Abstract
Waldenström macroglobulinemia (WM) is a serious and frequently fatal B-cell malignancy associated with an elevated monoclonal IgM protein in the serum. Many of the mechanisms leading to this disease are not yet known. B-lymphocyte stimulator (BLyS) is a TNF family member that is critical for maintenance of normal B-cell development and homeostasis. BLyS is overexpressed in a variety of B-cell malignancies and has been shown to inhibit apoptosis in malignant B cells. It also regulates immunoglobulin secretion by normal B cells. To determine the relevance of BLyS in WM, we examined the role of BLyS in WM patient samples. Malignant B cells were found to bind soluble BLyS and variably express the receptors BAFF-R, TACI, and BCMA. We also found expression of BLyS in bone marrow specimens by immunohistochemistry and elevated serum BLyS levels in patients with WM. BLyS, alone or in combination with cytokines that induce immunoglobulin production, was found to increase IgM secretion by malignant B cells. Furthermore, BLyS was found to increase the viability and proliferation of malignant B cells from WM patients. Due to the role of BLyS in WM, strategies to inhibit BLyS may potentially have therapeutic efficacy in these patients.
Introduction
Waldenström macroglobulinemia (WM) is an uncommon disorder characterized by the production of a monoclonal IgM protein, a lymphoplasmacytic infiltrate in the bone marrow, and associated symptoms such as anemia, lymphadenopathy, and hyperviscosity.1-4 Despite significant clinical advances in the treatment of WM, it remains incurable and most patients succumb to disease progression. Thus, there is an increasing need for novel effective therapies. An important component in the development of new therapies is an understanding of the mechanism(s) that underlies resistance to apoptosis, leading to prolonged survival of malignant B cells and tumor cell accumulation in the bone marrow. Additionally, the biology underlying the increased immunoglobulin production in WM is highly relevant. Our current lack of curative therapies in this disease is at least in part due to our current lack of knowledge regarding signals that regulate survival and immunoglobulin production in malignant B cells.
B-lymphocyte stimulator (BLyS), also known as B-cell-activating factor of the TNF family (BAFF),5 is a TNF family member expressed by monocytes, macrophages, dendritic cells,5-7 and neutrophils.8 BLyS has been shown to be critical for the maintenance of normal B-cell development and homeostasis.9,10 Early studies examining the effects of BLyS on B-cell physiology suggested that it costimulates B-cell proliferation and immunoglobulin secretion.5 Three receptors have been identified as receptors for BLyS10-12 : B-cell maturation antigen (BCMA),13 transmembrane activator and CAML interactor (TACI),14 and BAFF-R.15 BCMA and BAFF-R are predominantly expressed on B lymphocytes, while TACI can be found on B cells as well as activated T cells.14 BAFF-R has been identified as the main BLyS receptor responsible for peripheral B-cell homeostasis and specifically binds BLyS, whereas BCMA and TACI can also bind the related molecule A proliferation-inducing ligand (APRIL).16-20 Similarities between BLyS-/- mice and BAFF-R signaling-deficient mice (A/WySnJ)21-23 or BAFF-R-/- mice24-26 further support the notion that BAFF-R is the predominant BLyS receptor.
Because of the role BLyS plays in normal B-cell development and homeostasis, several studies have addressed whether BLyS plays a role in the pathogenesis of various B-cell malignancies. BLyS has been shown to bind the surface of malignant B cells from patients with B-cell chronic lymphocytic leukemia (B-CLL),27 non-Hodgkin lymphoma (NHL),28,29 and multiple myeloma.30 B-CLL B cells express BAFF-R and TACI,27 whereas multiple myeloma cells express BAFF-R, BCMA, and TACI.30 The ability of BLyS to bind to malignant cells suggests a functional significance of the receptor-ligand binding. BLyS enhances the survival of B-CLL cells27 and myeloma cell lines31 in vitro. Furthermore, BLyS alone induces the proliferation of myeloma cell lines31 and, in combination with B-cell receptor cross-linking, enhances the proliferation of primary follicular B cells.32
The significance of BLyS in B-cell survival and homeostasis, in addition to the finding that malignant B cells bind BLyS, raises the possibility that BLyS along with its receptors may be involved in the growth and survival of malignant B cells in WM. The critical role that BLyS plays in the regulation of IgM production by normal B cells and the association between elevated serum BLyS and increased IgM levels also suggest a significant role for this molecule in WM. This study was therefore undertaken to determine whether BLyS and its receptors are expressed on malignant cells in patients with WM and to determine whether BLyS levels are elevated in the sera from WM patients. We also sought to determine a functional role for the BLyS-receptor ligand in patients with WM.
Materials and methods
Cells and reagents
The Wayne State University Waldenström macroglobulinemia (WSU-WM) cell line33 was a kind gift from Dr Ayad Al-Katib (Grosse Pointe, MI). Bone marrow and tissue biopsy mononuclear cells were isolated as previously described27 from patients with WM, who provided written informed consent. This study was approved by the Mayo Clinic Foundation Institutional Review Board. ZymoGenetics (Seattle, WA) provided biotinylated BLyS, biotinylated mouse IgG control, and soluble TACI-Ig fusion protein. RED670- and phycoerythrin (PE)-conjugated streptavidin were purchased from Gibco (Carlsbad, CA) and Caltag (Burlingame, CA), respectively. Anti-BAFF-R, anti-BCMA, and anti-TACI biotinylated antibodies were purchased from R & D Systems (Minneapolis, MN).
Flow cytometry
Cells (0.5 × 106) were washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 0.05% sodium azide and incubated with 0.1 μg biotinylated recombinant BLyS, or 0.5 μg biotinylated anti-BCMA, anti-BAFF-R, anti-TACI, or mouse immunoglobulin control for 30 minutes at 4°C. Cells were washed and incubated with streptavidin-PE for 30 minutes at 4°C, washed, and analyzed using FACSCalibur and CellQuest software (Becton Dickinson, San Jose, CA).
Immunohistochemistry
Paraffin-embedded bone marrow or tumor specimens obtained from patients with WM were cut into 4-μm sections. The sections were deparaffinized in xylene and rehydrated with water through a graded alcohol series. The sections were pretreated with 10 mM citrate buffer, pH 6.0, for 30 minutes, cooled for 5 minutes, and then rinsed well with cold running tap water. To block endogenous peroxidase activity, sections were incubated for 10 minutes in methanol/H2O2 and then rinsed with tap water. To block nonspecific staining, sections were incubated with PBS with 5% human serum for 10 minutes. Anti-BLyS (R & D Systems) and nonimmune rabbit serum (10 μg/mL; DakoCytomation California, Carpinteria, CA) were added to slides for 30 minutes. The slides were then rinsed with APK wash solution (Ventana Medical Systems, Tucson, AZ) and then incubated with biotinylated goat anti-rabbit IgG (1:300) for 15 minutes and rinsed with APK. SA-HRP (1:300) was added to all slides for 15 minutes followed by rinsing with APK. BLyS was visualized using 3,3′-diaminobenzidine (DakoCytomation California) and counterstaining with hematoxylin. The slides were coverslipped and mounted with Cytoseal 280 (Stephens Scientific, Kalamazoo, MI). All slides were observed with light microscopy (Olympus AX70, 200 ×/aperture 0.46, 400 ×/aperture 0.75, 600 ×/aperture 0.80; Olympus America, Melville, NY) with images captured with a SPOT RT camera and software (Diagnostic Instruments, Burlingame, CA).
Assessment of cell viability by annexin-V and propidium iodide (PI) double staining
Cells isolated from bone marrow of WM patients (0.5 × 106 cells/mL) were incubated in 48-well plates (0.5 mL/well) in RPMI supplemented with 10% FCS. Cells were cultured in the presence/absence of 100 ng/mL BLyS at 37°C. After 7 days of culture, cells were assayed for viability using 1 μg annexin-V-FITC (Caltag) for 20 minutes at 4°C. Cells were washed once in annexin-V binding buffer and then stained with 0.5 μg PI and immediately analyzed by flow cytometry. Data acquisition was done on a FACSCalibur and analysis was done using CellQuest software (Becton Dickinson).
BLyS ELISA
Serum samples were collected from healthy donors (n = 41) and WM patients (n = 25). Enzyme-linked immunosorbent assay (ELISA) plates (Nunc Maxisorp; Nalge Nunc International, Rochester, NY) were coated with 100 μL 1-μg/mL anti-human BLyS (monoclonal antibody [mAb] clone E4732; ZymoGenetics) overnight at 4°C. Wells were washed with PBS/0.05% Tween and blocked with 175 μL Superblock (Pierce Chemical, Rockford, IL). Patient serum samples were diluted 1:5 in RPMI/20% normal human serum (NHS) and 100 μL was added to each well in duplicate and incubated for 1 hour at room temperature. Wells were washed and biotinylated mouse anti-BLyS (mAb clone E4731, 1 μg/mL; Zymo-Genetics) was added to each well. Wells were again washed and HRP-conjugated streptavidin (Chemicon, Temecula, CA) was added to the wells for 1 hour, washed, and developed with Turbo TMB-ELISA (Pierce Chemical). The reaction was stopped by addition of 1 N H2SO4 and results were measured with a plate reader (Molecular Devices, Palo Alto, CA) and analyzed using Softmax software (version 2.34; Molecular Devices). BLyS serum levels were calculated from a standard curve generated with serially diluted recombinant human BLyS (ZymoGenetics) in 20% normal human sera. Data are presented using patients who had detectable levels of BLyS: 38 healthy donors and 9 WM patients.
Tumor cell culture and quantifying secreted IgM
To detect the levels of secreted IgM, 0.5 × 106 positively selected CD19+CD138+ cells from WM were cultured in 48-well plates. Cells were treated as previously described.34 Briefly, cells were either left untreated, treated with 100 ng/mL BLyS, treated with cytokines (20 U/mL IL-2, 5 ng/mL IL-6, 50 ng/mL IL-10, and 2 ng/mL IL-12) that stimulate immunoglobulin production, or treated with both BLyS and cytokines. One week later, supernatants were harvested and used to detect the presence of IgM using human IgM ELISA Kit (Bethyl Laboratories, Montgomery, TX) following manufacturer recommendations.
Proliferation assay
CD19+CD138+ cells from WM patients were cultured in 96-well round-bottom microtiter plates (Costar, Cambridge, MA) at a density of 1 × 105 cells/well in the presence of 100 ng/mL BLyS, 10 μg/mL anti-Ig (Jackson Immunoresearch, West Grove, PA), both BLyS and anti-Ig, or media alone. Inhibition of BLyS was achieved by adding 10 μg/mL soluble TACI-Ig fusion protein. Cells were cultured for 5 days at 37°C in the presence of 5% CO2. Cultures were pulsed with 1 μCi (0.037 MBq) tritiated thymidine (3H-TdR, 5.0 Ci [185 GBq]/mmol; Amersham, Piscataway, NJ) for 18 hours prior to harvesting, and 3H-TdR incorporation levels were determined using a Beckman scintillation counter (GMI, Ramsey, MN).
Statistical analysis
Comparisons between groups were based on χ2 tests for nominal variables; the Wilcoxon rank-sum test or the Kruskal-Wallis test was used for continuous variables. For all statistical tests, P less than .05 was considered significant. Analysis was performed on Statview software (SAS Institute, Cary, NC).
Results
Expression of BLyS receptors, BAFF-R, TACI, and BCMA, in WM
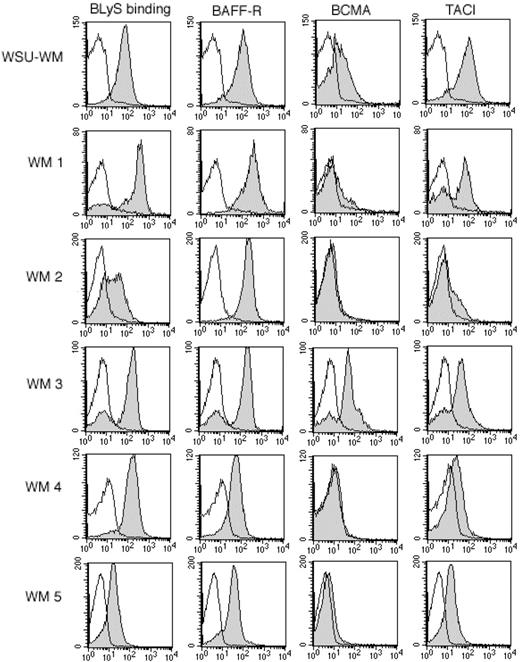
To determine the relevance of the BLyS receptor-ligand system in WM, we examined malignant B cells from 12 patients with WM and the WSU-WM cell line33 (cell line derived from a patient with WM) for their ability to bind soluble BLyS and for the expression of the known BLyS receptors, BAFF-R, BCMA, or TACI (Figure 1). The WSU-WM cell line was analyzed and found to bind BLyS and express high levels of BAFF-R and TACI, and lower levels of BCMA. Malignant B cells isolated from patients with WM were found to bind BLyS (12/12 patients) and express BAFF-R (12/12). Of the 12 patients tested, 3 patients expressed intermediate levels of TACI and 6 patients expressed low levels (Table 1). BCMA, which is highly expressed on normal plasma cells,10 was detectable (8/12 patients) at variable levels, with only 2 patients expressing BCMA at intermediate levels (Table 1). The ability of malignant B cells to bind BLyS, as well as the presence of BAFF-R, TACI, and BCMA on cells from patients with WM, suggests a role for this receptor-ligand system in this disease.
Expression of BAFF-R, BCMA, and TACI on WM cells. Tumor cells from the WM cell line (WSU-WM) and WM patients were stained with biotin-conjugated BLyS, anti-BAFF-R, anti-BCMA, or anti-TACI for 30 minutes at 4°C, then washed and incubated for 30 minutes with PE-streptavidin. Isotype and fluorochrome controls were done in parallel with each sample (white histograms). Gray histograms correspond to CD19+CD138+ cells from a representative example of each subtype. Results shown are from 5 representative WM samples, out of a total of 12 patient samples analyzed.
Expression of BAFF-R, BCMA, and TACI on WM cells. Tumor cells from the WM cell line (WSU-WM) and WM patients were stained with biotin-conjugated BLyS, anti-BAFF-R, anti-BCMA, or anti-TACI for 30 minutes at 4°C, then washed and incubated for 30 minutes with PE-streptavidin. Isotype and fluorochrome controls were done in parallel with each sample (white histograms). Gray histograms correspond to CD19+CD138+ cells from a representative example of each subtype. Results shown are from 5 representative WM samples, out of a total of 12 patient samples analyzed.
Expression of BLyS in bone marrow specimens of patients with WM
Next, we evaluated the expression of BLyS by immunohistochemistry in bone marrow specimens from patients with WM. BLyS expression in 3 Waldenström patients was compared with the expression in normal bone marrow. As shown in Figure 2, the lymphoplasmacytic cell infiltrate in the bone marrow of patients with WM stained for BLyS expression, while minimal diffuse expression was seen in bone marrow specimens from healthy controls. Our findings indicate that BLyS is expressed in bone marrow specimens from WM patients and support the hypothesis that BLyS is important in the biology of this disease.
Elevated serum BLyS levels in Waldenström patients
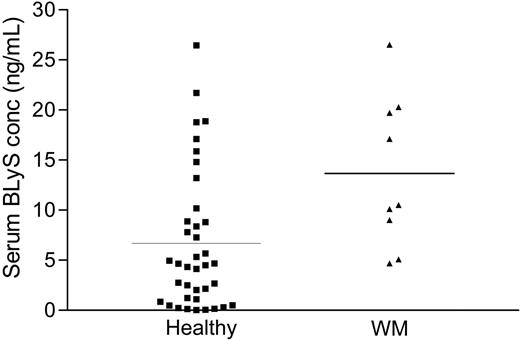
Because BLyS plays a crucial role in B-cell development, survival, and maintenance, we postulated that serum BLyS levels in patients with WM would be significantly elevated when compared with age- and sex-matched controls. We determined the serum BLyS levels by ELISA in stored serum specimens from patients with WM, and compared them with serum levels in stored healthy controls. As shown in Figure 3, serum BLyS levels in Waldenström patients (mean: 13.66 ± 2.75 ng/mL) were significantly higher (P < .01) than those reported in healthy controls (mean: 6.68 ± 2.64 ng/mL),35 further supporting the hypothesis that BLyS is important in WM. The average value of BLyS detected in our control samples (6.68 ng/mL) is similar to values reported by others.32,36
BLyS up-regulates immunoglobulin production in WM
Initial studies on the effects of BLyS on normal B-cell physiology suggest that it costimulates B-cell proliferation and immunoglobulin secretion. We therefore hypothesized that addition of BLyS to malignant B cells from WM patients would enhance their ability to secrete IgM. We cultured positively selected CD19+CD138+ cells from 5 WM patients for 1 week in the presence of either BLyS alone, a cocktail of cytokines that induce immunoglobulin secretion (IL-2, IL-6, IL-10, and IL-12), or a combination of BLyS and cytokines together. Using an ELISA to test for levels of secreted IgM in the supernatants of cultured cells, we found a subtle increase in the levels of IgM secreted by malignant B cells in the presence of BLyS when compared with untreated cells (P = .01) (Figure 4). As expected, culture of WM cells with cytokines resulted in increased IgM secretion compared with the nil control (P < .001). To understand the contribution of BLyS, we next combined BLyS with the cytokine cocktail and saw a significant increase in the level of IgM secreted compared with cytokines alone (P = .001). Although baseline IgM levels secreted by cells from different patients varied, the pattern of increase in IgM secretion when BLyS was added alone or in combination with cytokines was consistent. Studies using the WSU-WM cell line showed undetectable baseline levels of IgM that did not increase when either BLyS or cytokines were added.
BLyS expression in bone marrow specimens of WM patients. Immunohistochemical analysis of BLyS expression in 3 WM bone marrow specimens (WM 6-WM 8) was performed using anti-BLyS mAb as described in “Materials and methods.” A similar analysis was performed on a normal bone marrow specimen (NBM). Original magnification for images shown: × 600.
BLyS expression in bone marrow specimens of WM patients. Immunohistochemical analysis of BLyS expression in 3 WM bone marrow specimens (WM 6-WM 8) was performed using anti-BLyS mAb as described in “Materials and methods.” A similar analysis was performed on a normal bone marrow specimen (NBM). Original magnification for images shown: × 600.
Elevated BLyS levels in the sera of healthy and WM patients. Serum BLyS levels were analyzed by ELISA in specimens obtained from healthy individuals (n = 38) and from WM patients (n = 9). Results are presented as individual values of each patient, and the bar represents the value of the mean.
Elevated BLyS levels in the sera of healthy and WM patients. Serum BLyS levels were analyzed by ELISA in specimens obtained from healthy individuals (n = 38) and from WM patients (n = 9). Results are presented as individual values of each patient, and the bar represents the value of the mean.
Elevated IgM levels in the presence of BLyS. CD19+CD138+ cells (0.25 × 106 cells/well) from WM patients (n = 5) were cultured in 48-well plates in the presence of 100 ng/mL BLyS, cytokines (20 U/mL IL-2, 5 ng/mL IL-6, 5 ng/mL IL-10, and 2 ng/mL IL-12), a combination of BLyS + cytokines, or media alone. One week following treatment, supernatants were harvested and used to test for IgM secretion using ELISA. Results for 5 WM patients are presented as means ± standard deviation (SD) of duplicate wells.
Elevated IgM levels in the presence of BLyS. CD19+CD138+ cells (0.25 × 106 cells/well) from WM patients (n = 5) were cultured in 48-well plates in the presence of 100 ng/mL BLyS, cytokines (20 U/mL IL-2, 5 ng/mL IL-6, 5 ng/mL IL-10, and 2 ng/mL IL-12), a combination of BLyS + cytokines, or media alone. One week following treatment, supernatants were harvested and used to test for IgM secretion using ELISA. Results for 5 WM patients are presented as means ± standard deviation (SD) of duplicate wells.
BLyS protects malignant B cells from apoptosis
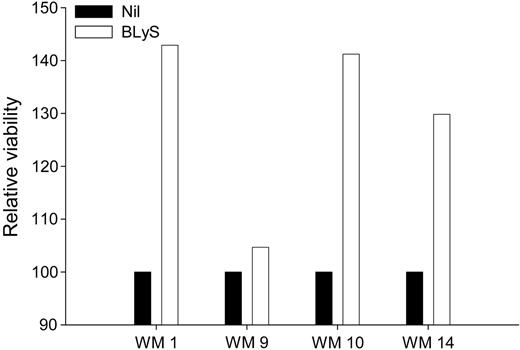
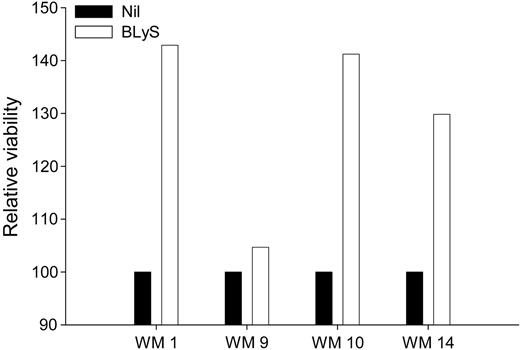
Because BLyS attenuates apoptosis in normal B cells, and BLyS protects other types of malignant B cells from apoptosis,28,37 we next examined the effect of BLyS on the viability of freshly isolated cells from patients with WM. CD19+CD138+ cells from 4 WM patients were either left untreated or stimulated with BLyS for 1 week. Cell viability was assessed using annexin-V/PI double staining and cell viability was normalized relative to the media-alone control. There was a significant (P = .001) increase in the relative viability of malignant WM B cells that were cultured in the presence of BLyS for 7 days, compared with cells grown in media alone (Figure 5). These results again suggest that BLyS promotes survival of malignant B cells in patients with WM. These data are in accordance with previous work demonstrating the significance of BLyS in the survival of malignant lymphoma,28,29 multiple myeloma,30,31 and B-CLL cells.27
BLyS promotes survival of WM cells. CD19+CD138+ cells (0.25 × 106 cells/well) from WM patients (n = 4) were cultured in 48-well plates in RPMI 10% FBS in the presence or absence of 100 ng/mL BLyS. One week following treatment, the cells were harvested and assayed for viability using annexin-V/PI staining. Cell viability in the presence of BLyS was normalized to the media-alone control and is presented as relative viability.
BLyS promotes survival of WM cells. CD19+CD138+ cells (0.25 × 106 cells/well) from WM patients (n = 4) were cultured in 48-well plates in RPMI 10% FBS in the presence or absence of 100 ng/mL BLyS. One week following treatment, the cells were harvested and assayed for viability using annexin-V/PI staining. Cell viability in the presence of BLyS was normalized to the media-alone control and is presented as relative viability.
BLyS enhances the proliferation of malignant cells in vitro
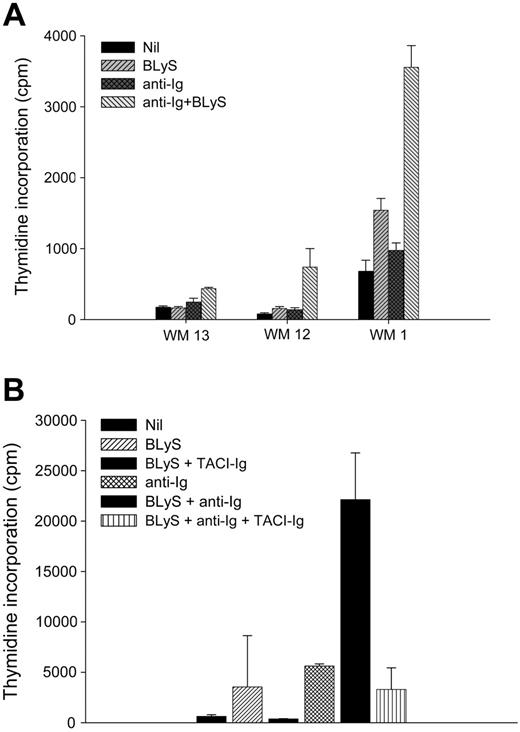
To further characterize the functional role of BLyS in WM, we next determined if BLyS could induce proliferation of malignant B cells from Waldenström patients in vitro. CD19+CD138+ cells were purified from 3 different patients with WM. Cells were then cultured in the presence or absence of BLyS, and the proliferation status was assessed after 5 days (Figure 6A). As previously seen,30,32 BLyS (P = .181) or anti-Ig (P = .068) alone had minimal effects on proliferation compared with the nil control. However, proliferation was significantly enhanced in the presence of both anti-Ig and BLyS (P < .001). The increase in proliferation was abrogated when BLyS was bound using a decoy receptor TACI-Ig (Figure 6B). These results show that BLyS enhances the proliferative capacity of WM B cells in vitro and suggest that the presence of BLyS in the microenvironment of WM may enhance the proliferation of the malignant cells in vivo.
Discussion
Under normal conditions, lymphocytes must strictly regulate growth and apoptosis to provide adequate immunologic defenses against infections, while not overwhelming the organism with inappropriate cell numbers. BLyS has been shown to be critical for maintenance of normal B-cell development and homeostasis.10 In normal B cells, BLyS signaling drives plasmacytoid differentiation, and BLyS has been shown to elicit immunoglobulin class switching.38 Furthermore, plasmacytic differentiation is also associated with up-regulation of BCMA, one of the BLyS receptors, and BLyS has been shown to enhance the survival of plasmablasts generated from human memory B cells.39 Serum levels of BLyS have also been found to correlate with circulating antibody levels in patients with other B-cell-mediated diseases, such as systemic lupus erythematosus (SLE).40 Additionally, systemic BLyS administration in BALB/c mice resulted in a 5-fold increase in serum IgM levels. IgM secretion in mice after exposure to antigen was significantly inhibited when BLyS was bound using TACI-Ig as a decoy receptor.
BLyS promotes proliferation of WM cells. (A) CD19+CD138+ cells (0.1 × 106 cells/well) from WM patients (n = 3) were cultured in 96-well round-bottom plates in the presence or absence of 100 ng/mL BLyS or 10 μg/mL anti-Ig Ab, each alone or in combination, for 5 days at 37°C in the presence of 5% CO2. Values represent the mean of triplicate values ± SD. (B) TACI-Ig fusion protein blocks BLyS-induced proliferation of malignant B cells. A fourth WM patient sample (WM15) was used to show that BLyS-induced proliferation of malignant B cells is inhibited in the presence of TACI-Ig fusion protein.
BLyS promotes proliferation of WM cells. (A) CD19+CD138+ cells (0.1 × 106 cells/well) from WM patients (n = 3) were cultured in 96-well round-bottom plates in the presence or absence of 100 ng/mL BLyS or 10 μg/mL anti-Ig Ab, each alone or in combination, for 5 days at 37°C in the presence of 5% CO2. Values represent the mean of triplicate values ± SD. (B) TACI-Ig fusion protein blocks BLyS-induced proliferation of malignant B cells. A fourth WM patient sample (WM15) was used to show that BLyS-induced proliferation of malignant B cells is inhibited in the presence of TACI-Ig fusion protein.
The ability of BLyS to modulate normal B-cell homeostasis has been well documented.10-12 However, it is less clear how BLyS influences the growth and survival of malignant B cells. Previous reports have shown the expression of BLyS receptors in multiple myeloma and non-Hodgkin lymphoma.29,30,32 We have analyzed malignant B cells from patients with WM and found them to express BAFF-R and TACI and to bind BLyS (Figure 1; Table 1). This is similar to previous reports showing expression of BAFF-R and TACI and BLyS binding to normal peripheral blood B cells.30 This pattern of BLyS binding and expression of TACI in WM is similar to that seen in multiple myeloma.30 However, in multiple myeloma, BAFF-R expression was undetectable on multiple myeloma cell lines and varied on freshly isolated multiple myeloma cells.30 Malignant B cells from WM patients variably expressed BCMA (Figure 1), which was initially reported to be expressed by mature B cells.41 Later studies suggest that BCMA is expressed at relatively high levels among early transitional B cells and at very low levels on late transitional B cells and mature splenic B cells.42 Additionally, recent studies suggest that BCMA is an important receptor for the survival of long-lived bone marrow plasma cells.43 These data are in accordance with others who have shown that tonsillar, myeloma, and plasmacytoma B cells express relatively high levels of BCMA.16,30 WM B cells are therefore similar to multiple myeloma cells in the expression pattern of TACI, BAFF-R, and BCMA.
Previous studies by our group, as well as others, suggest that elevated BLyS levels are found in B-cell inflammatory disorders and malignancies.29,32,35,40,44,45 We therefore used immunohistochemistry to detect the presence of BLyS in bone marrow specimens from patients with WM and found increased BLyS expression when compared with normal bone marrow controls (Figure 2). However, the overall BLyS levels detected were fairly low compared with that seen in multiple myeloma.30 While it is thought that stromal cells within the bone marrow microenvironment are a predominant source of BLyS in mice, we have detected low levels of BLyS expression in normal human bone marrow sections in this study (Figure 2) and others.30 Currently, we do not know which component of the tumor microenvironment is responsible for BLyS expression, and it will be of interest to determine this in future studies. Next, we determined serum BLyS levels in patients with WM and found substantially elevated BLyS levels compared with healthy controls (Figure 3). Elevated levels of BLyS in either serum or the bone marrow microenvironment may support survival and growth of malignant WM cells and could therefore be a useful therapeutic target.
In our studies, to determine the functional significance of BLyS in WM we found that BLyS, in combination with cytokines that stimulate immunoglobulin secretion, enhances IgM secretion (Figure 4). The cocktail of cytokines used for our studies has been previously implicated in immunoglobulin production or in WM. It has been shown that IL-246 and IL-647,48 are elevated in the sera of WM patients, and IL-10 has been suggested to be a growth factor for malignant B cells in germinal centers.47 While IL-12 has not been implicated in WM, it has been shown to bind human and mouse B cells,49 up-regulate antibody production in mice,50 and along with soluble IL-6R, results in IgM secretion by plasma cells.51 The ability of BLyS to enhance IgM secretion may be in part due to its capacity to attenuate apoptosis of B cells. However, regardless of the mechanism, the ability of BLyS to drive IgM secretion in combination with the finding of elevated serum BLyS levels suggests that it may contribute to elevated serum IgM, which is seen as hallmark of this disease. We also show that BLyS promotes the survival and proliferation of WM B cells (Figures 5, 6). Taken together, our functional studies further substantiate the relevance of this molecule in the biology of WM. Although our studies have focused on the role of BLyS in WM, APRIL may also play a role. APRIL has significant homology to BLyS, binds TACI and BCMA, and has been shown to stimulate malignant B cells from multiple myeloma and lymphoma patients.30,31,52,53 Studies are currently under way to better understand the significance of APRIL in WM.
In conclusion, we have shown that malignant B cells from patients with WM express the receptors for BLyS and can bind soluble BLyS. We have also shown that serum BLyS levels are elevated in patients with WM when compared with healthy controls. Furthermore, we have presented data that show that BLyS up-regulates immunoglobulin secretion in specimens obtained from patients with WM. Based on these data, we anticipate that strategies to inhibit BLyS will have significant potential therapeutic efficacy in WM. BLyS blockade in WM patients may help decrease the IgM protein in the serum. Additionally, it is likely to decrease the viability and survival of malignant cells in WM, thereby reducing the number of cells that are secreting IgM. BLyS may be blocked using soluble TACI-Ig as a decoy receptor and the use of high-affinity peptide binders to BLyS has recently been suggested.54 Regardless of the method, our data suggest that inhibition of BLyS is likely to have a positive result in WM.
Prepublished online as Blood First Edition Paper, November 22, 2005; DOI 10.1182/blood-2005-09-3552.
Supported in part by grants CA92104 and CA97274 from the National Institutes of Health and a grant from the International Waldenström Macroglobulinemia Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.