Comment on Evans et al, page 3593
Selective cleavage of the αLβ2 integrin from neutrophils and monocytes during an in vivo human inflammatory response points to a novel mechanism for regulating leukocyte detachment.
Within the past 5 years, since the elucidation of the first integrin crystal structure,1 there has been extraordinary progress in understanding mechanisms by which integrin-mediated cell adhesion is turned on.2 However, there is considerably less understanding of integrin adhesion reversal and cell detachment. Regulated deactivation of integrins, “membrane ripping,” and cytoskeletal rearrangements all may be involved. In some cases, an intracellular protease, calpain, helps to disassemble integrin-cytoskeletal complexes during cell detachment.3 On the extracellular side of the membrane, there have been occasional reports of single-chain integrin cleavage (involving either α or β chains alone). However, proteolytic cleavage and release of intact integrin αβ heterodimers have not previously been observed under physiologic conditions. In this issue of Blood, Evans and colleagues now provide evidence for shedding of an active heterodimeric fragment of integrin αLβ2 into the cell supernatant. This surprising result suggests a novel mechanism for cessation of integrin-dependent adhesion, to enable rapid detachment of leukocytes from substrate ICAM-1 on endothelium.
Cantharidin (Spanish fly) is a lipid-soluble blister beetle irritant that has been used for more than 100 years to promote skin blistering.4 This model of cutaneous inflammatory response in humans yields a substantial number of infiltrating leukocytes (0.1-10 × 106 cells/blister).
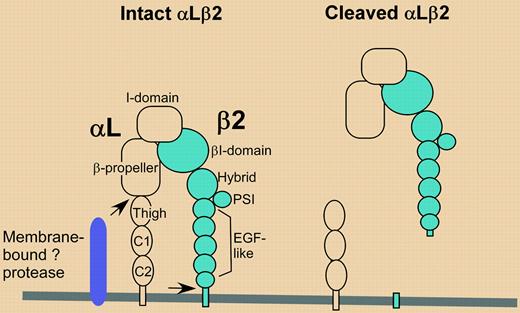
Evans et al noticed that expression of integrin αLβ2 was markedly diminished on the surface of neutrophils and monocytes in blister fluid. Analysis of cell-free blister fluid revealed that membrane-bound integrin heterodimer (αL, 180 kDa; β2, 90 kDa) had been converted to a soluble heterodimer of 110 kDa and 86 kDa, respectively (see figure). Meanwhile, a piece of the αL subunit (∼ 70 kDa) was left behind on the cell. The observed shedding was remarkably specific on 3 levels. First, only αLβ2 integrin, and not αMβ2 or α4β1 integrins, was shed from neutrophils and monocytes. Second, αLβ2 was not shed from lymphocytes present in the same blister fluid. Third, comparable αLβ2 shedding was observed in nearly all (67 of 68) individuals treated with cantharidin but was not so obvious in another type of human inflammatory infiltrate. In synovial samples from arthritis patients, there was no evidence for decreased αLβ2 on the surface of leukocytes, and only one of 15 samples showed biochemical evidence for conversion of αLβ2 to its shed form.FIG1
In cantharidin blister fluid, αLβ2 is cleaved at 2 (or more) sites (arrows) by one or more proteases present on the surface of neutrophils and monocytes. A functionally active heterodimeric fragment is released, leaving behind a C-terminal αL stump. A C-terminal piece of β2 also might be left behind, but this was not detected.
In cantharidin blister fluid, αLβ2 is cleaved at 2 (or more) sites (arrows) by one or more proteases present on the surface of neutrophils and monocytes. A functionally active heterodimeric fragment is released, leaving behind a C-terminal αL stump. A C-terminal piece of β2 also might be left behind, but this was not detected.
As is typically the case for original research findings, many questions arise. For example, how is shedding achieved? Shedding activity was not conferred on cells by incubation with cell-free blister fluid, implying the existence of cis-acting proteases on the monocytes and neutrophils. Although only one protease is depicted in the figure, it is difficult to imagine a single protease cleaving both αL, at a site approximately 10 to 15 nm above the membrane, and β2, at a site within 1 to 2 nm of the membrane. Also, why is this result obvious in the cantharidin blister model but not in other samples of inflammatory fluid? Evans et al suggest that in other clinical samples, shed αLβ2 may be rapidly degraded and newly synthesized αLβ2 may quickly replace that which was removed. However, another possibility is that cantharidin stimulation induces a unique profile of proteins, including proteases, on the surface of neutrophils and monocytes. In this regard, cDNA microarray analysis of cantharidin-treated HL-60 neutrophils revealed a wide range of specific gene expression changes.5 It will be important in future studies to assess the general relevance of αLβ2 shedding. Available diagnostic anti-αL monoclonal antibodies might be used in a 2-color flow cytometry screen of inflammatory infiltrates, potentially revealing the presence of leukocytes that have lost epitopes on the cleaved N-terminal part of αL while retaining epitopes residing on the αL stump, left behind after proteolysis. ▪