We conducted a retrospective analysis of 968 adults with acute myeloid leukemia (AML) on 5 recent Southwest Oncology Group trials to understand how the nature of AML changes with age. Older study patients with AML presented with poorer performance status, lower white blood cell counts, and a lower percentage of marrow blasts. Multidrug resistance was found in 33% of AMLs in patients younger than age 56 compared with 57% in patients older than 75. The percentage of patients with favorable cytogenetics dropped from 17% in those younger than age 56 to 4% in those older than 75. In contrast, the proportion of patients with unfavorable cytogenetics increased from 35% in those younger than age 56 to 51% in patients older than 75. Particularly striking were the increases in abnormalities of chromosomes 5, 7, and 17 among the elderly. The increased incidence of unfavorable cytogenetics contributed to their poorer outcome, and, within each cytogenetic risk group, treatment outcome deteriorated markedly with age. Finally, the combination of a poor performance status and advanced age identified a group of patients with a very high likelihood of dying within 30 days of initiating induction therapy. The distinct biology and clinical responses seen argue for age-specific assessments when evaluating therapies for AML.
Introduction
Both the nature of acute myeloid leukemia (AML) and the health of the patient change with age. It is axiomatic that older patients are more likely to have more comorbidities and have a poorer performance status than younger patients. As we and others have noted, when compared with the disease in younger adults, AML in older patients is more likely to be preceded by a myelodysplastic phase, more frequently has unfavorable cytogenetics, more commonly expresses multidrug resistance, and responds less well to chemotherapy.1,2 Although these conclusions are generally accepted, less is known about the extent of these associations and how they vary by decade of patient age. Therefore, we have analyzed the clinical and biologic features of a large number of adult patients with previously untreated AML entered on 5 Southwest Oncology Group Studies.
Patients, materials, and methods
Clinical and biologic features of AML in 968 patients entered onto 5 Southwest Oncology Group (SWOG) trials, 2 dealing with patients age younger than 55 (S9034, 16-55 years; S9500, 18-55 years) and 3 restricted to patients older than 56 (S9031, S9333, and S0112), were analyzed.3-7 All 5 studies were restricted to patients with previously untreated AML. Patients with acute promyelocytic leukemia were excluded. Patient performance status and laboratory values reflected the status of the patient at the time of study entry, within 72 hours of initiation of therapy. All 5 trials required centralized morphologic and cytogenetic review. The specific protocols and treatment outcomes of 4 of the 5 trials have been published (results of S0112 have not yet been published).3-7
Collection and quality control of patient pretreatment and outcome data were performed according to standard SWOG procedures. Quantitative factors were treated as continuous variables in regression analyses but grouped when necessary for descriptive tables and figures. Comparison of dichotomous variables was based on the chi-square approximation of the Fisher exact test. Overall survival (OS) was measured from randomization until death from any cause, with observations censored for patients last known alive. Disease-free survival (DFS) was measured from the date the complete response was established until the relapse of leukemia or death from any cause, with observations censored for patients last known to be alive without report of relapse. Distributions of OS and DFS were estimated by the method of Kaplan and Meier.1,7 For OS and DFS, comparisons between groups were based on the log-rank test. All P values are 2-tailed. Results are based on data available as of August 2005.
The SWOG Cytogenetics Committee reviewed all pretreatment cytogenetic studies included in this analysis. Patients were accepted if they met the following criteria: all had to be processed by at least 2 different methods and at least 20 metaphase cells had to be analyzed, unless an abnormal clone was diagnosable with fewer cells. Patients are divided into favorable, intermediate, and unfavorable cytogenetic categories.4 The favorable category includes patients with the inv(16) and t(8;21) abnormalities, unless complex cytogenetic abnormalities were also present. The intermediate category includes patients with normal cytogenetics, trisomy 8, a few other single-chromosome deletions, and sex chromosome losses. The unfavorable category includes patients with complex (3 or more) aberrations, aberrations or losses of 5 and 7, inv(3q), t(6;9), t(9;22), and others.
Results
Demographics of the 968 patients are presented in Table 1. There was no change in sex ratio with age. However, the proportion of nonwhites entered onto these studies declined with age. In addition, the performance status of patients dropped with age, with a smaller proportion of patients with a performance status of 0 among the elderly. Somewhat surprisingly, the proportion of patients with de novo presentation of AML did not decrease in the very elderly (age, > 75) compared with patients age 56 to 65 or 66 to 75.
Demographics and presentation of AML, by age
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 368 | 246 | 274 | 80 |
| Sex, male, no. (%) | 193 (52) | 139 (57) | 154 (56) | 44 (55) |
| Race, white, no. (%) | 318 (86) | 216 (88) | 254 (93)* | 73 (91) |
| Presentation, de novo, no. (%) | NA† | 192 (78) | 208 (76) | 62 (78) |
| Performance status, no. (%) | ||||
| 0 | 129 (35) | 72 (29) | 73 (27) | 14 (18) |
| 1 | 180 (49) | 112 (46) | 126 (46) | 40 (50) |
| 2 | 46 (13) | 34 (14) | 52 (19) | 14 (18) |
| 3 | 9 (2) | 24 (10) | 19 (7) | 11 (14) |
| Unknown | 4 (2) | 4 (2) | 4 (1) | 1 (1) |
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 368 | 246 | 274 | 80 |
| Sex, male, no. (%) | 193 (52) | 139 (57) | 154 (56) | 44 (55) |
| Race, white, no. (%) | 318 (86) | 216 (88) | 254 (93)* | 73 (91) |
| Presentation, de novo, no. (%) | NA† | 192 (78) | 208 (76) | 62 (78) |
| Performance status, no. (%) | ||||
| 0 | 129 (35) | 72 (29) | 73 (27) | 14 (18) |
| 1 | 180 (49) | 112 (46) | 126 (46) | 40 (50) |
| 2 | 46 (13) | 34 (14) | 52 (19) | 14 (18) |
| 3 | 9 (2) | 24 (10) | 19 (7) | 11 (14) |
| Unknown | 4 (2) | 4 (2) | 4 (1) | 1 (1) |
AML indicates acute myeloid leukemia; NA, not available.
One patient from S9031 has unspecified race.
Not recorded for patients on studies S9034 and S9500.
Hematologic characteristics of the disease at the time of entry onto the study are shown in Table 2. There was a trend toward a higher peripheral white blood cell count (P = .004) and percentage of peripheral blasts (P < .001) among patients younger than age 56. Otherwise, no significant differences were seen among the different age groups.
Hematologic values
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 368 | 246 | 274 | 80 |
| Laboratory values, median (range) | ||||
| Hgb level, g/dL* | 9.2 (1.7-25.0) | 9.0 (4.3-14.4) | 9.1 (5.2-14.9) | 9.4 (6.2-12.4) |
| WBC count, × 109/L† | 18.6 (0.3-360.0) | 12.0 (0.7-274.0) | 10.0 (0.6-298.0) | 12.7 (0.6-215.6) |
| PLT count, × 109/L‡ | 49 (3-999) | 49 (5-1200) | 61 (2-684) | 59 (12-537) |
| Peripheral blasts, %§ | 39 (0-99) | 26 (0-99) | 24 (0-99) | 24 (0-97) |
| Marrow blasts, %∥ | 70 (1-99) | 61 (9-99) | 65 (0-99) | 60 (0-96) |
| FAB classification of local diagnosis, no. (%) | ||||
| M1 | 78 (21) | 65 (26) | 64 (23) | 20 (25) |
| M2 | 123 (33) | 80 (33) | 90 (33) | 21 (26) |
| M4 | 106 (29) | 50 (20) | 53 (19) | 18 (23) |
| M5 | 35 (10) | 25 (10) | 22 (8) | 10 (13) |
| M6 | 9 (2) | 5 (2) | 11 (4) | 3 (4) |
| M7 | 0 | 5 (2) | 4 (1) | 1 (1) |
| M0 | 3 (1) | 12 (5) | 21 (8) | 6 (8) |
| Other AML | 1 (0) | 4 (2) | 9 (3) | 1 (1) |
| Unknown or other | 13 (4) | 0 (0) | 0 (0) | 0 (0) |
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 368 | 246 | 274 | 80 |
| Laboratory values, median (range) | ||||
| Hgb level, g/dL* | 9.2 (1.7-25.0) | 9.0 (4.3-14.4) | 9.1 (5.2-14.9) | 9.4 (6.2-12.4) |
| WBC count, × 109/L† | 18.6 (0.3-360.0) | 12.0 (0.7-274.0) | 10.0 (0.6-298.0) | 12.7 (0.6-215.6) |
| PLT count, × 109/L‡ | 49 (3-999) | 49 (5-1200) | 61 (2-684) | 59 (12-537) |
| Peripheral blasts, %§ | 39 (0-99) | 26 (0-99) | 24 (0-99) | 24 (0-97) |
| Marrow blasts, %∥ | 70 (1-99) | 61 (9-99) | 65 (0-99) | 60 (0-96) |
| FAB classification of local diagnosis, no. (%) | ||||
| M1 | 78 (21) | 65 (26) | 64 (23) | 20 (25) |
| M2 | 123 (33) | 80 (33) | 90 (33) | 21 (26) |
| M4 | 106 (29) | 50 (20) | 53 (19) | 18 (23) |
| M5 | 35 (10) | 25 (10) | 22 (8) | 10 (13) |
| M6 | 9 (2) | 5 (2) | 11 (4) | 3 (4) |
| M7 | 0 | 5 (2) | 4 (1) | 1 (1) |
| M0 | 3 (1) | 12 (5) | 21 (8) | 6 (8) |
| Other AML | 1 (0) | 4 (2) | 9 (3) | 1 (1) |
| Unknown or other | 13 (4) | 0 (0) | 0 (0) | 0 (0) |
Hgb indicates hemoglobin; WBC, white blood cell; PLT, platelet; FAB, French-American-British; AML, acute myeloid leukemia.
n = 949.
n = 968.
n = 965.
n = 931.
n = 907.
The results of cytogenetic studies for the 759 patients with evaluable cytogenetics are presented in Table 3 and Figure 1. Nineteen patients with uncommon cytogenetic abnormalities could not be classified in a cytogenetic risk category. As shown, the percentage of patients with favorable cytogenetics dropped from 17% in patients younger than age 56 to only 4% in patients older than age 75. Correspondingly, the proportion of patients with unfavorable risk cytogenetics increased markedly from 35% in patients age younger than 56 to 51% in patients older than age 75. Much of the increase in unfavorable cytogenetics was due to a marked increase in the proportion of patients with loss of part or all of chromosomes 5 or 7, a finding seen in 12% of patients younger than age 56 but in 34% of patients older than age 75.
Cytogenetic results
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . | P* . |
|---|---|---|---|---|---|
| No. patients | 323 | 183 | 199 | 54 | |
| Cytogenetic risk group, no. (%) | < .001† | ||||
| Favorable | 51 (16) | 10 (5) | 10 (5) | 2 (4) | |
| Intermediate | 149 (46) | 101 (55) | 110 (55) | 24 (44) | |
| Unfavorable | 108 (33) | 70 (38) | 78 (39) | 27 (50) | |
| Unknown | 15 (5) | 2 (1) | 1 (1) | 1 (2) | |
| Specific abnormalities, no. (%) | |||||
| -5 or 5q- | 21 (7) | 27 (15) | 28 (14) | 14 (26) | < .001 |
| -7 or 7q- | 28 (9) | 35 (19) | 36 (18) | 12 (22) | < .001 |
| 17p | 6 (2) | 16 (9) | 14 (7) | 6 (11) | .001 |
| t(8;21) | 22 (7) | 7 (4) | 4 (2) | 0 (0) | .019 |
| inv(16) | 31 (10) | 4 (2) | 7 (4) | 4 (7) | .002 |
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . | P* . |
|---|---|---|---|---|---|
| No. patients | 323 | 183 | 199 | 54 | |
| Cytogenetic risk group, no. (%) | < .001† | ||||
| Favorable | 51 (16) | 10 (5) | 10 (5) | 2 (4) | |
| Intermediate | 149 (46) | 101 (55) | 110 (55) | 24 (44) | |
| Unfavorable | 108 (33) | 70 (38) | 78 (39) | 27 (50) | |
| Unknown | 15 (5) | 2 (1) | 1 (1) | 1 (2) | |
| Specific abnormalities, no. (%) | |||||
| -5 or 5q- | 21 (7) | 27 (15) | 28 (14) | 14 (26) | < .001 |
| -7 or 7q- | 28 (9) | 35 (19) | 36 (18) | 12 (22) | < .001 |
| 17p | 6 (2) | 16 (9) | 14 (7) | 6 (11) | .001 |
| t(8;21) | 22 (7) | 7 (4) | 4 (2) | 0 (0) | .019 |
| inv(16) | 31 (10) | 4 (2) | 7 (4) | 4 (7) | .002 |
Patients on SWOG trial S0112 are not included. Only patients with evaluable karyotypes are included.
Test for heterogeneity among ago groups (age was not treated as a continuous variable for these tests).
Test for patients with known risk group.
Multidrug resistance, as measured by MRK staining,8 was found in 33% of AMLs in patients age younger than 56, but in between 57% and 62% of cases in the higher age categories (Table 4). For this multidrug resistance analysis, MRK staining data were limited to the 404 patients entered onto studies S9500 and S9333 because different methodologies were used in the other studies.
MRK-positive leukemias
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients* | 76 | 130 | 156 | 42 |
| MRK, D at least 0.15, no. (%) | 25 (33) | 80 (62) | 60 (61) | 24 (57) |
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients* | 76 | 130 | 156 | 42 |
| MRK, D at least 0.15, no. (%) | 25 (33) | 80 (62) | 60 (61) | 24 (57) |
Data from study S9500 only for patients younger than 56 years and data from study S9333 only for patients older than 56 years.
The likelihood of death within the first 30 days of initial remission induction according to age and performance status is summarized in Table 5. As shown, age had, at most, a modest effect for patients with an excellent performance status, but for patients with a performance status of 2 or 3, age had a dramatic effect, with 82% of patients older than age 75 with a performance status of 3 dying within 30 days of the initiation of induction.
Mortality within 30 days of initiation of induction
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 364 | 242 | 270 | 79 |
| Early deaths*by performance status, no./no. total patients (%) | ||||
| 0 | 3/129 (2) | 8/72 (11) | 9/73 (12) | 2/14 (14) |
| 1 | 6/180 (3) | 6/112 (5) | 20/126 (16) | 7/40 (18) |
| 2 | 1/46 (2) | 6/34 (18) | 16/52 (31) | 7/14 (50) |
| 3 | 0/9 (0) | 7/24 (29) | 9/19 (47) | 9/11 (82) |
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 364 | 242 | 270 | 79 |
| Early deaths*by performance status, no./no. total patients (%) | ||||
| 0 | 3/129 (2) | 8/72 (11) | 9/73 (12) | 2/14 (14) |
| 1 | 6/180 (3) | 6/112 (5) | 20/126 (16) | 7/40 (18) |
| 2 | 1/46 (2) | 6/34 (18) | 16/52 (31) | 7/14 (50) |
| 3 | 0/9 (0) | 7/24 (29) | 9/19 (47) | 9/11 (82) |
Patients with known prestudy performance status are included.
Within 30 days of registration to the trial.
The percentage of complete response, percentage of resistant disease, median disease-free survival, and median overall survival for patients according to age group are shown in Table 6. Patients younger than age 56 treated on S9034 and S9500 received more aggressive chemotherapy than did older patients, making comparisons between patients younger and older than age 56 difficult. There were no protocol-directed differences in dosing in patients age 56 to 65, 66 to 75, and older than age 75. As shown, response rates dropped with age, as did the median overall survival.
Treatment outcomes
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 368 | 246 | 274 | 80 |
| Response, no. (%) | ||||
| CR | 235 (64) | 113 (46) | 108 (39) | 26 (33) |
| Resistant disease | 99 (27) | 91 (37) | 101 (37) | 29 (36) |
| Median overall survival, no. (95% CI) | 18.8 (14.9-22.6) | 9.0 (8.1-10.2) | 6.9 (5.4-7.7) | 3.5 (1.4-6.1) |
| No. patients with CR | 235 | 113 | 108 | 26 |
| Median disease-free survival, no. (95% CI) | 21.6 (15.8-25.5) | 7.4 (6.5-8.8) | 8.3 (6.3-10.2) | 8.9 (5.8-10.8) |
. | Younger than 56 y . | 56-65 y . | 66-75 y . | Older than 75 y . |
|---|---|---|---|---|
| No. patients | 368 | 246 | 274 | 80 |
| Response, no. (%) | ||||
| CR | 235 (64) | 113 (46) | 108 (39) | 26 (33) |
| Resistant disease | 99 (27) | 91 (37) | 101 (37) | 29 (36) |
| Median overall survival, no. (95% CI) | 18.8 (14.9-22.6) | 9.0 (8.1-10.2) | 6.9 (5.4-7.7) | 3.5 (1.4-6.1) |
| No. patients with CR | 235 | 113 | 108 | 26 |
| Median disease-free survival, no. (95% CI) | 21.6 (15.8-25.5) | 7.4 (6.5-8.8) | 8.3 (6.3-10.2) | 8.9 (5.8-10.8) |
CI indicates confidence interval; CR, complete response.
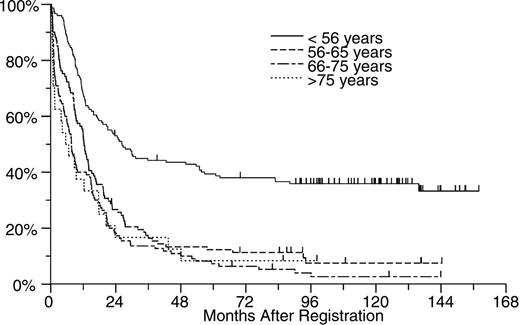
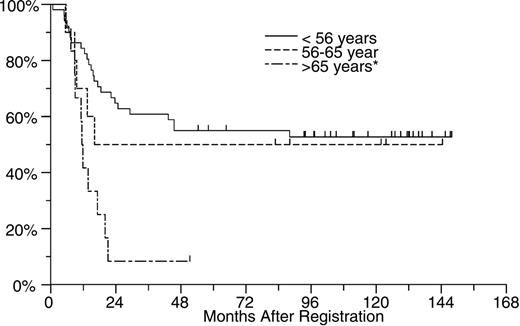
In an effort to determine whether the poorer outcome of treatment in older patients was predominantly due to the higher incidence of cases with unfavorable cytogenetics, we looked at treatment outcome within each cytogenetic risk group. As shown in Figures 2, 3, and 4, age appeared to have an effect within each cytogenetic risk group. As shown in Figure 2, the likelihood of prolonged survival is low for any patient with unfavorable cytogenetics regardless of age but slightly better for those younger than age 56. Among patients with intermediate risk cytogenetics, patients younger than 56 did markedly better than older individuals (Figure 3). Among patients with good risk cytogenetics (ie, those with t(8;21) and inv(16)) age appears to have a powerful effect on outcome. Among this subgroup of patients, those older than age 65 did much worse than younger patients (Figure 4).
Discussion
The results presented in this study confirm and extend previous reports showing that the biology of AML changes with age. When presenting in older study patients, AML was a less proliferative disease with lower white blood cell counts and peripheral blast percentages, the spectrum of cytogenetic abnormalities changed with a much higher incidence of abnormalities involving chromosomes 5, 7, and 17 and a lower incidence of the translocations associated with favorable treatment outcomes, and the incidence of expression of p-glycoprotein markedly increased. Also, as noted by others, patient performance status deteriorated with age. The effects of age both on the patient and the disease resulted in a higher incidence of death early after induction therapy, a lower rate of complete response, and a lower chance for long-term survival as the age of the patients increased.
A limitation of this and most other reports attempting to describe features of AML is that the data are derived only from those patients actually entered onto clinical trials. As Mengis et al9 reported, patients entered onto therapeutic trials may comprise only a fraction of patients with AML seen at any treatment center and tend to be younger with fewer comorbidities than those patients not included in such trials. Although the clinical trials included in this report had broad entry criteria with no restrictions for secondary leukemia or performance status, and no upper age limit for those trials designed for patients older than age 55, the patient population described undoubtedly includes only a subset of patients with AML seen at the participating institutions. For example, although the median age at diagnosis for AML in the United States is 68 years, the median age of patients entered onto the trials presented here was 61.10 Further, although according to SEER statistics patients older than age 65 represent approximately 55% of AML cases, only 37% of the patients entered onto the Southwest Oncology Group trials reported here was older than age 65. Nonetheless, although the data presented here may not reflect the nature of AML across the entirety of the disease, the study does illustrate the nature of the disease among patients currently referred for and entered onto therapeutic trials.
Southwest Oncology Group Leukemia Committee percentage of patients in cytogenetic risk groups by age category. The percentage includes only patients with a known cytogenetic risk group.
Southwest Oncology Group Leukemia Committee percentage of patients in cytogenetic risk groups by age category. The percentage includes only patients with a known cytogenetic risk group.
The lower white blood cell count and percentage of marrow blasts in older patients reported in the current study have not previously been specifically noted but are consistent with data reported from sequential United Kingdom Medical Research Council (MRC) trials.11,12 These lower counts may relate to AML in the elderly more often arising from a myelodysplastic syndrome as observed by the MRC.13 Supporting this possibility is the observation that the prestudy white blood cell counts were significantly higher in the 462 patients with de novo AML than in the 138 patients with secondary AML (12 600/mm3 versus 9100/mm3). However, it is also possible that these results reflect a selection bias if, for example, physicians were reluctant to place older patients with higher white blood cell counts and percentage of blasts on clinical trials.
A high incidence of unfavorable cytogenetic abnormalities among older patients with AML has previously been noted. Moorman et al14 noted a marked increase in deletions of all types with age, but most notably deletions in chromosomes 5 and 7. Schoch et al15 reported an exponential increase in the incidence of AML with complex aberrant karyotypes with age. The MRC similarly reported a marked increase in the incidence of complex aberrant karyotypes as well as abnormalities of chromosome 5 in older individuals and, similar to the present study, a relative rarity of favorable cytogenetic changes in older patients with AML.13
The reasons for the association of increasing age with chromosomal abnormalities involving –5 or 5q–, –7 or 7q–, and 17p in AML are not understood. Loss of all or part of the long arm of chromosome 5 occurs relatively frequently in a variety of myeloid malignancies, including myelodysplasia and alkylating agent–induced AML. The 5q–syndrome of myelodysplasia has been linked to 5q32, whereas many of the therapy-related cases show deletions centering at band 5q31 leading to longstanding speculation that this is a site of a tumor suppressor gene.16 Other cases have been linked to abnormalities at 5q13.17 Abnormalities of 17p13 resulting in loss of the normal TP53 allele are also common in treatment-related AML and are often accompanied by deletion or loss of 5q.18 Monosomy 7 and 7q–are also frequently seen in myelodysplasia and therapy-related AML. Additionally, these abnormalities occur frequently in myeloid malignancies arising in patients with Fanconi anemia, neurofibromatosis type 1, and severe congenital neutropenia. Analogous to the situation with chromosome 5, the frequent involvement of 7q32 has led to speculation that this also might be the site of a tumor suppressor gene.19 Our current study shows a remarkable similarity between AML in the elderly and AML associated with marrow damage and stress, as previously noted by others.20 If aging is, as has been hypothesized, largely the consequence of accumulated genetic damage to stem cells, then the similarities between secondary AML and AML in the elderly are understandable. However, this does not explain why involvement of chromosomes 5 and 7 are so prominent in both settings.
The health of patients entered into any clinical trial or group of trials can be influenced by the trial entry criteria as well as the expectations of the physician and patient. The trials for older patients with AML included in this report involved daunomycin and cytarabine or mitoxantrone plus etoposide given at doses typically used for patients older than age 55. The modest increase in the population of patients with poor performance status with increasing age is hardly surprising and almost certainly underestimates the true effect of age on performance status. More impressive is the apparently marked interaction between age and performance status on the incidence of an early fatal outcome after beginning induction. In patients with excellent performance status, age appeared to have at most a modest effect on the incidence of death early after induction, but, for patients with poor performance status, age appeared to have a profound effect on outcome, with the likelihood of early death increasing from 0% in patients younger than age 56 with performance status of 3, to 82% in patents older than age 75 with a similar performance status.
OS by age for patients with unfavorable risk cytogenetics. Patients younger than 56 years (n = 108) had a median survival of 11 months, patients aged 56 to 65 years (n = 70) had a median survival of 5 months, and patients aged 66 to 75 years (n = 78) had a median survival of 4 months, as did the 27 patients older than 75 years.
OS by age for patients with unfavorable risk cytogenetics. Patients younger than 56 years (n = 108) had a median survival of 11 months, patients aged 56 to 65 years (n = 70) had a median survival of 5 months, and patients aged 66 to 75 years (n = 78) had a median survival of 4 months, as did the 27 patients older than 75 years.
A final observation made in this study is that the poorer treatment outcomes seen in the elderly did not appear to be solely the result of an increased incidence of early death and a higher incidence of poor-risk cytogenetics in this age group. Within each cytogenetic risk group, even after accounting for an increased incidence of early death, results tended to deteriorate with increasing age. Similar results have recently been reported by Buchner et al21 and Schoch et al22 in studies of patients treated on German AML Cooperative Group trials. Older individuals tend to tolerate chemotherapy less well than younger individuals and accordingly are often treated with lower doses of chemotherapy. Thus, receipt of less therapy might also contribute to the poorer outcome seen in this age group. However, there may be more to it than that. For example, Figure 4 shows that treatment outcome in patients with t(8;21) and inv(16) AMLs worsens with age, yet patients in the 56 to 65 age group who did relatively well were treated using exactly the same regimens as patients older than age 65 who did poorly. Further, as illustrated by the shape of the survival curves in Figure 4, there were relatively few deaths during induction therapy in any of the age groups. Instead, most of the failures in the older patients appear to be the consequence of persistent or recurrent disease. A recent review of 373 cases of core binding factor AMLs from SWOG, the Eastern Cooperative Oncology Group, and MD Anderson confirms this observation in a large group of patients.23 In that analysis, the incidence of drug resistance as a cause of induction failure and the incidence of relapse following successful remission induction increased significantly with increasing patient age. Almost identical results have been reported by the German AML Cooperative Group.22
Why should the outcome of what appear to be molecularly similar core binding factor (CBF) leukemias worsen with age? Although there is, of course, no clear answer, several hypotheses can be raised. We and others have postulated that AML in younger patients may result from a limited number of mutational events restricting the diversity of leukemia subclones and leaving many cell functions, including apoptotic apparatus, relatively intact. In contrast, AML in the elderly may more often be the result of a string of mutational events leading to multiple leukemic subclones with the opportunity to develop multiple mechanisms of chemoresistance. Perhaps CBF leukemias in the elderly occur in already malignant, disordered hematopoietic progenitors.
OS by age for patients with intermediate risk cytogenetics. Patients younger than 56 years (n = 149) had a median survival of 26 months, patients aged 56 to 65 years (n = 101) had a median survival of 12 months, patients aged 66 to 75 years (n = 110) had a median survival of 8 months, and patients older than 75 years (n = 24) had a median survival of 7 months.
OS by age for patients with intermediate risk cytogenetics. Patients younger than 56 years (n = 149) had a median survival of 26 months, patients aged 56 to 65 years (n = 101) had a median survival of 12 months, patients aged 66 to 75 years (n = 110) had a median survival of 8 months, and patients older than 75 years (n = 24) had a median survival of 7 months.
OS by age for patients with favorable risk cytogenetics. The median overall survival for patients younger than age 56 (n = 51) and those aged 56 to 65 (n = 10) has not been reached, while the median survival for those older than age 65 (n = 12) was 12 months.
OS by age for patients with favorable risk cytogenetics. The median overall survival for patients younger than age 56 (n = 51) and those aged 56 to 65 (n = 10) has not been reached, while the median survival for those older than age 65 (n = 12) was 12 months.
A slightly different hypothesis is that the nature of AML is determined in part by the age of the hematopoietic stem cell in which it occurs. Although our understanding of the aging of hematopoietic stem cells is somewhat sketchy, available data both in animal models and in humans suggest a number of changes in stem cells with age. Yeast genomes become inherently unstable with age.24 Murine hematopoietic stem cells in elderly animals appear to be more frequently in cycle, perhaps because of diminished numbers in the most primitive compartment.25 Telomere lengths tend to shorten with age, and shortened telomeres have been associated with diminished genetic stability.26 Epigenetic changes with aging have been demonstrated with lymphocytes but have not been carefully studied in early hematopoietic progenitors.27 Finally, there may be an accumulation of non–clonal mutational events in stem cells with aging. Thus, it may be that the development of leukemia in an old stem cell will inevitably result in a malignancy with greater genetic instability and clonal diversity. The presence of fewer normal stem cells to compete with the malignant clone and to repopulate the marrow following therapy may also contribute to the worse outcome seen in older patients. Whether an aging microenvironment may also influence the nature of AML in the elderly is an additional question of interest.
The improvements achieved in the treatment of younger patients with AML have not affected older patients. The data presented here, illustrating a marked effect of age, even after taking into account known biologic characteristics of AML, argue that careful age-specific assessments need to be made when evaluating new therapeutic strategies for this disease.
Prepublished online as Blood First Edition Paper, February 2, 2006; DOI 10.1182/blood-2005-09-3724.
Supported in part by the National Cancer Institute (PHS Cooperative Agreement grant nos. DHHS: CA38926, CA32102, CA20319, CA46368, CA12213, CA46282).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal