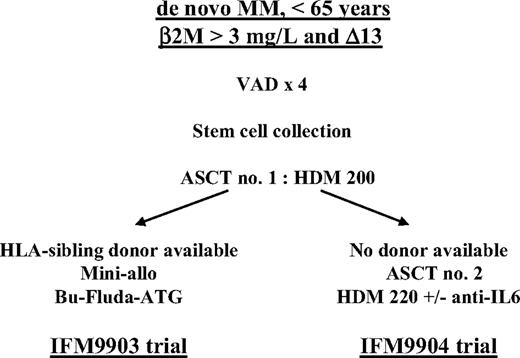
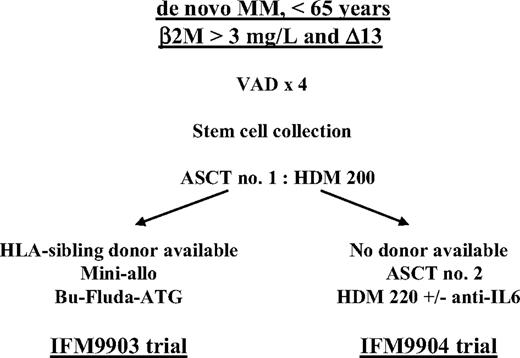
The Intergroupe Francophone du Myélome (IFM) initiated 2 trials in 1999 to study patients with high-risk (β2-microglobulin level greater than 3 mg/L and chromosome 13 deletion at diagnosis) de novo multiple myeloma. In both protocols, the induction regimen consisted of vincristine, doxorubicin, and dexamethasone (VAD) followed by first autologous stem cell transplantation (ASCT) prepared by melphalan 200 mg/m2. Patients with an HLA-identical sibling donor were subsequently treated with dose-reduced allogeneic stem cell transplantation (IFM99-03 trial), and patients without an HLA-identical sibling donor were randomly assigned to undergo second ASCT prepared by melphalan 220 mg/m2 and 160 mg dexamethasone with or without anti–IL-6 monoclonal antibody (IFM99-04 protocol). Two hundred eighty-four patients—65 in the IFM99-03 trial and 219 in the IFM99-04 trial—were prospectively treated and received at least one course of VAD. On an intent-to-treat basis, overall survival (OS) and event-free survival (EFS) did not differ significantly in the studies (medians 35 and 25 months in the IFM99-03 trial vs 41 and 30 months in the IFM99-04 trial, respectively). With a median follow-up time of 24 months, the EFS of the 166 patients randomly assigned in the tandem ASCT protocol was similar to the EFS of the 46 patients who underwent the entire IFM99-03 program (median, 35 vs 31.7 months), with a trend for a better OS in patients treated with tandem ASCT (median, 47.2 vs 35 months; P = .07). In patients with high-risk de novo MM, the combination of ASCT followed by dose-reduced allogeneic transplantation was not superior to tandem dose–intensified, melphalan-based ASCT.
Introduction
Autologous stem cell transplantation (ASCT) is considered the standard of care for younger patients with multiple myeloma (MM).1-3 Melphalan 200 mg/m2 (mel200) is considered the optimal conditioning regimen.4 The randomized IFM94 trial showed that double transplantation significantly improved overall survival (OS) and event-free survival (EFS) compared with single ASCT.5 Allogeneic stem cell transplantation is a possible curative approach for patients with MM. Compared with ASCT, the relapse rate is lower, and some patients survive disease free for the long term.6 The possible advantage of allogeneic transplantation is a well-proven graft-versus-myeloma (GVM) effect by immunocompetent donor lymphocytes.7-9 However, despite better control of infectious complications and of graft-versus-host disease (GVHD), the transplant-related mortality (TRM) rate of allogeneic transplantation is still 15% to 40%.10-12 Therefore, allogeneic transplantation is only an option for younger patients with an HLA-identical sibling. The introduction of reduced-intensity conditioning regimens, with a reduction in immediate TRM and stable engraftment, has renewed interest in the use of allogeneic transplantation for myeloma. Recent studies have reported encouraging results with tandem autograft and dose-reduced allograft approaches in MM patients.13,14
In patients with newly diagnosed MM, a number of biologic markers or genetic abnormalities have been shown to adversely influence outcome after ASCT, including high levels of β2-microglobulin (β2m), C-reactive protein (CRP), or lactate dehydrogenase (LDH) level, increased plasma cell labeling index, hypodiploidy, chromosome 13 deletion (Δ13), translocation (4;14), or a combination of these factors.2,4,12,15-17 In a retrospective trial of 110 patients treated with high-dose therapy (HDT) followed by ASCT, Facon et al16 have shown that a subgroup of patients— approximately 25% to 30% of patients younger than 65 with de novo disease and with high β2-microglobulin levels and high Δ13 (identified by fluorescence in situ hybridization [FISH]) at the time of diagnosis—had poor outcomes, with median survival and progression-free survival (PFS) times of 25 and 15 months, respectively. To treat this subgroup of patients at high risk, the IFM group designed 2 specific trials in 1999 that were based on genetic randomization. When an HLA-identical sibling donor was identified at diagnosis, the patient was offered dose-reduced allogeneic stem cell transplantation after a single melphalan-based (melphalan 200 mg/m2 [mel200]) ASCT/IFM99-03 trial. Patients who had no donor underwent tandem autologous transplantation with mel200 followed by further dose-increased melphalan 220 mg/m2 (mel220) and dexamethasone (DXM), with or without the anti–IL-6 monoclonal antibody (BE-8)/IFM99-04 trial.18 Here we present the results of the IFM 99-03 trial and compare both protocols.
Patients and methods
Eligibility
The IFM99-03 and the IFM99-04 trials were conducted from April 2000 to August 2004. Patients younger than 65 years who had Durie-Salmon stage I (one bone lesion), II, or III myeloma and initial biologic features Δ13 (FISH analysis) and β2-microglobulin levels greater than 3 mg/L were eligible and registered at the time of diagnosis. FISH analysis15 and β2-microglobulin studies were carried out centrally at the University of Nantes (H. Avet-Loiseau). Exclusion criteria were previous treatment for myeloma, another cancer, abnormal cardiac function (indicated by systolic ejection fraction less than 50%), chronic respiratory disease (indicated by vital capacity or carbon monoxide diffusing capacity less than 50% of predicted), abnormal liver function (indicated by serum bilirubin level greater than 2 mg/dL [35 μM] or alanine aminotransferase or aspartate aminotransferase level more than 4 times the upper limit of normal), and psychiatric disease.
VAD and first ASCT
After registration in the study (Figure 1), patients were initially treated with continuous intravenous infusion of 0.4 mg vincristine and 9 mg/m2 doxorubicin over a 24-hour period for 4 consecutive days, with 40 mg oral DXM per day on days 1 through 4 (VAD regimen). Three or 4 cycles of VAD were administered at 4-week intervals. After initial chemotherapy, patients with a performance status below World Health Organization grade 3 and adequate cardiopulmonary, hepatic, and renal functions underwent peripheral blood stem cell (PBSC) collection. Stem cells were collected after G-CSF priming (10 μg/kg daily for 6 days). Daily apheresis was continued until at least 5 × 106 CD34 cells/kg were collected. After PBSC collection, patients underwent first ASCT prepared by mel200.
IFM99-03 trial
After the first ASCT, patients who had an available HLA-identical sibling donor no older than 65 years and with no severe comorbid illness were treated according to the IFM99-03 protocol. After an interval of 2 months, patients received dose-reduced conditioning consisting of busulphan 2 mg/kg daily administrated orally over 2 days, fludarabine 25 mg/m2 daily for 5 days, and antithymocyte globulin (Imtix; Genzyme, Cambridge, MA) 2.5 mg/kg daily over 12 hours on days –5, –4, –3, –2, and –1, followed by allogeneic PBSC on day 0. Graft-versus-host prophylaxis consisted of cyclosporin A (3 mg/kg daily, given from day –1 to day +100 after transplantation) and short-course methotrexate. The dose of cyclosporin A was adjusted to serum levels. Cyclosporin A was tapered from day 60 and was discontinued, if possible, at day 100. Methotrexate (10 mg/m2) was given on days 1, 3, and 6 after transplantation. Chimerism studies were performed using FISH in sex-mismatched pairs, and polymerase chain reaction analyses of polymorphic microsatellite regions were performed in sex-matched pairs. On day 90, additional donor lymphocyte infusions (DLIs) were scheduled for patients with incomplete chimerism or persistent disease. Standard criteria were used for grading of acute and chronic GVHD.19
IFM99-04
After the first ASCT, patients without an HLA-identical sibling donor were randomly assigned to either of the 2 HDT groups.18 Randomization was stratified according to the center and was conducted through fax communications. In arm A, patients underwent second ASCT prepared by the combination of DXM 40 mg/d over 4 days plus mel220 infused over 30 minutes 48 hours before stem cell reinfusion. In arm B, patients underwent second ASCT prepared by the combination of mel220, DXM, and the addition of B-E8 (250 mg total dose). No maintenance therapy was given after the second ASCT.
Ethics considerations
IFM99-03 and IFM99-04 were approved by the local institutional ethics committee of the University of Grenoble and the University of Nantes, respectively, approved by the institutional review boards of all participating centers, and approved and registered by the official French agency for health security (Agence Française de Sécurité Sanitaire et des Produits de Santé [AFSSAPS]). Patients gave written informed consent.
Assessment of response
Response criteria have been defined previously.5,18 Complete response (CR) was defined as lack of detectable paraprotein by serum and urine electrophoresis and 5% or less plasma cells with normal morphologic features in a bone marrow aspirate. Very good partial response (VGPR) was defined as a 90% decrease in the serum paraprotein level; partial response (PR) was defined as a 50% decrease in the paraprotein level or a 90% decrease in the level of Bence-Jones protein (including patients with Bence-Jones protein alone), or both; minimal response (MR) was defined as a 25% decrease in the paraprotein level; stable disease (SD) was defined as no change in the paraprotein level; progressive disease (PD) was defined as a 25% increase in the paraprotein level; and relapse was defined as the reappearance of paraprotein, the recurrence of bone marrow infiltration, or both in a patient who had had complete response and as a 50% increase above the plateau level of paraprotein in 2 samples obtained 4 weeks apart in a patient who had had a response.
FISH analysis
FISH analysis of 13q14 was performed on highly purified human myeloma cells, as previously described.16
Statistical analysis
In the IFM99-04 trial, the primary end point was to compare the CR rates achieved after the second ASCT (HDT with or without anti–IL-6 monoclonal antibody BE-8).18 Secondary end points were to compare both arms regarding OS and EFS to study the feasibility and the toxicity of tandem transplantation with 2 different dosages of melphalan (mel200 and mel220). Assuming the complete response rate to be 25% in the DXM + mel220 arm, the study required 200 patients to have 80% power to detect an absolute improvement of 15% in the CR rate in the mel220 + DXM + anti–IL-6 mAb arm. The recruitment target was 200 randomly assigned patients. Two interim analyses were planned, the first after the first 50 patients to check feasibility and toxic death rate and the second after 140 randomly assigned patients to check OS and EFS. The board of the IFM group agreed to stop the trial in September 2004 when 165 patients had been randomly assigned in consideration of the lack of difference regarding primary and secondary end points of the study.
In the IFM99-03 trial, the objectives were to evaluate the feasibility and the TRM of dose-reduced allograft. The recruitment target was 60 patients. An interim analysis was planned after the first 20 patients to check feasibility and toxic death rate. The protocol was to have stopped if the TRM rate was greater than 30% at 6 months. At the IFM99-04 trial stopping date of September 2004, 65 patients had been included in the IFM99-03 protocol, and the board of the IFM group agreed to stop the trial on the same date to evaluate the results and to allow comparison of the 2 programs.
Overall survival was calculated from the start of therapy to death from any cause. Data on patients who were alive at the time of analysis were censored in the survival analysis on the last date they were known to be alive. Event-free survival was calculated from the start of therapy to disease progression, disease relapse, or patient death. Data on patients who had not experienced disease progression or relapse were censored on the last date they were known to be alive and event free. Comparisons of frequencies between groups were performed using the χ2 test and the Fisher exact test. Median values were compared with the use of the Wilcoxon rank-sum test. Survival was estimated with the Kaplan-Meier product limit method, and curves were compared with the stratified log-rank test. A cut-off date of May 15, 2005, was used for survival analysis.
Results
From April 2000 to September 2004, 284 patients from 48 centers met eligibility criteria, were registered, and received at least one course of VAD. Sixty-five (22.9%) patients had an available HLA-identical sibling donor and were included in the IFM99-03 trial, and 219 (77.1%) patients were included in the IFM99-04 trial. Among these 219 patients, 4 patients had an HLA-identical sibling but were not included in the IFM 99-03 trial because of underlying disease in the donor: progressive solid tumor (n = 2), ongoing infection (n = 1), psychiatric disease (n = 1). Moreover, HLA typing was not performed in 24 patients included in the IFM99-04 trial because of patient refusal (n = 5), family refusal (n = 3), physician decision (n = 5), potential sibling donor older than 70 years (n = 6), or unknown causes (n = 5). Table 1 shows the baseline characteristics of the patients included in both studies. Patients were older in the tandem ASCT trial (median age, 58 vs 54 years; P = .006), and the β2-microglobulin level was also higher in the latter group (median, 4.9 mg/L vs 4.1 mg/L; P = .049).
IFM99-03 trial
Sixty-five patients were included in the study. Nineteen (29.2%) did not complete the entire program (ASCT followed by reduced-intensity allogeneic transplantation) because of progressive disease (n = 7), donor refusal (n = 2), recipient refusal (n = 3), ongoing infection (n = 4), or unknown causes (n = 3).
For the 46 patients who completed the entire program, the median time between diagnosis and ASCT was 153 days (range, 120-226 days), and it was 73 days (range, 44-92 days) between ASCT and dose-reduced allograft.
Engraftment and chimerism
Peripheral blood was used as the source of stem cells in all 46 patients. Engraftment occurred in 100% of the patients. Chimerism data were available for 29 patients. At best, 25 (86.2%) patients achieved full donor chimerism at day 60, but this decreased to 21 (72.4%) patients at subsequent analysis on day 120. All patients with persistent partial chimerism had progressive disease.
Graft-versus-host disease
Acute GVHD occurred in 15 (32.6%) patients, with 4 (8.7%) experiencing grade I and 11 (23.9%) grade II-IV disease. Forty-two patients were evaluable for chronic GVHD (cGVHD), of whom 3 (7.1%) developed limited and 15 (35.7%) developed extensive cGVHD. Five of 15 patients with extensive cGVHD were observed after DLI.
Transplant-related mortality
Five (10.9%) patients died of procedure-related complications; 2 died before day 100 of infections (viral pneumonitis [n = 1], septicemia with brain metastasis [n = 1]), and 3 died after day 100 of infections associated with GVHD (bacterial pneumonitis [n = 2], disseminated aspergillosis [n = 1]).
Response after transplantation
Forty-five patients (1 early death) were evaluable for response assessment after allogeneic transplantation. One month after ASCT, 17 (37.8%) patients were in CR (n = 5) or VGPR (n = 12), 21 (46.6%) were in PR, and 7 (15.5%) had stable or progressive disease. Two months after allogeneic transplantation, 28 (62.2%) were in CR (n = 15) or VGPR (n = 13), 9 (20%) were in PR, and 8 (17.8%) had stable or progressive disease.
Overall and event-free survival
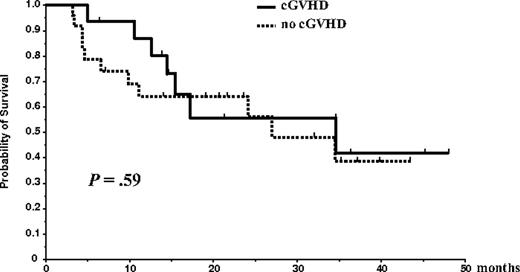
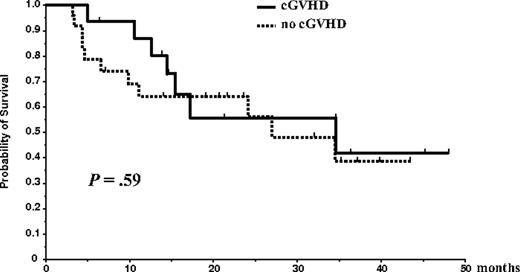
At the reference date of May 15, 2005, the median OS time for the entire group of 65 patients was 35 months; at 56 months, the survival rate was 33.1%. Median EFS time from diagnosis for the entire group of 65 patients was 25 months, and the 5-year EFS rate was 0%. Overall survival and EFS of the 46 patients who underwent allogeneic transplantation were not different from those of the 19 patients who could not receive the allogeneic graft (OS median, 35 vs 36.6 months [P = .66]; EFS median, 31.7 vs 23 months [P = .14]). Disease progression or relapse occurred in 26 of 46 (56.5%) patients. The cumulative probability of progression was 57% at 3 years, and the projected 5-year probability of relapse was 100%. There was a trend for delayed progression among patients who developed cGVHD (median time to progression from the date of allogeneic transplantation, 24 months in patients without cGVHD vs 32 months in patients with cGVHD [P = .14]), but this delayed progression time did not translate to better OS (median survival from the date of allogeneic transplantation, 27 months in patients without cGVHD vs 34 months in patients with cGVHD [P = .59]; Figure 2). For acute GVHD, no effect on OS or relapse rate was observed for presence or severity of disease. Of 46 patients who underwent the entire program, 22 have died, 5 of TRM and 17 of disease progression. Median follow-up time for living patients who underwent the entire program was 28 months (range, 11-57 months).
Donor lymphocyte infusion
Seventeen patients received DLIs. In 8 patients, DLI was performed for persistent disease without GVHD at day 90. The starting dose in each case was 1 × 107 CD3+ cells/kg. Two patients received a second DLI at an increased dose of 5 × 107 CD3+ cells/kg, and one patient received a third dose of 1 × 108 CD3+ cells/kg. In 5 patients, DLI induced disease response (CR or VGPR), and 3 patients had progressive disease despite DLI infusions. Nine patients received DLI later in the course of the disease, at the time of relapse; no responses were observed.
IFM99-04 trial overall results
Patient characteristics, response rates, TRM, OS, and EFS have been described elsewhere.18 Briefly, 53 of 219 (24.2%) enrolled patients did not proceed to randomization to receive or not receive anti–IL-6 mAb because of severe complications or disease progression before second ASCT. Thus, 166 (75.8%) patients were randomly assigned (85 patients in arm A and 81 patients in arm B) and were treated according to the entire protocol. Response rates (CR + VGPR) were 16% after VAD induction therapy, 34% after the first ASCT prepared by mel200, and 51% after second ASCT. The addition of BE-8 to the second conditioning regimen did not increase the response rate. The TRM rate was 5%: 6 patients died during induction therapy with VAD, 2 patients died during first ASCT, and 3 patients died during second ASCT (1 in arm A and 2 in arm B).
Overall survival and event-free survival
At the reference date of May 25, 2005, the median OS for the entire group of 219 patients was 41 months, and the survival rate at 56 months was 44.4%.18 Median EFS from diagnosis for the entire group of 219 patients was 30 months, and the EFS rate at 5 years was 0%.18 Median follow-up time for living patients who were randomly assigned was 24 months (9-59 months). EFS was nearly identical in both arms of the study (median, 35 months in arm A and 31 months in arm B and 0% at 59 and 57 months, respectively; P = .39). OS was not statistically different in arm A and arm B (46% vs 51% at 54 months, respectively; P = .90).
Comparison of studies
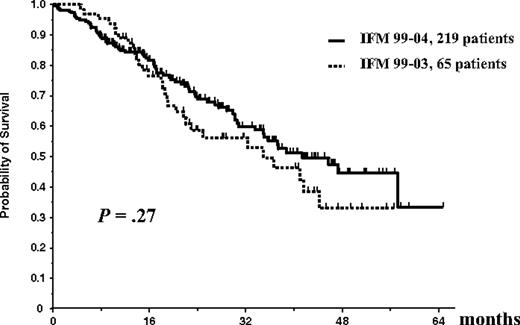
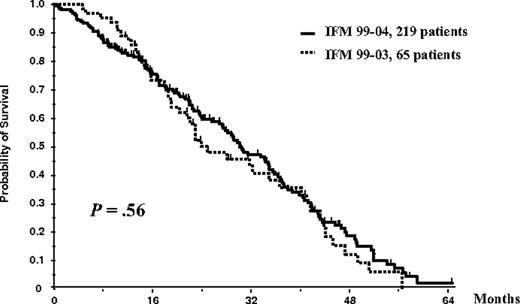
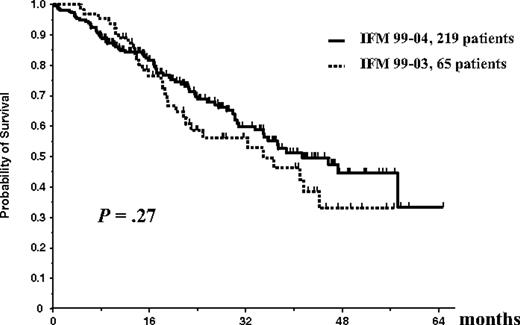
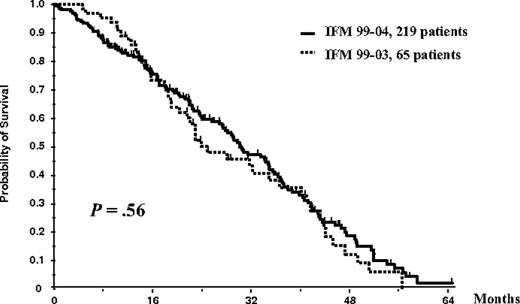
On an intent-to-treat basis, considering the entire population of 284 patients (65 in the IFM99-03 protocol and 219 in the IFM99-04 trial), OS did not differ significantly between studies (median survival, 35 months in the IFM99-03 trial vs 41 months in the IFM99-04 protocol; P = .27; Figure 3). Similarly, considering the entire population of 284 patients, EFS did not differ significantly (median EFS, 25 months in the IFM99-03 trial vs 30 months in the IFM99-04 protocol; P = .56; Figure 4).
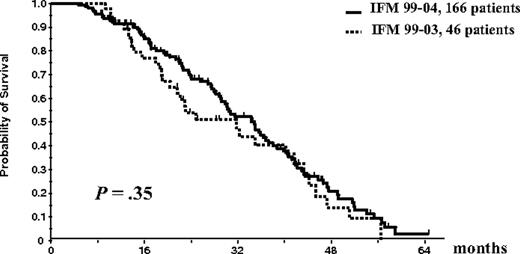
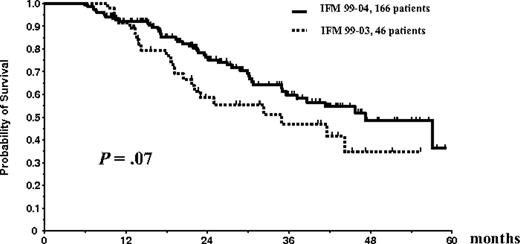
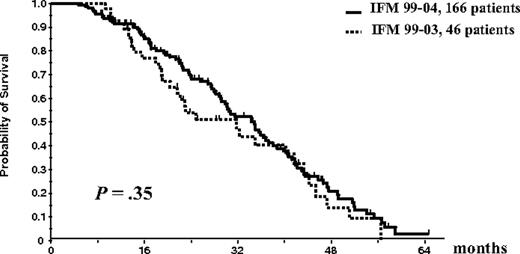
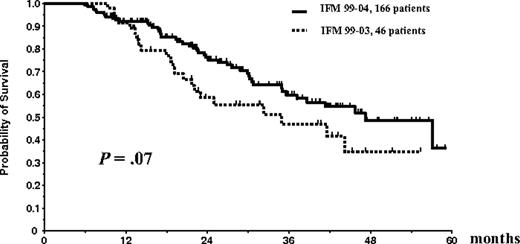
Overall survival and EFS were similar in the 2 treatment arms of the IFM99-04 trial. Thus, the results of patients in arm A (n = 85) and arm B (n = 81) were pooled for comparison with those of the 46 patients included in the IFM99-03 study who underwent the entire ASCT plus allogeneic transplantation program. EFS of the 166 patients randomly assigned in the tandem ASCT protocol was similar to the EFS of the 46 patients who underwent the entire IFM99-03 program (median, 35 months vs 31.7 months; P = .35; Figure 5). There was a trend for better OS for the randomly assigned patients in the tandem transplantation trial than for patients treated with the combination of ASCT followed by mini-allogeneic transplantation (median OS, 47.2 months vs 35 months; P = .07; Figure 6).
Discussion
Despite improvements in the results of conventional myeloablative allogeneic transplantation in the late 1990s,10 this treatment is associated with a high TRM rate and a low survival rate in patients with multiple myeloma (MM).11,12 In case-matched retrospective6 and prospective comparisons,20,21 overall short-term results of allogeneic transplantation have always been considered inferior to those of ASCT, primarily because of high TRM, even when allogeneic transplantation is used as frontline therapy. However, the immunologic effect of the graft, so-called graft-versus-myeloma (GVM), has been clearly demonstrated by the use of DLI, and long-term remission, including molecular remission, can be obtained.7-9 Reduced-intensity conditioning was developed to decrease the toxicity and the TRM associated with myeloablative allogeneic transplantation while maintaining the GVM effect. Several groups have studied the impact of a tandem approach combining the reduction of tumor burden obtained by ASCT and the GVM effect of mini-allotransplantation.13,14,22 Nevertheless, few data are available regarding outcomes of this cytoreductive autografting followed by reduced-intensity genoidentical allogeneic transplantation as part of initial therapy in patients with MM. In the series described by Maloney et al,14 48% of the patients had undergone more than one treatment regimen, and the median time between diagnosis and ASCT was 282 days. In the German study of tandem auto–mini-ASCT,13 the median time from diagnosis to autotransplantation was 13 months. In the recent retrospective trial from the European Bone Marrow Transplantation (EBMT) group,22 most of the 229 patients who received dose-reduced allografts had received at least one previous autograft, but the median time from diagnosis to allotransplantation was 1.6 years (range, 2-11 years). Nevertheless, even in patients with advanced disease, results of combination ASCT plus dose-reduced allografts were encouraging. The TRM rate was low (range 4%14 -11%13 ). The CR rate and the 2-year estimated survival rate were 73% and 74%, respectively, in the series of Kröger et al.13 The overall response rate was 81%, and the 2-year estimated survival rates were 78% and 46% in patients who survived in CR at a median follow-up of 18 months in the Maloney et al14 series.
Given the good results of tandem ASCT in patients at “standard risk,”5,16 the IFM group considered that the risk for TRM and cGVHD were not justified in these patients. Therefore, we decided to study the impact of combination auto/mini-allotransplantation in a subset of high-risk patients with genoidentical siblings and to prospectively compare this approach with tandem melphalan-based dose-intensified ASCT in the same subgroup of patients who did not have available sibling donors. Our study confirmed that this strategy is relatively safe; the 100-day mortality rate was 4.3%, and the overall TRM rate was 10.9%. These results are comparable to those previously published.13,14,22 However, this low TRM rate did not translate to better long-term survival; the 2-year estimated survival rate was 58%, but the 5-year estimated EFS rate was 0% without any plateau. This was attributed to a high relapse rate primarily because of the selection of patients with poor prognosis at the time of diagnosis. All patients included in the present trials presented with high β2-microglobulin level and chromosome 13 deletion, which have already been described as adverse prognostic factors in patients who receive dose-reduced allograft.23 In the Seattle series,14 32% of patients had β2-microglobulin levels greater than 2.5 mg/L, and chromosome 13 data were not available. Median β2-microglobulin level among the patients in the German study13 was 3 mg/L, and again chromosome 13 status was not mentioned in that cohort of patients. Another possible explanation for this high relapse rate is that the conditioning regimen of the IFM99-03 trial, unlike the regimens usually proposed in dose-reduced allograft for MM,13,22 did not include melphalan and lost its cytotoxic activity. However, melphalan was used before ASCT for tumor burden reduction; in addition, we wanted to use dose-reduced allograft primarily for its immune effects. The use of high-dose ATG given for 5 days during the preparative regimen might have contributed to the low incidence of GVHD by its in vivo T cell depletion,13,24 but it might also have inhibited, for a prolonged time, donor cytotoxic cells responsible for the GVM effect. The development of cGVHD seemed to be associated with a delayed progression time. Disappointingly, however, there was no convincing plateau in any of the survival curves, efficacy was only transient, and disease usually progressed despite transplantation. Therefore, a logical interpretation could be that the GVM effect in our series served only to delay disease progression.
Results of the IFM99-04 have been discussed in detail elsewhere,18 but the OS and EFS rates were superior to the 2-year and 18-month survival and EFS rates previously described in high-risk patients treated by ASCT.16,25,26 This could be attributed to the dose intensity of mel420 (mel200 plus mel220). Median EFS was 30 months in this cohort of 219 high-risk patients, identical to the median EFS of the double transplantation arm of the IFM94 trial in which 200 patients underwent a tandem mel140, mel140 + 8 Gy total body irradiation program.5 In this study, patients with de novo MM were included regardless of β2-microglobulin level or chromosome 13 abnormality. Nevertheless, the median OS time was 58 months, much longer than the median OS time of 41 months of the IFM99-04 trial, indicating that in patients at high risk, the duration of survival after relapse was short and salvage regimens were less frequently active in relation with disease severity. In such patients, relapse after tandem HDT was explosive and often refractory.
Compared with those of the IFM99-04 protocol, the results of the IFM99-03 trial could be interpreted negatively. Tandem ASCT followed by dose-reduced allograft was not superior to the tandem ASCT protocol, either for EFS or for OS, without any plateau on survival curves. The dose-reduced allograft used in this study carried the risk for cGVHD, which dramatically decreased the quality of life of patients. Therefore, it should not be recommended for patients like those in the specific subgroup of patients at high risk discussed in this study. The main objective of the IFM99-03 trial was to reduce TRM. This goal was achieved, but tumor control, as previously discussed, was only transient, and long-term results must be improved. Apart from focusing on a subgroup of patients with better prognosis at diagnosis or from using a conditioning regimen with cytotoxic antimyeloma activity or without high-dose ATG evaluated in other ongoing European trials, it may be possible to explore systematically the immunologic effects of prophylactic DLI–enhancing reconstitution of donor T cells, conversion to donor hematopoiesis, and antitumor immunity, as has been reported in the context of myeloablative bone marrow transplantation.27 Some authors have also reported that adding a drug such as thalidomide to DLI could improve the antimyeloma effect in patients who undergo reduced-intensity conditioning.28 Other novel antimyeloma agents could be investigated. It has recently been reported in a murine model that the proteasome inhibitor bortezomib, given at the time of allogeneic SCT, was able to inhibit acute GVHD and to retain the graft-versus-tumor effect.29 All these promising strategies should be explored in the context of prospective clinical studies.
Appendix
The following additional centers and investigators from The Intergroupe Francophone du Myélome and the Swiss Group for Clinical Cancer Research participated in this study: Amiens, France, Centre Hospitalier Général, V. Salles; Avignon, France, Centre Hospitalier Général, G. Lepeu; Bourg-en-Bresse, France, Centre Hospitalier Général, M. C. Perrin; Brussels, Belgium, Centre Universitaire Saint Luc, A. Ferrant; Brussels, Belgium, University Hospital, Chantal Doyen; Clermont-Ferrand, France, Centre Hospitalier Régional et Universitaire, C. Chaleteix; Dunkerque, France, Centre Hospitalier Général, M. Wetterwald; Genève, Switzerland, Hôpital Universitaire, T. Matthés; Haine Saint-Paul, Belgium, Centre Hospitalier Jolimont, P. Delannoy; La Roche sur Yon, France, Centre Hospitalier Général, M. Tiab; Le Havre, France, Centre Hospitalier Général, M. Zarnitsky; Marseille, France, Centre Paoli-Calmette, A. M. Stoppa; Metz, France, Centre Hospitalier Général, V. Dorvaux; Pau, France, Clinique des Pyrénées, D. Schlaiffer; Percy-Clamart, France, Hôpital des Armées, B. Souleau; Perpignan, France, Centre Hospitalier Général, X. Vallantin; Poitiers, France, Centre Hospitalier Régional et Universitaire, F. Guilhot; Quimper, France, Centre Hospitalier Général, J. P. Vilque; Reims, France, Centre Hospitalier Régional et Universitaire, B. Kolb; Rennes-Sud, France, Centre Hospitalier Régional et Universitaire, B. Grosbois; Saint-Etienne, France, Centre Hospitalier Régional et Universitaire, J. Jaubert; Suresnes, France, Hôpital Foch, M. Janvier; Vannes, France, Centre Hospitalier Général, H. Jardel; Annecy, France, Centre Hospitalier Général, C. Martin; Institut Curie, France, Centre Anti-cancéreux, J. Decaudin; Laval, France, Centre Hospitalier Général, M. Jacomi; Boulogne-sur-mer, France, Centre Hospitalier Général, X. Agape; Caen, France, Centre Anti-cancéreux, A. M. Pény; Colmar, France, Centre Hospitalier Général, B. Audhuy; Brussels, Belgium, Institut Bordet, P. Bron; Bruges, Belgium, M. Lauvagie; Paris, France, Hôpital Saint-Louis, P. Brice; Draguignan, France, Centre Hospitalier Général, B. Valenza; Caen, France, Polyclinique du Parc, X. Levaltier; Le Mans, France, Centre Hospitalier Général, M. Duguet; Blois, France, Centre Hospitalier Général, P. Rodon; Tours, France, Centre Hospitalier Régional et Universitaire, L. Benboubker; Nice, France, Centre Hospitalier Régional et Universitaire, J. G. Fuzibet, J. P. Cassutto; Besançon, France, Centre Hospitalier Régional et Universitaire, L. Voillat; Bobigny, France, Centre Hospitalier Régional et Universitaire, P. Casassus; Rouen, France, Centre Hospitalier Régional et Universitaire, M. Monconduit; Paris, France, Centre Régional et Universitaire Saint-Antoine, L. Garderet; Lausanne, Switzerland, University Hospital, S. Leyvraz.
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-09-3869.
A complete list of the members of Intergroupe Francophone du Myélome and the Swiss Group for Clinical Cancer Research appears in the “Appendix.”
Supported by grants from the Programme Hospitalier National de Recherche Clinique.
All the authors are members of the Intergroupe Francophone du Myélome group or the Swiss Group for Clinical Cancer Research and were involved in the conception and design of the study, the provision of study patients, and manuscript review and approval. F.G., J.-L.H., and P.M. analyzed the data and wrote the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Hervé Avet-Loiseau for conducting FISH analysis and for reviewing the manuscript.