, B), IL-5 treatment; and Etx (light gray shading, D), eotaxin treatment.
Intriguingly, our data show that 25% of human peripheral blood eosinophils express the inhibitory receptor p140, whereas nasal polyp eosinophils do not. A member of the killer immunoglobulin-like receptor (KIR) family, p140 is known to inhibit NK functions in response to HLA-A alleles such as HLA-A3 and HLA-A11.39 It is detected on NK-cell subsets, on CD3+/CD8+ cells in peripheral blood, and on CD4+ cutaneous T-cell lymphoma cells.39-41 Therefore, its limited expression on the peripheral blood eosinophils is not atypical. Since HLA-A3, -B7, and DRw2 display a decreased frequency in intrinsic asthma,42 it is possible that this expression pattern can influence p140+ eosinophil activation thresholds.
Cross-linking of IRp60 recruits SHP-1 but not SHP-2. Eosinophils that were treated with anti-IRp60 or isotype control antibody followed by sheep anti-mouse F(ab′)2 were washed and IL-5 (50 ng/mL) was added for the indicated time points. In panel A, after the activation step, cells were lysed and precleared. Thereafter, IRp60 was immunoprecipitated (IP), analyzed by SDS-PAGE, transferred to a membrane, and blotted with antibodies to phospho tyrosine (pY), SHP-1, and SHP-2 (IB). Data shown are representative for 1 of 2 experiments.
Cross-linking of IRp60 recruits SHP-1 but not SHP-2. Eosinophils that were treated with anti-IRp60 or isotype control antibody followed by sheep anti-mouse F(ab′)2 were washed and IL-5 (50 ng/mL) was added for the indicated time points. In panel A, after the activation step, cells were lysed and precleared. Thereafter, IRp60 was immunoprecipitated (IP), analyzed by SDS-PAGE, transferred to a membrane, and blotted with antibodies to phospho tyrosine (pY), SHP-1, and SHP-2 (IB). Data shown are representative for 1 of 2 experiments.
Recently, 2 inhibitory pathways that regulate eosinophil biology have been characterized. Nutku et al12 have shown that siglec-8 actively induces rapid apoptosis of human eosinophils. Importantly, siglec-8 is not a “classical” inhibitory receptor, as it contains both an ITIM and an immunoreceptor tyrosine-based switch motif (ITSM) that may recruit either inhibitory phosphatases (such as SHP-1 and/or SHP-2) or activatory molecules (such as SAP and/or EAT-2).43 Therefore, the interaction between these intracellular components may tune the outcome of siglec-8 activation on human eosinophils, directing it toward apoptosis. In addition, Fulkerson et al13,14 have demonstrated that murine CCR3 is capable of delivering inhibitory signals upon binding to Mig. However, to the best of our knowledge, there have been no reports concerning negative signals delivered by “traditional” exclusive ITIM-bearing inhibitory receptor pathways in human eosinophils.
Hence we studied the ability of IRp60 to inhibit eosinophil chemotaxis, survival, and activation in response to eotaxin GM-CSF and IL-5. We observed that cross-linking of IRp60 on the surface of eosinophils significantly inhibited all these responses. In addition, IRp60 was able to inhibit LTB4-induced eosinophil transmigration. However, cross-linking of IRp60 did not inhibit TNF-α-mediated activation of human eosinophils (data not shown), suggesting that IRp60 is not able to inhibit all signaling modules. It is possible that IRp60 cross-linking may block src kinase-dependent activation pathways, leaving others such as TNF receptor family signaling pathways unaffected.
In survival experiments, but not the transmigration or the mediator-release assays, it was observed that increasing concentrations of IL-5/GM-CSF augmented the inhibitory response of IRp60 in a concentration-dependent fashion. Moreover, in eosinophils from 2 donors, high concentrations of IL-5/GM-CSF (50 ng/mL) did not require additional IRp60 cross-linking by a sheep anti-mouse F(ab′)2 antibody. In these cases, incubating the cells with an anti-IRp60 mAb alone was sufficient to establish a minor but statistically significant inhibitory effect (0.5-fold increase in apoptotic cells, P < .05; data not shown). However, additional cross-linking of IRp60 amplified the inhibitory response significantly (6.5-fold increase in apoptotic cells; P < .001).
The ability of IL-5/GM-CSF to increase ITIM-bearing receptor function has been described.12 To effectively induce apoptosis of freshly isolated eosinophils, siglec-8 needs to be cross-linked by an additional secondary antibody. However, when the cells are exposed to IL-5/GM-CSF, no additional cross-linking is needed.12 Importantly, there is a major difference between siglec-8 and IRp60. IRp60 does not actively induce apoptosis but rather inhibits the effects of IL-5/GM-CSF on eosinophil survival (data not shown). The ability of IL-5/GM-CSF to enhance these responses elicited by inhibitory receptors is probably due to the diverse intracellular signaling cascades that are recruited upon activation and their cross talk with phosphatases recruited by inhibitory receptors.44 Therefore, we speculate that the potency of IRp60 inhibition may be influenced by different intracellular signaling mechanisms and their cross talk.
We showed that IRp60 strongly suppresses eosinophil activation in response to IL-5/GM-CSF. While not all eosinophils of our donors were activated by either IL-5 or GM-CSF, in those that were stimulated by IL-5/GM-CSF, cross-linking of IRp60 inhibited activation. Although the overall inhibitory effect on cytokine release might have been slightly affected by increased eosinophil apoptosis at 18 hours (Figure 2B), these would not account for the complete inhibition that was observed, as only 16% to 19% of the cells were apoptotic when cytokine release was measured.
One of the main functions attributed to eosinophils in allergic settings such as bronchial asthma and atopic dermatitis is to modulate fibroblast properties; one of these properties is to enhance fibroblast proliferation.45-48 As expected, supernatants from IRp60-treated eosinophils exhibited an attenuated mitogenic effect on fibroblasts, emphasizing the biologic relevance of eosinophil inhibition through IRp60.
We were then interested in elucidating the downstream events of IRp60's effects. Eotaxin binding to CCR3 causes marked calcium influx. Although cross-linking of IRp60 inhibited eosinophil transmigration, it did not inhibit calcium influx. This observation is compatible with that of Fulkerson et al13 who showed that inhibition of eosinophil transmigration does not always correlate with inhibition of calcium influx.
IL-5 and eotaxin have been shown to activate several eosinophil signaling pathways including JAK2, Src-family, and MAP kinases.31,49 Thus, we were interested in investigating whether IRp60 inhibits downstream effector mechanisms. We found that IRp60 potently inhibited JAK2 phosphorylation as well as p38 and ERK1/2 phosphorylation in response to IL-5 and eotaxin stimulation.
Certainly, IRp60 has been shown to recruit SHP-1 and SHP-2, both of which are able to dephosphorylate docking sites for Src-family kinases on ITAM-bearing receptors and receptor tyrosine kinases (such as c-kit).50 Interestingly, in eosinophils, IRp60 recruited SHP-1 but not SHP-2; thus, indicating a prominent role for SHP-1 in IRp60 downstream effects.
The inhibitory effect(s) of IRp60 on IL-5/GM-CSF/IL-3 and CCR3 signaling does not rule out its potential to inhibit activatory signals coming from other receptors as well.
Recently it was shown that LIR3/ILT5 inhibits LIR7 activation of human basophils.51 Since the inhibitory machinery is essentially the same between LIR3/ILT5 and IRp60, we may speculate that IRp60 could also suppress the effects of other potent activatory receptors such as LIR734 and 2B4,21 as well as those of additional cytokine and growth factor receptors present on human eosinophils. In fact, we have found that cross-linking of IRp60 can significantly inhibit IgE-dependent activation of human cord blood-derived mast cells. Furthermore, cross-linking of IRp60 inhibits c-kit-mediated survival of human cord blood-derived mast cells.52 The finding that IRp60 can inhibit both mast cell and eosinophil activation strengthens once more the importance of this receptor in allergic and T-helper 2-associated immune responses.
In summary, IRp60 regulates critical checkpoints in eosinophil functions, namely recruitment, survival, activation, and subsequent fibroblast proliferation. Therefore, we believe that IRp60 is a novel pathway that might be exploited therapeutically in the future for suppressing the activity of human eosinophils in allergic diseases.
Prepublished online as Blood First Edition Paper, October 27, 2005; DOI 10.1182/blood-2005-07-2926.
Supported by an Applied Science Grant from the Yissum Research Development Company (Hebrew University) and a grant from the Italian Ministry of Foreign Affairs for joint research with Israel.
A. Munitz and I.B. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Howard Katz (Harvard University) and Dr Marco Colonna (Washington University) for many important discussions and suggestions; the Fondazione Compagnia di San Paolo, Turin, Italy; Roni Gilad for technical assistance; and Madelyn Segev for her editorial assistance.

![Figure 3. IRp60 inhibits eosinophil-mediated fibroblast proliferation. Eosinophils previously incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by the cross-linker sheep anti-mouse F(ab′)2 were washed and incubated with various concentrations of IL-5/GM-CSF (50 ng/mL) for 18 to 20 hours. Cell supernatants were assessed for their ability to induce 3T3 fibroblast proliferation as assessed by [3H]-thymidine incorporation. Data are expressed as mean CPM/well ± SD (n = 3; **P < .005).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-07-2926/2/m_zh80050691790003.jpeg?Expires=1763475505&Signature=HdIq3igei~I6jRsuZ9Qbm6h5dTY4Up8bknKz5~eEoPcd1L5CXEoKUF4EE5xOXtriizRNdadVVu1UmZ3r2PScuie4F1Hi9nfXt~B8RSjmluH0x26x9oo1vtadl6etyflPzAu3MVzvniQTzwZOB2sBsK2KYsJwjXqxpyh-9kAOpThQQ1UhNqywRrD3VBq0cCc8acAVgEiNZMd1p2Xqo8ploB~k5QpHDx0Rw1vfwwy4DIPN-kWG~y2EVbjQX-R9HGFHtAZtAkltmbb3SpWddSy3MtmcAwL2kufWOhaiuGj2-sZ1qIrQz7YDplY0tq6vutoDe2R0jONa5LKkHrp9DQXGkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. IRp60 does not influence eotaxin-induced calcium influx. Eosinophils previously incubated with either anti-IRp60 or control antibodies (isotype, anti-p58.2) followed by the cross-linker sheep anti-mouse F(ab′)2 were washed and loaded with Calcium Green-1AM. The cells were allowed to flow freely in the cytometer until the indicated time point at which eotaxin (1-100 ng/mL) was added (↑). [Ca2+] is expressed as mean fluorescent intensity (MFI). NA indicates not activated; Etx, eotaxin activated (n = 3). Data are expressed as relative cell count ± SD and are representative of 1 of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-07-2926/2/m_zh80050691790004.jpeg?Expires=1763475505&Signature=NqCrOnIiFB2KOycEr1dzoz8l2eo8kPbkjSzOXFN8qsXyYv1Lpo0l1TGA8xT9mHGlglTjOWn4GO2sK0djVS2w7y3XadHZynwmVQoN6-npvzvwhXm1CHVpgjVU4C~zqpva~S46LAYkpL2dHXMrxs~7~Tg84tIGjUv8NN1SMXKAFvwuGN7zc8XEOPlasiVJ-A-I8uQV3yWHvWJgGqmPMLDwRCbdm-3vqI1w3u2nMwULMyyktXEF-QyRfdB7WETkf7hx24AkeDqUJnjB~5xW0Wez9SYyYzbUkmVzkIrknFTggbeeIEUr-59yNdmhodta90NI8nLY9oJRC22ti4u7j4WPHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)