Abstract
Plasmacytoid dendritic cells (pDCs) play an important role in innate and adaptive immunity, prompting interest in mechanisms controlling the production of this lineage of cells. Notch signaling via one of the Notch ligands, delta-like 1 (delta-1), influences the hematopoietic development of several lymphoid and myeloid lineages, but whether or not delta-1 affects the formation of pDCs is unknown and was tested here. Human CD34+ progenitor cells were cultured onto delta-1–expressing OP9 stroma in the presence of flt-3 ligand and IL-7, and this efficiently generated BDCA-2+ CD123+ CD4+ CD11c– cells with the characteristic morphology of pDCs, expressing toll-like receptor-9 (TLR9), pre-Tα mRNAs, and secreting CpG-induced IFN-α. Delta-1 augmented the numbers of BDCA-2+ cells produced without affecting their proliferation, and the effect was blocked by γ-secretase inhibition. The development of pDCs was stroma-, delta-1–, and cytokine-dependent and could be induced from committed lymphoid progenitor cells, which responded to delta-1 by opposite changes in pDC- and B-cell production. Our results identify delta-1 as a novel factor enhancing pDC hematopoiesis and delineate a new role for Notch signaling in lymphopoiesis by showing its opposite effect on pDC and B lineage determination.
Introduction
Dendritic cells (DCs) constitute a complex system of antigen-presenting cells (APCs) providing the immune system with multiple possibilities for antimicrobial and antiviral host defenses (recently reviewed by Colonna et al1 ). Within this system, plasmacytoid DCs (pDCs) are distinguished from so-called conventional DCs by the expression of specific microbial pattern recognition receptors and by the unique ability to secrete high levels of type I interferon (IFN) in response to viruses. Being immature precursor cells, pDCs poorly stimulate T cells and may be tolerogenic but, once activated, they can direct various types of T-cell responses depending on associated pathogenic or inflammatory signals. In humans, pDCs are recognized by expression of the C-type lectin BDCA-2, BDCA-4/neuropilin-1, CD4, and high levels of the IL-3Rα chain (CD123). They have low levels of major histocompatibility complex (MHC) class II antigens and lack expression of CD1a or CD11c, which are found on conventional DCs.2 Several lymphoid transcripts are expressed in pDCs, such as pre–T-cell receptor α (pre-TCRα) chain, λ5, IgH D-J gene rearrangements, and Spi-B, and not found in most conventional DCs.3 Altogether, pDCs constitute a distinct and important component of innate and adaptive immunity. They have been implicated not only in viral defenses but also in autoimmunity as well as immune regulations occurring in solid tissue and bone marrow (BM) transplantation.1,4 Delineating the molecular mechanisms that contribute to the production of pDCs could therefore provide new insights into the physiopathology and therapies of such conditions.
The hematopoietic development of DC lineages seems strikingly flexible compared with other leukocytes. Recent studies have formally established that pDCs, like conventional DCs, have diverse origins and can arise from progenitor cells engaged toward lymphoid or myeloid differentiation programs.5-8 One challenge is to identify the soluble factors and cell-contact–dependent signals that regulate the development of specific DC progenitor cells. The cytokine flt-3 ligand (FL) has emerged as a major regulator of pDC development in vivo and in vitro.8-10 Cell-to-cell interactions are generally important during developmental processes, particularly for lymphoid cells. Interactions between Notch receptors and Notch ligands constitute an evolutionarily conserved example of interactions between precursor cells and their surrounding microenvironment. Notch receptors (Notch 1-4) and their ligands (classified into 2 families of proteins: Jagged/serrate [Jagged 1 and 2] and delta [delta-like 1 (delta-1), 3, and 4]) are widely expressed in the hematopoietic system (recently reviewed by Radtke et al11 ). Notch signaling is a prominent regulator of the immune system essentially through marked effects on T/B lymphopoiesis and terminal T-cell differentiation.12-17 One of the Notch ligands, delta-1, is a major inducer of Notch signaling in lymphoid cells. Stromal cell lines expressing delta-1 support T-cell differentiation,18-20 facilitate T/natural killer (NK) precursor development, and block B lymphopoiesis.21,22 In contrast to lymphoid cells, much less is known about the effects of delta-1 or Notch signaling in DC development. In humans, delta-1 acts as a negative regulator of monocyte/macrophage differentiation23 and induces monocyte apoptosis specifically in the presence of M-CSF.24 Delta-1 does not seem to affect the differentiation of myeloid precursor cells into conventional DCs.23 Whether delta-1 has a role in pDC development is not known. Because delta-1 affects lymphoid progenitor cells, and because pDCs have some lymphoid characteristics, we hypothesized that delta-1 may regulate pDC formation. We adapted a culture system used for lymphoid differentiation18-20,22 based on M-CSF–deficient OP9 stromal cells and the cytokines FL and IL-7 to test if human delta-1 affected human pDC hematopoiesis. Our results show that delta-1 supports pDC formation from hematopoietic progenitor cells and is particularly efficient on committed lymphoid progenitor cells, which differentiate into pDCs while B lymphopoiesis is blocked. Our results therefore delineate a new role for this Notch ligand in lymphopoiesis.
Materials and methods
Hematopoietic progenitor cells
Samples of umbilical cord blood (UCB) and adult BM were obtained in accordance with the French bioethics laws and the French National Bioethics Committee and with approval from the Banque de Tissus pour la Recherche scientific review committee. For BM samples, informed consent was provided in accordance with the Declaration of Helsinki. Mononuclear cells (MNCs) were isolated by density centrifugation (Lymphoprep; AbCys, Paris, France). UCB CD34+ cells were purified using magnetic-activated cell sorting and microbead-conjugated CD34 antibodies according to the manufacturer's recommendations (Miltenyi Biotec, Bergisch Gladbach, Germany) and generally cryopreserved before use. BM lymphoid progenitor cells were prepared from cryopreserved BM MNCs using flow cytometry cell sorting (MoFlo, Dako Cytomation, Trappes, France) with the following antibodies: APC-conjugated CD34 (clone 581, BD Biosciences, Le pont de Claix, France), FITC-conjugated lineage (Lin) markers (TCRαβ, clone T10B9.1A-31, BD Biosciences; CD14, clone 3C10E12 prepared in our laboratory (ATCC, Manassas, VA); CD40 clone 5C3, BD Biosciences; CD19, clone SJ25-C1, Caltag Laboratories, Burlingame, CA; CD20, clone H147, Caltag Laboratories), and PE-conjugated CD10 (clone SJ-1B4, Caltag Laboratories).
Generation of OP9 stroma cell lines
The cDNA encoding human delta-1 (delta-like 1 accession AF003522 - gi 10518496) was kindly provided by S. Artavanis-Taskonas (Harvard Medical School, Charlestown, MA). The open reading frame (ORF) (EcoRV-XbaI 2897 bp fragment) was cloned into the BamHI site of the multiple cloning site of the self-inactivating pWPIReGFP bicistronic HIV-1–derived lentiviral transfer vector, kindly provided by P. Salmon (University of Geneva, Switzerland) and allowing concomitant expression of human delta-1 under the control of the EF1-α promoter and of the green fluorescent protein (GFP) through an ECMV internal ribosomal entry site (IRES) sequence. The vector encoding only GFP was used as control. VSV-G–pseudotyped lentiviral vector stocks were produced by quadritransfection of 293T cells as described.25 The same parental stock of OP9 cells26 was transduced either with delta-1/GFP-encoding lentiviral vector to produce OP9-Del1 cells or with the control GFP vector to produce OP9-C control cells. Lines expressing high levels of transgene were enriched by flow cytometry cell sorting based on GFP expression. OP9 cells are known to lack expression of murine delta-1.22 High levels of human delta 1 expression were confirmed in OP9-Del1 cells, but absent in OP9-C cells, by real-time polymerase chain reaction (PCR). Primers and probes sequences were hDel1 F: CTCCTGAGGTCCTCGACGC; hDel1 R: CGACGTCACGGAAGGCAG; hDel1 P: ACAGCCTGTCGCGGCCCG; expression of target gene was normalized using the endogenous gene B2M (Taqman PDAR reagent; Applied Biosystems, Foster City, CA) (data not shown).
Coculture assay
Cocultures were initiated by seeding 2 × 104 to 3 × 104 CD34+ cells per well of a 24-well plate into which OP9-Del1 or OP9-C cells were seeded the day before at 28 000/cm2. Culture medium (0.5 mL per well of R10 medium) consisted of RPMI 1640 supplemented with 10% FCS (Hyclone, Logan, UT), l-glutamine, and penicillin/streptomycin (Gibco BRL, Life Technologies, Paisley, Scotland) that was supplemented with recombinant human FL and human interleukin-7 (IL-7) (5 ng/mL each; R&D Systems, Minneapolis, MN) unless otherwise indicated. In some experiments, γ-secretase inhibitor (N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester [DAPT]) was added every 3 to 4 days to culture medium (10 μg/mL; Calbiochem, EMD Biosciences, San Diego, CA), and controls consisted of treating cultures with the same concentration of DMSO carrier. Cells were collected at different time points after forceful pipetting to disrupt stroma, counted, and used for subsequent analyses and experimental assays. For transwell experiments, CD34+ cells were separated from the OP9 stroma by 0.4 μm pore size membrane inserts (Corning BV, Life Sciences, Schiphol-Rijk, The Netherlands).
Flow cytometric analysis
Directly conjugated mouse anti–human monoclonal antibodies (mAbs) included APC-conjugated CD19 (clone SJ25-C1, Caltag Laboratories), HLA-DR (clone TU36, Caltag Laboratories), CD14 (clone TuK4, Caltag Laboratories), PE-conjugated CD56 (clone B159, BD Biosciences), CD1a (clone VIT6B, Caltag Laboratories), CD11c (clone BU15, Caltag Laboratories), CD4 (clone S3.5, Caltag Laboratories), FITC-conjugated CD3 (clone S4.1, Caltag Laboratories), and isotype control mAbs. BDCA-2 FITC, CD123 PE, BDCA-4 APC, and BDCA-1 FITC were from Miltenyi Biotec. Cells were stained with mAbs for 30 minutes on ice and washed twice with PBS, 0.2% BSA, and 0.02% NaN3. Cellular staining was measured on a FACSCalibur instrument (BD Biosciences), and data were analyzed using CellQuest software, with results expressed as percentages of cells staining above background staining obtained with irrelevant mAbs. Stromal cells and dead cells were excluded from analysis. For cell-cycle experiments, DNA content was detected using Hoechst 33342 DNA binding dye (Molecular Probes, Eugene, OR). Cells were resuspended at 1 × 106/mL in HBSS, 10% FCS, 1 g/L glucose, 2 mM Hepes, and 10 μg/mL Hoechst 33342 during 90 minutes at 37°C. Cells were washed and labeled with directly conjugated BDCA-2 FITC antibodies. Simultaneous analysis of DNA content and surface phenotype was performed on a dual laser LSR instrument (UV 488 nm; BD Biosciences).
RT-PCR
Total RNA was extracted from 5 × 105 total cocultured cells using Wizard SV total RNA isolation system (Promega, Madison, WI) and was reverse transcribed using random hexamers according to the manufacturer's instructions (SuperScript first strand synthesis system for reverse transcriptase–PCR; Invitrogen, Paisley, Scotland).
PCR primers sequences were as follows: TLR9 F: TTATGGACTTCCTGCTGGAGGTGC; TLR9 R: CTGCGTTTTGTCGAAGACCA; pre-Tα F: GGCACACCCTTTCCTTCT; pre-Tα R: GCAGGTCCTGGCTGTAGAAGC; TLR4 F: CTGCAATGGATCAAGGACCA; TLR4 R: TCCCACTCCAGGTAAGTGTT; HPRT F: TATGGACAGGACTGAACGTCTTGC; HPRT R: GACACAAACATGATTCAAATCCCTGA. PCR products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining.
In vitro activation
For in vitro activation, 2 × 105 to 5 × 105 total cocultured cells were transferred to a well of a 24-well tissue culture plate in R10 medium supplemented or not with a mixture of recombinant human CD40L trimer (1 μg/mL; a kind gift from Immunex) and IL-1β (10 ng/mL; R&D Systems), CpG oligodeoxynucleotide type A (ODN 2216, 2 μM), poly I:C (50 μg/mL), or peptidoglycan (PGN) (10 μg/mL) (InvivoGen, San Diego, CA). After 24 to 48 hours, cell-supernatant fluids were collected and stored frozen at –20°C until used to measure IL-8 (BD PharMingen, San Diego, CA) and IFN-α (Biosource, Camarillo, CA) contents by enzyme-linked immunosorbent assay (ELISA) according to manufacturer's instructions. Purified pDCs (BDCA-2+ CD123+ cells or BDCA-4+ CD123+ cells) obtained from coculture by flow cytometry cell sorting were activated in culture medium supplemented with IL-3 (25 ng/mL) and for 72 hours as described.27
Statistical analysis
The statistical analysis of data was performed by the paired t test using 95% confidence interval for significance.
Results
Delta-1 induces human CD34+ cells to differentiate into pDCs
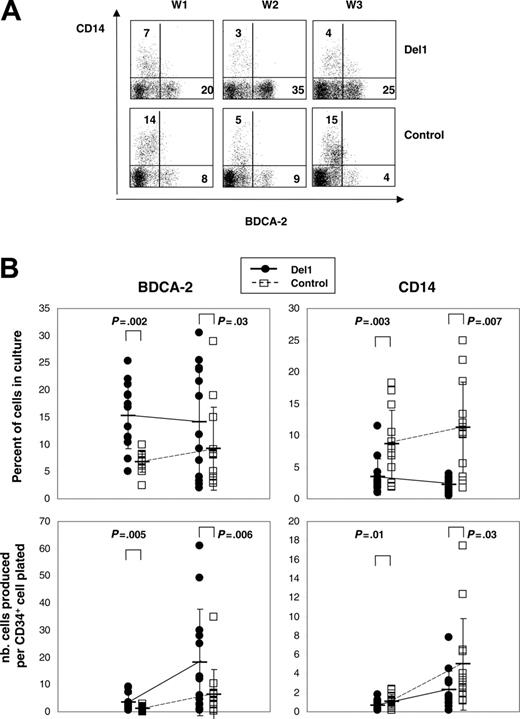
To examine the effects of human delta-1 on pDC hematopoiesis, we compared how M-CSF–deficient OP9 stromal cells expressing or not expressing this molecule affected growth and differentiation of multipotent hematopoietic progenitor cells in cultures supplemented with FL and IL-7. In this system, UCB CD34+ cells grew and differentiated over time. In 20 days, total cell numbers expanded 98-fold ± 57-fold on OP9-Del1 cells, which was higher than 61-fold ± 52-fold on OP9-C stroma (n = 17 experiments, P < .001). Flow cytometric analyses were performed at different time points to monitor hematopoietic differentiation through the expression of mature cell markers on cultured cells. Representative kinetics studies showed that BDCA-2, a marker of pDCs, was readily detected after 1 week in both OP9-Del1 and OP9-C cultures and was expressed, as expected, on a well-defined population lacking the CD14 antigen (Figure 1A). The percentage of BDCA-2+ cells was significantly higher in OP9-Del1 cultures than in control cultures as measured at day 10 (15% ± 6% versus 7% ± 2%) and at day 20 (14% ± 10% versus 9% ± 8%) in a total of 13 separate experiments (Figure 1B). Over time, higher total numbers of BDCA-2+ cells were generated in OP9-Del1 cultures compared with control (Figure 1B). In approximately 3 weeks, we calculated that a single CD34+ cell yielded in theory 18 BDCA-2+ cells in the OP9-Del1 system, compared with 6 BDCA-2+ cells in control cultures.
Differentiation of human CD34+ cells into BDCA-2+ cells. UCB CD34+ cells were cultured on either OP9-Del1 or OP9-C stroma for up to 3 weeks in the presence of FL and IL-7 cytokines. (A) Flow cytometric analysis of the expression of BDCA-2 and CD14 on the cultured cells at 1, 2, and 3 weeks (W) of culture. These plots represent 1 representative kinetic experiment of 2. Numbers indicate the percentage of cells in each quadrant. Irrelevant Ig controls provided less than 2% background staining. (B) Percentages and numbers of BDCA-2+ and CD14+ cells produced after 10 and 20 days of culture. The data are from 13 different experiments, each represented by a symbol. Average values are indicated by a bar with representation of standard deviation values. Statistical analysis compared the control and Del1 groups at each time point, and the P values of the paired t test are indicated.
Differentiation of human CD34+ cells into BDCA-2+ cells. UCB CD34+ cells were cultured on either OP9-Del1 or OP9-C stroma for up to 3 weeks in the presence of FL and IL-7 cytokines. (A) Flow cytometric analysis of the expression of BDCA-2 and CD14 on the cultured cells at 1, 2, and 3 weeks (W) of culture. These plots represent 1 representative kinetic experiment of 2. Numbers indicate the percentage of cells in each quadrant. Irrelevant Ig controls provided less than 2% background staining. (B) Percentages and numbers of BDCA-2+ and CD14+ cells produced after 10 and 20 days of culture. The data are from 13 different experiments, each represented by a symbol. Average values are indicated by a bar with representation of standard deviation values. Statistical analysis compared the control and Del1 groups at each time point, and the P values of the paired t test are indicated.
Because CD34+ cells decreased similarly over time in both OP9-Del1 and OP9-C cultures, the results suggested that delta-1 enhanced the differentiation of hematopoietic progenitor cells into BDCA-2+ cells or, alternatively, that delta-1 expanded committed cells. Comparisons of cell-cycle analyses on BDCA-2+ cells produced in OP9-Del1 or OP9-C cultures showed identical distribution of cells in the various phases of the cell cycle and, in particular, no augmentation of cells in G2-M in cells produced on OP9-Del1 stroma (Figure 2). These results argue against an effect of delta-1 on BDCA-2+ cell expansion and support the interpretation of enhanced hematopoietic differentiation of CD34+ cells into the BDCA-2+ cell lineage.
BDCA-2+ cells generated onto OP9-Del1 displayed characteristic pDC features
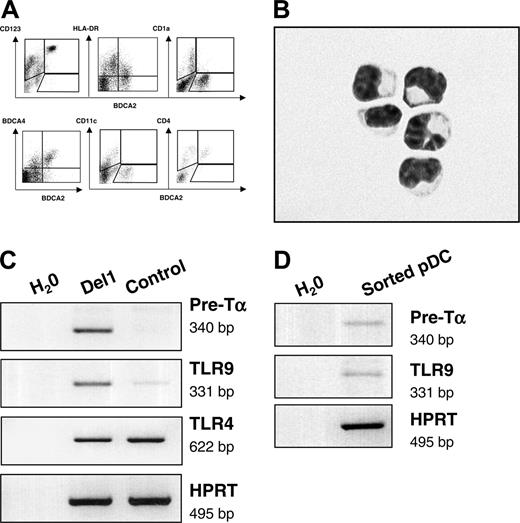
The entire population of cells expressing BDCA-2 that was produced onto OP9-Del1 stroma also displayed high levels of CD123 as well as BDCA-4 and CD4, intermediate levels of HLA-DR, and lack of expression of CD11c, CD1a, and CD14 antigens (Figures 1A and 3A). Overall, this is a cell-surface phenotype that defines so-called circulating precursors of pDCs.1,2 As a confirmation, and to exclude trivial artifacts from dying OP9 cells, we purified cells coexpressing the human-specific CD45 marker with BDCA-2 and CD123 from OP9-Del1 cocultures. We found that such cells displayed the characteristic morphology of immature pDCs, with small size and a high nucleus-cytoplasm ratio (Figure 3B). The molecular signature of pDCs was evident in OP9-Del1 cocultures with the detection of transcripts for pre-TCRα chain and toll-like receptor-9 (TLR9) mRNAs (Figure 3C). These transcripts were more abundantly expressed in the presence of delta-1, consistent with the augmented numbers of BDCA-2+ cells.
pDCs express specific microbial pattern recognition receptors such as TLR9 for recognition of DNA with high CpG content. Conventional DCs express TLRs 2 to 5 for recognition of bacterial products such as peptidoglycan or of poly I:C, which mimics viral double-stranded RNA.28 Activation of the bulk of cultured cells with various stimuli consisting of TLR ligands or of inflammatory stimuli confirmed the existence of a functional response via TLR9 because CpG oligonucleotides induced the secretion of high levels of IFN-α (Table 1). Such production of IFN-α was statistically higher in OP9-Del1 cultures than in controls, in a range that was compatible with the enhancement of pDC numbers. In such bulk cultures, delta-1 did not seem to affect the cellular capacity for IFN-α production, because the calculated production of IFN-α per BDCA-2+ cell was 0.19 ± 0.15 pg per cell in OP9-Del1 cultures versus 0.06 ± 0.06 pg per cell in OP9-C cultures (n = 5), which was statistically not significantly different. The induction of IFN-α was specific to CpG stimulation, as expected from published studies.28 We also directly confirmed that OP9-Del1–generated pDCs could produce IFN-α after CpG stimulation. Homogeneous populations of purified BDCA-2+ CD123+ or BDCA-4+ CD123+ cells were isolated by flow cytometry cell sorting, stimulated with CpG and IL-3 (IL-3 was used to maintain viability), and IFN-α was produced in all experiments (n = 3), averaging 0.05 ± 0.02 pg per cell, which is in the range of calculated values from bulk cultures. These values are also consistent with the literature, because pDCs produced in stroma-free cultures and activated with herpes virus produce an estimated 0.1 pg per cell IFN-α.27 Using these sorted cells we also confirmed the expression of pre-Tα and TLR9 mRNA (Figure 3D), which further demonstrated their pDC characteristics. In addition, the cells produced on OP9-Del1 were activated via CpG or via CD40L and IL-1, and both of these stimuli augmented the levels of CD86 cell-surface expression on the BDCA-2+ cell population (Table 2), providing evidence that phenotypic maturation could be induced on these cells. Thus, our data showed that delta-1 was an effective signal that enhanced the differentiation of hematopoietic progenitor cells into functional pDCs.
Cell-cycle analysis. UCB CD34+ cells were cultured on either OP9-Del1 (▪) or OP9-C stroma (▦), and after 7 to 14 days cells were stained with BDCA-2 and Hoescht to measure cell cycle by flow cytometry. Results represent the means ± SD values of gated BDCA-2+ cells in the different phases of cell cycle obtained from 4 independent experiments. A representative histogram of the cell cycle of BDCA-2+ cells cultured onto OP9-Del1 stroma is inserted.
Cell-cycle analysis. UCB CD34+ cells were cultured on either OP9-Del1 (▪) or OP9-C stroma (▦), and after 7 to 14 days cells were stained with BDCA-2 and Hoescht to measure cell cycle by flow cytometry. Results represent the means ± SD values of gated BDCA-2+ cells in the different phases of cell cycle obtained from 4 independent experiments. A representative histogram of the cell cycle of BDCA-2+ cells cultured onto OP9-Del1 stroma is inserted.
Effects of delta-1 on other hematopoietic lineages of cells
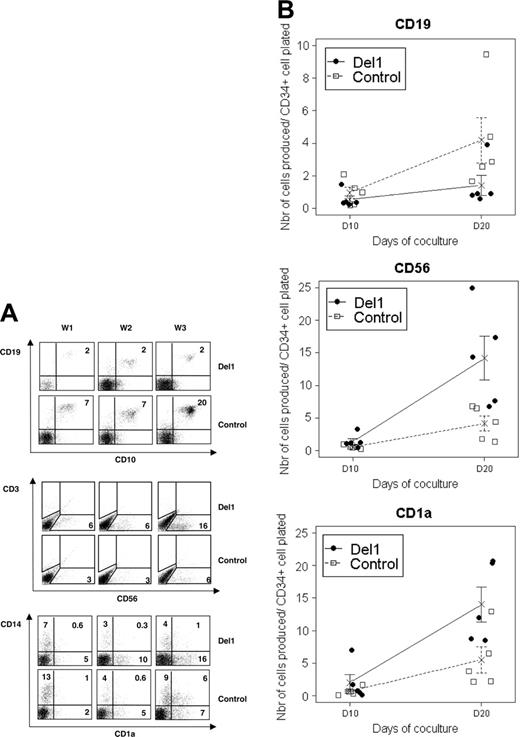
Multiple types of hematopoietic cells were generated in the OP9 coculture system, including cells of lymphoid lineages. Delta-1 significantly decreased CD19+ CD10+ B-cell differentiation because, at day 20, the percentages of CD19+ B cells in OP9-Del1 cultures were 1% ± 1% versus 6% ± 3% in OP9-C cultures (P = .02, n = 5) and, overall, approximately 3 times fewer CD19+ cells were produced in OP9-Del1 cultures compared with controls (P = .05) (Figure 4). In opposition, the production of CD56+ cells, putatively NK cells, was significantly augmented by delta-1 both in proportion (11% ± 5% versus 4% ± 1%, P = .04, n = 5) and total numbers of cells, because approximately 3.5 times more CD56+ cells were produced in 3 weeks onto OP9-Del1 stroma compared with controls (P = .03, n = 5) (Figure 4). T lymphocytes were not examined in these experiments. However, separate studies in our laboratory showed that our OP9-Del1 cells support the differentiation of murine lineage-negative bone marrow progenitor cells into CD3+ CD8+ T cells in 3 weeks, as expected from prior reports.18 Furthermore, our OP9Del1 cells support the differentiation of human CD34+ CD38– UCB progenitor cells into CD7+ CD3+ human T cells after more than 3 weeks of culture, as reported19,20 (Supplemental Figure S1, available on the Blood website; click on the Supplemental Figure link at the top of the online article).
As for myeloid cells, monocytic cells expressing CD14 were produced in small proportions, which were significantly reduced by delta-1, as shown in Figures 1 and 4. For instance, at day 20, CD14+ cells represented, on average, 2% ± 1% of cells in OP9-Del1 cultures versus 11% ± 7% in OP9-C cultures (P < .001, n = 13 experiments). We also identified populations of CD1a+ cells that were generated in these cultures (Figure 4). In the absence of other T-cell markers, the population of CD1a+ CD14– cells most likely represented conventional DCs rather than immature T cells. Indeed, such CD1a+ cells also coexpressed BDCA-1, CD11c, and high levels of HLA-DR antigens, which are characteristic DC markers (data not shown). Besides, we also observed CD1a+ CD14+ cells, resembling the intermediate cells generated during DC differentiation from CD14+ cells.29 There was no statistically significant effect of delta-1 on the numbers of total CD1a+ cells produced (n = 5). However, further analysis of subtypes of CD1a+ cells should be performed to determine if an effect of delta-1 exists on subsets of conventional DCs. At the functional level, cytokine secretion profiles confirmed that monocytes and conventional DCs were also generated with pDCs in the culture system. Stimulation of the cultures with the proinflammatory stimuli CD40L and IL-1 or with the TLR2 ligand PGN induced the secretion of, respectively, IL-12 and IL-8 cytokines, which were found at similar levels in OP9-Del1 and OP9-C cultures (Table 1). Both stimuli are known to activate cytokine secretion in CD1a+ interstitial DCs or Langerhans cells.28,30 The expression of mRNA for TLR4, a receptor known to be present on CD1a+ DCs and monocytes, appeared to be similar in both cultures (Figure 3C). Altogether, delta-1 significantly modulated the differentiation of hematopoietic progenitor cells into pDCs, CD14+ cells, B cells, and CD56+ lymphoid lineages in the same time frame. However, at the functional level, delta-1 did not appear to be a strong modulator of activities attributed to conventional DCs in this particular experimental setup.
Hematopoiesis induced by delta-1 is Notch-, contact-, and cytokine-dependent
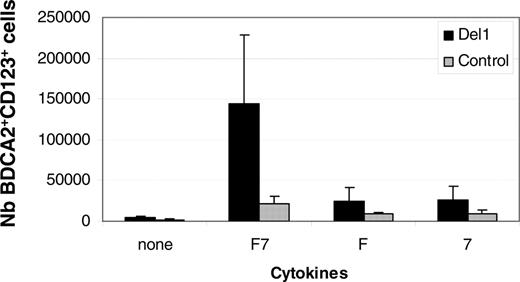
To confirm that delta-1 affected hematopoietic progenitor cell differentiation into pDCs through Notch signaling, we tested the effects of DAPT, an inhibitor of the γ-secretase responsible for the cleavage of Notch induced by ligand binding and a well-accepted inhibitor of the Notch signaling pathway.31 Treatment of cultures with DAPT significantly reduced the percentages and numbers of BDCA-2+ CD123+ cells produced in culture (Figure 5A). Concomitantly, and as expected, DAPT enhanced the levels of CD14+ cells while reducing that of CD56+ cells, thus validating the system (Figure 5B). To characterize the mode of action of delta-1, we compared cocultures performed by direct cell contact or through a transwell insert that physically separated progenitor cells and stroma. Results showed that direct contact between CD34+ cells and stroma was essential for the efficient production of BDCA-2+ pDCs (data not shown). The presence of stroma was confirmed to be essential because culturing CD34+ cells in the presence of FL and IL-7 cytokines but without OP9-Del1 cells did not generate pDCs (data not shown). While FL was known to be essential for the production of pDCs,9,32 the importance of IL-7, a lymphoid growth factor, in pDC differentiation was not known. Results showed that removal of either IL-7 or FL compromised the production of BDCA-2+ cells (Figure 6), demonstrating that a cooperation is needed between these 2 signals and delta-1 to efficiently support pDC formation.
BDCA-2+ pDCs that develop onto OP9-Del1 stromal cells have characteristic markers of pDCs. (A) Flow cytometric analysis on the cells produced in OP9-Del1 coculture, representative of 3 experiments. (B) Purified pDCs were obtained by flow cytometric isolation of CD45+ CD123+ BDCA-2+ cells from OP9-Del1 coculture. Cells were then spun on glass slides and stained with Wright Giemsa. Photograph was obtained with a Leica DFC320 digital color camera mounted onto a Leica DMRB microscope (Leica Microsystems, Wetzlar, Germany) using a 60×/1.32 NA oil objective and Leica IM 50 version 4.0 software. (C) Analysis of mRNA transcripts expressed by 1 × 106 cells obtained at day 20 of culture of CD34+ cells onto OP9Del1 or OP9-C. (D) mRNA transcripts expressed by sorted BDCA-4+ CD123 + cells.
BDCA-2+ pDCs that develop onto OP9-Del1 stromal cells have characteristic markers of pDCs. (A) Flow cytometric analysis on the cells produced in OP9-Del1 coculture, representative of 3 experiments. (B) Purified pDCs were obtained by flow cytometric isolation of CD45+ CD123+ BDCA-2+ cells from OP9-Del1 coculture. Cells were then spun on glass slides and stained with Wright Giemsa. Photograph was obtained with a Leica DFC320 digital color camera mounted onto a Leica DMRB microscope (Leica Microsystems, Wetzlar, Germany) using a 60×/1.32 NA oil objective and Leica IM 50 version 4.0 software. (C) Analysis of mRNA transcripts expressed by 1 × 106 cells obtained at day 20 of culture of CD34+ cells onto OP9Del1 or OP9-C. (D) mRNA transcripts expressed by sorted BDCA-4+ CD123 + cells.
The production of pDCs is induced from lymphoid progenitor cells
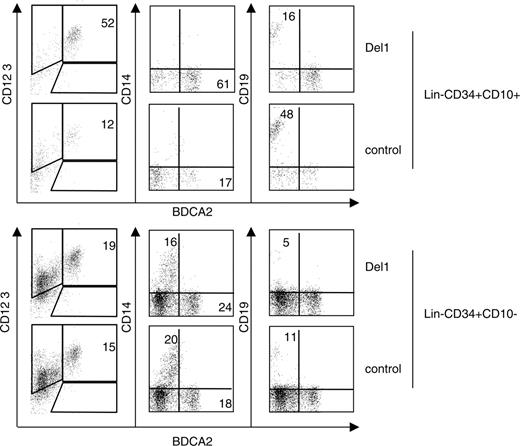
Because delta-1 significantly modulated pDC- and lymphoid-cell fates, we wondered if this signaling pathway could directly target a population of lymphoid progenitor cells. Several developmental origins of pDCs have been demonstrated in the murine system. In particular, BM lymphoid progenitor cells constitute one population capable of producing pDCs in vivo and in vitro in the presence of FL.5 In humans, a BM committed lymphoid progenitor-cell population (CLP) that displays T, B, DC, and NK-cell potential can be isolated on the basis of expression of CD34 and CD10 and lack of CD19 and other lineage markers.33 This lymphoid progenitor cell is more abundantly found in BM than UCB and, therefore, BM CLPs were tested here. The coculture of this CLP onto OP9-C in the presence of FL and IL-7 rapidly generated large proportions of CD19+ B cells (Figure 7), thus confirming its strong B lymphoid commitment.34 CLPs were able to produce pDCs. The presence of delta-1 strongly enhanced the proportion of BDCA-2+ CD123++ pDCs in CLP cultures (average, 43% ± 7% versus 20% ± 17% of cells in OP9-Del1 versus OP9-C cultures, n = 3 experiments) while consistently reducing B lymphoid differentiation (average, 19% ± 13% versus 41% ± 23% of cells in OP9-Del1 versus OP9-C cultures, n = 3 experiments) (Figure 7). There was little expansion, and total cell numbers produced could not be determined reliably in these experiments. These results demonstrate that pDCs can be produced directly and rapidly from a population of committed human BM lymphoid progenitor cells. Depending on the presence of delta-1, the CLPs were able to differentiate into B cells or into pDCs within the same time frame and in comparable proportions. The near complete lack of CD14+ cell production confirmed the peculiar lymphoid-restricted developmental potential of the CLPs, contrasting with the control population of CD34+ Lin– CD10– CD19– progenitor cells that comprise myeloid and multipotent hematopoietic progenitor cells (Figure 7). On such cells and at the time points examined, delta-1 had a modest effect on pDC- or B-cell formation. Therefore, at present, our results essentially show that delta-1 acts on lymphoid-committed cells to regulate pDC and B-cell lineage differentiation in opposite fashion.
Various hematopoietic lineages are produced on OP9-Del1 and OP9-C coculture. Cultures on OP9-Del1 (•) or OP9-C (□) were analyzed for expression of CD1a/CD14, CD19/CD10, and CD3/CD56 markers to identify monocytes, DCs, B cells, and NK cells. (Right) The yields of CD19+, CD56+, and CD1a+ cells normalized to the input of a single CD34+ cell after 10 and 20 days of culture. Data are from 5 different experiments, and average values are indicated by an X with representation of standard deviation values.
Various hematopoietic lineages are produced on OP9-Del1 and OP9-C coculture. Cultures on OP9-Del1 (•) or OP9-C (□) were analyzed for expression of CD1a/CD14, CD19/CD10, and CD3/CD56 markers to identify monocytes, DCs, B cells, and NK cells. (Right) The yields of CD19+, CD56+, and CD1a+ cells normalized to the input of a single CD34+ cell after 10 and 20 days of culture. Data are from 5 different experiments, and average values are indicated by an X with representation of standard deviation values.
pDC hematopoiesis is blocked by γ-secretase inhibition. (A) Representative fluorescence-activated cell sorter (FACS) analysis showing the modulation of BDCA-2 and CD14 expression by the γ-secretase inhibitor DAPT (γSI) or DMSO control. (B) Average percentages and total numbers of BDCA-2+ CD123+ cells ± SD produced in 3 independent experiments in the presence or not of γ-secretase inhibition with concomitant analysis of the changes in percentages of CD56+ NK cells and CD14+ monocytes in these cultures.
pDC hematopoiesis is blocked by γ-secretase inhibition. (A) Representative fluorescence-activated cell sorter (FACS) analysis showing the modulation of BDCA-2 and CD14 expression by the γ-secretase inhibitor DAPT (γSI) or DMSO control. (B) Average percentages and total numbers of BDCA-2+ CD123+ cells ± SD produced in 3 independent experiments in the presence or not of γ-secretase inhibition with concomitant analysis of the changes in percentages of CD56+ NK cells and CD14+ monocytes in these cultures.
pDC hematopoiesis is FL- and IL-7–dependent. UCB CD34+ cells were cultured onto OP9-Del1 (▪) or OP9-C (▦) in the presence of FL alone (F), IL-7 alone (7), or both FL and IL-7 (F7). Results show the average ± SD of the number of BDCA-2+ CD123+ cells produced at day 20 per well from 3 independent experiments.
pDC hematopoiesis is FL- and IL-7–dependent. UCB CD34+ cells were cultured onto OP9-Del1 (▪) or OP9-C (▦) in the presence of FL alone (F), IL-7 alone (7), or both FL and IL-7 (F7). Results show the average ± SD of the number of BDCA-2+ CD123+ cells produced at day 20 per well from 3 independent experiments.
Delta-1 enhances the development of bone marrow–derived committed lymphoid progenitor cells into pDCs. Common lymphoid progenitor cells were purified from bone marrow using flow cytometric sorting of Lin– CD34+ CD10+ cells. The control Lin– CD34+ CD10– cells were also obtained and tested. Cells were seeded onto the indicated OP9 stroma cells lines in the presence of FL and IL-7. The progeny was harvested at day 14 and analyzed by flow cytometric analysis for expression of CD19, CD14, and BDCA-2 markers. Numbers indicate the percentage of each subset in quadrants.
Delta-1 enhances the development of bone marrow–derived committed lymphoid progenitor cells into pDCs. Common lymphoid progenitor cells were purified from bone marrow using flow cytometric sorting of Lin– CD34+ CD10+ cells. The control Lin– CD34+ CD10– cells were also obtained and tested. Cells were seeded onto the indicated OP9 stroma cells lines in the presence of FL and IL-7. The progeny was harvested at day 14 and analyzed by flow cytometric analysis for expression of CD19, CD14, and BDCA-2 markers. Numbers indicate the percentage of each subset in quadrants.
Discussion
This study describes the Notch ligand delta-1 as a pDC development cofactor and shows that this signal is particularly efficient at triggering the differentiation of human lymphoid progenitor cells into pDCs. Delta-1 provides a positive signal for hematopoietic development into the pDC lineage by augmenting the proportions and the absolute numbers of pDCs produced from progenitor cells without having a demonstrable effect on expansion of committed pDCs. The induction of differentiation is highly likely; however, we cannot exclude that delta-1 acts in part through enhanced survival of pDC precursors. The term “cofactor” is used because the efficiency of the delta-1 signal is dependent upon the presence of both FL and IL-7 cytokines.
The effects of delta-1 are blocked by γ-secretase inhibition, which strongly implicates the Notch signaling pathway in the control of human pDC development. The genes and signals resulting from Notch activation and susceptible to control pDC lineage determination are not defined yet. It is commonly accepted that ligands binding to the Notch receptors engage a core signaling pathway, which results in the nuclear translocation of a signal-transducing Notch intracellular domain (NICD) that heterodimerizes with CBF-1 (also known as CSL or RBP-Jk), converting this repressor into an activator that subsequently regulates several target genes.35 One known Notch target gene is the PTCRA gene,36 which is relevant to pDCs, thus providing indirect evidence that Notch signaling can occur in this lineage. Further evidence that delta-1 has induced Notch signaling in our system comes from observing the modulation of lineages other than pDCs, as expected from the literature.21,22 The production of CD19+ B cells and of CD14+ cells was reduced while that of CD56+ cells was enhanced by delta-1, and the reversal of these effects by γ-secretase inhibition is documented here on the NK and monocyte lineages. The modulation of B, NK, and monocyte production is known to be Notch dependent, being reproduced with enforced Notch signaling via NICD expression or via enforced expression of the Notch target genes HES1 and HES5.15,37 The relevance of delta-1 in pDC development in vivo has yet to be evaluated. These cells were not examined in mice conditionally ablated for delta-1, which otherwise lacked splenic marginal zone B cells but had normal T-cell development.38 The lack of effect of delta-1 ablation on T cells was explained by a redundant activity of delta-4. Thus, by analogy, Notch ligands other than delta-1 could regulate pDC development, although this is unknown at the moment. The inactivation of Notch 1 through the Mx-Cre recombinase strategy suggests that this receptor is not essential for DC formation for interstitial DCs, Langerhans DCs, or pDCs.39 However, mutant models using such IFN-mediated conditional deletion may be ill-adapted to the study of pDCs because the Cre-inducing agent, poly I:C, has a major effect on this lineage of cells.40 Thus, alternative methods of investigation of Notch signaling on pDC development in vivo seem required.
Several recent studies have established that pDCs, like conventional DCs, have complex developmental origins. A lymphoid origin, initially postulated on the basis of lymphoid transcripts in pDCs,3 is now clearly established in the murine and human systems, because pDCs can be directly obtained by in vitro culture or by in vivo transplantation of lymphoid lineage–restricted progenitor cells.5-7 We confirm and extend these findings by showing that human CLPs effectively produce pDCs and identify delta-1 as a signal of differentiation for these cells. Delta-1 controlled a symmetric process of CLP differentiation toward pDC or B cells that occurred in a similar context and time frame and from a relatively homogeneous progenitor-cell population. Stromal contact and soluble factors played a determining role in pDC formation. The cytokine FL is known to be a major regulator of human or murine pDC development in vivo8 and in vitro.9,10,27 Flt-3 receptor is found on lymphoid and myeloid pDC progenitor cells, further supporting the relevance of using FL to generate pDCs.7,41 While the effects of FL on pDCs are well established, the enhancing activity of IL-7 had not been appreciated before. The effects of IL-7 on pDC production could be context dependent or progenitor specific. Indeed, prior studies have shown that IL-7 reduces the effects of FL on pDC development from primitive hematopoietic progenitor cells.9 The identification of human lymphoid progenitor cells as efficient precursor cells for pDCs may be an important aspect of the IL-7 response. Indeed, CLPs express the IL-7Rα chain, and high levels of IL-7Rα mRNA are detected in CLPs and in their pDC progeny, contrary to myeloid progenitor cells and their pDC descendance.7 However, a positive effect of delta-1 on IL-7R expression is unlikely, because we found no difference in IL-7R expression in OP9-C or OP9-Del1 cultures (data not shown). There is also an important myeloid differentiation pathway for pDC production that has been shown both in vitro with human cells7 and in vivo in mice.5 In mice, spleen and liver pDC populations were more effectively reconstituted after transplantation of committed myeloid progenitor cells (CMPs) than CLPs. However, this was not the case in the thymus where CLP-derived pDC reconstitution was 9-fold greater than for CMPs.5 Being present on murine MHC class II–positive thymic epithelium,42 delta-1 may provide a physiologically relevant signal regulating the fate of CLPs entering the thymus. Through the reciprocal control of pDC-versus B-cell development, the presence or the absence of delta-1 may constitute an essential aspect of the niche in which lymphoid progenitor cells develop. A recent study in mice seems to contradict our findings, reporting an inhibitory effect of delta-1 on the production of pDCs from early lymphoid progenitor cells of bone marrow.43 However, these murine BM lymphoid cells were cultured for short periods (8 days) using FL only, and pDCs were produced from IL-7Rα–deficient cells, which contrasts with our observations of an essential need for IL-7 in the human system.7 One confounding factor in the murine study is that pDCs were identified by cell-surface markers that happen to be shared by lymphoid progenitor cells, making it difficult to segregate the effects of delta-1 on these 2 cell types. Furthermore, those putative pDCs did not produce high levels of IFN-α, unlike in our study. It is therefore possible that delta-1 could have predominantly affected an undifferentiated progenitor-cell compartment in this murine study. Another element of variability seems to be the amount of delta-1 that is provided to the progenitor cells in the initial steps of culture. In our hands, we could abolish the enhancing effect of delta-1 by plating the same numbers of CD34+ cells onto far fewer OP9-Del1 cells compared with our usual conditions (A.G., unpublished preliminary observations, November 2005). Thus, future experiments should determine the levels of delta-1/Notch signaling that are required to modulate pDC development. In addition, future studies should investigate the possibility that delta-1 regulates pDC progenitor-cell pathways in addition to the lymphoid progenitor-cell pathway that we document.
The OP9-Del1 culture system constitutes a practical tool to efficiently produce various types of human DCs, which is useful for developmental and immune studies. So-called conventional DCs are produced in similar amounts as in control cultures based on CD1a+ cell-surface phenotype, TLR4 expression, or IL-12 production. This is consistent with prior studies showing little effect of delta-1 on this type of DC.23 A distinguishing feature of the OP9-Del1 system is its efficiency for production of pDCs based on phenotype and functional activity, because it is superior to previously reported systems. Starting with total UCB CD34+ cells, each CD34+ cell yields 18 pDCs in 3 weeks, which is 3 times the number obtained in optimized liquid cultures supplemented with thrombopoietin and FL that reportedly generate 6 pDCs per CD34+ cell in the same time frame.27 The pDCs produced onto OP9-Del1 appear to be functional as determined essentially by their cytokine production, which constitutes an underpinning mechanism for the antiviral and immunomodulatory effects of pDCs.4 However, more studies are needed to fully evaluate the immune activities induced by pDCs developed onto delta-1. This system provides an opportunity to study the role played by Notch ligands in immune responses through effects on pDCs in addition to their well-recognized roles in the regulation of lymphopoiesis and Th1 T-cell differentiation.17
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-03-0970.
Supported by INSERM, Association Francaise contre les myopathies, and Société Française d'Hématologie (A.O.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Graziella Griffith for help with vector construction, Philippe Rameau for help with flow cytometry, Sonia Poirault for help with cell-cycle experiments, and Roseline Yao for help with pDC cultures. We also acknowledge Drs A. Turhan (Institut Gustave Roussy, Villejuif), P. Salmon (University of Geneva), S. Artavanis-Tsakonas (Harvard Medical School, Charlestown, MA), and Immunex (Seattle, WA) for the kind gifts of reagents. The generosity and help from the obstetrics staff at Hopital Louise Michel (Evry, France), Association Française contre les Myopathies (AFM) Tissue Bank, and Genethon tissue processing personnel are also acknowledged.