We studied 25 patients with myelofibrosis with myeloid metaplasia and 19 patients with secondary myelofibrosis associated with pulmonary hypertension (PH). In these 2 groups, we compared the peripheral-blood CD34 count, the clonality of granulocytes and platelets in peripheral blood, the mutational status of the JAK2 kinase gene, and the morphology of the peripheral blood and bone marrow. We found that the following were distinctive features of myelofibrosis with myeloid metaplasia but not of secondary myelofibrosis due to PH: high circulating CD34 cell count, the presence of clonal platelets and granulocytes and of peripheral-blood dacrocytes, and a JAK2 1849G>T (V617F) mutation. We conclude that these are intrinsic features of clonal progenitors present in patients with myelofibrosis due to myeloproliferative disorders and that these features are not due to the abnormal marrow architecture seen in secondary myelofibrosis.
Introduction
Myelofibrosis with myeloid metaplasia (MMM) is a clonal myeloproliferative disorder characterized by pancytopenia, splenomegaly, and myelofibrosis. Like all myeloproliferative disorders, it results from an acquired somatic mutation of a hematopoietic progenitor cell that results in clonal erythrocytes, platelets, and granulocytes. A single acquired point mutation of JAK2 1849G>T (V617F), a tyrosine kinase with a key role in signal transduction from growth factor receptors, occurs in 50% to 97% of patients with MMM, essential thrombocythemia (ET), and polycythemia vera (PV) (reviewed by Emanuel and Prchal1 ); however, this point mutation has also been reported in other disorders.2,3 Myelofibrosis also occurs in patients with pulmonary hypertension (PH), lupus erythematosus, and metastatic malignancies involving the bone marrow.4,5 Circulating CD34+ cell counts are typically elevated in patients with MMM, and this was found to correlate with prognosis in one study6 but not in another.7 The etiology of the high CD34+ cell count in blood is not known, but 2 pathophysiologic mechanisms have been hypothesized. The first is fibrous tissue distortion of the marrow architecture resulting in the displacement of the hematopoietic precursors into the circulation. The second is an adhesion defect specific to clonal MMM stem cells and progenitors resulting in their displacement from the marrow.8 To determine which of these 2 mechanisms was the more likely cause, we compared peripheral-blood CD34 counts between patients with clonal MMM and patients with polyclonal secondary myelofibrosis of equivalent severity associated with PH.5
Study design
In a prospective study approved by the institutional review board of Baylor College of Medicine, consecutive patients with MMM who met European Myelofibrosis Network consensus criteria9 for the disease were evaluated. Informed consent was provided according to the Declaration of Helsinki. Simultaneously, 19 previously identified patients with PH who were found to have bone marrow fibrosis, some of whom were the subject of a previous study,5 were also studied (Table 1). To establish normal CD34+ cell counts, we also studied healthy controls and patients with PV and ET without significant myelofibrosis.
Results: clonality, JAK2 mutation, and CD34+ cells in peripheral blood
. | CD34 %* . | . | JAK2 mutation . | . | . | Clonality† . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | No. . | Mean (95% CI)* . | No. . | Mutated (%) . | Homozygous (%) . | No. . | Clonal† . | ||||
| Myelofibrosis with myeloid metaplasia | 25 | 1.1 (0.58-1.64) | 23 | 17 (74) | 6 (26) | 14 | 14 | ||||
| Myelofibrosis secondary to PH | 19 | 0.08 (0.04-0.12) | 19 | 0 | 0 | 16 | 1 | ||||
| PV/ET without significant myelofibrosis | 6 | 0.06 (0.02-0.10) | 5 | 4 (80) | 0 | 4 | 4 | ||||
| Healthy controls | 23 | 0.05 (0.03-0.06) | — | — | — | — | — | ||||
. | CD34 %* . | . | JAK2 mutation . | . | . | Clonality† . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | No. . | Mean (95% CI)* . | No. . | Mutated (%) . | Homozygous (%) . | No. . | Clonal† . | ||||
| Myelofibrosis with myeloid metaplasia | 25 | 1.1 (0.58-1.64) | 23 | 17 (74) | 6 (26) | 14 | 14 | ||||
| Myelofibrosis secondary to PH | 19 | 0.08 (0.04-0.12) | 19 | 0 | 0 | 16 | 1 | ||||
| PV/ET without significant myelofibrosis | 6 | 0.06 (0.02-0.10) | 5 | 4 (80) | 0 | 4 | 4 | ||||
| Healthy controls | 23 | 0.05 (0.03-0.06) | — | — | — | — | — | ||||
CD34+ cells expressed as a percentage of peripheral-blood white cells.
Denominator only includes female patients with informative X-chromosome exonic polymorphisms; this can be studied only in females.
We analyzed their peripheral-blood morphology, bone marrow histology, the clonality of the granulocytes and platelets, and the mutational status of the JAK2 kinase gene in granulocytes, and we quantified the number of circulating CD34+ cells.
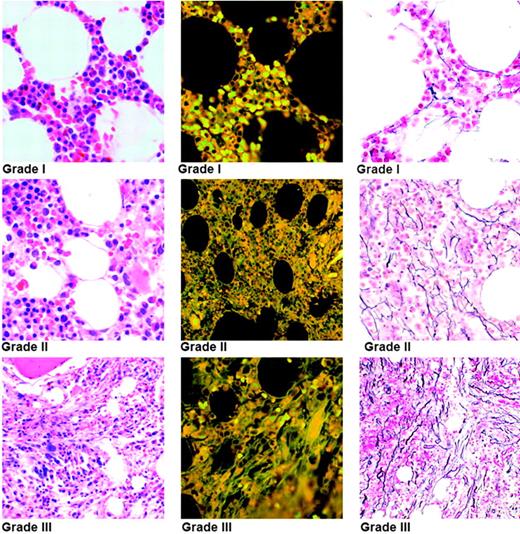
Three hematologists/hematopathologists blindly and independently analyzed all the marrow biopsy specimens and graded the severity of the fibrosis on reticulin-stained sections using a grading system based on a severity template (Figure 1). Fibrosis was graded in severity from 0 to 3, as follows: Grade 0 indicates no reticulin fibers; grade 1, few or rare noninterlacing reticulin fibers; grade 2, several interlacing reticulin fibers; and grade 3, many interlacing thick and undulating reticulin fibers. Because the severity of fibrosis in a biopsy specimen varies in different areas, 2 separate severity scores were determined: 1 for the predominant pattern and 1 for the minor pattern. A composite score was then determined by adding the scores for both patterns, with an absence of fibrosis indicated by a score of 0, mild fibrosis by a score of 1 or 2, moderate fibrosis by a score of 3 or 4, and severe fibrosis by a score of 5 or 6. Blood smears were scored for presence or absence of dacrocytes and myeloid and erythroid precursors.
Clonality studies were done on granulocytes and platelets from all females with an informative marker by performing the X-chromosome transcriptional polymorphism analyses of the allelic usage expression of 5 X-chromosome exonic polymorphic loci,10 which can only be studied in the females.
Peripheral-blood CD34+ cell counts were determined by flow cytometry. Lin– CD34+ cells were gated, analyzed, and reported as a percentage of white cells. Blood samples from 23 healthy individuals were used as controls. Analysis of variance was used to compare the mean CD34+ counts between the different groups.
Assays for allele-specific determination by a real-time polymerase chain reaction (PCR) on ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) were designed to quantify the somatic mutation JAK2 1849G>T and the wild-type JAK2 allele in isolated granulocytes. In allelic discrimination assays, during the PCR, the normal JAK2 1849G allele leads to the release of the VIC fluorescent label, while the mutated JAK2 1849T allele leads to the release of FAM fluorescent label. The assay mix contained the following probes and primers: (a) forward primer: 5′-AAGCTTTCTCACAAGCATTTGGTTT-3′; (b) reverse primer: 5′-AGAAAGGCATTAGAAAGCCTGTAGTT-3′; (c) VIC-labeled oligo: 5′-TCTCCACAGACACATAC-3′; and (d) FAM-labeled oligo: 5′-TCCACAGAAACATAC-3′. The reaction was performed in 96-well optical PCR plates in a total volume of 25 μL following the manufacturer's protocol.
INSERT TITLE. Marrow fibrosis/reticulin content is graded in the reticulin-stained section (right), the corresponding hematoxylin and eosin (H&E)–stained section (left), and autofluorescence of the H&E-stained section (center). The bone marrow images were taken with an Olympus BH2 microscope (Olympus, Tokyo, Japan) with a 20× objective and a final magnification of 100×. The numeric aperture of the Plan Apochromat lens was 0.70. Images were transferred by JPEG high resolution (24-bit color at 1200×) using 800 software Olympus DP-12 and Olympus DP-12 BW acquisition software. Adobe Photoshop CS version 8 (Adobe Systems, San Jose, CA) was used as word processing software.
INSERT TITLE. Marrow fibrosis/reticulin content is graded in the reticulin-stained section (right), the corresponding hematoxylin and eosin (H&E)–stained section (left), and autofluorescence of the H&E-stained section (center). The bone marrow images were taken with an Olympus BH2 microscope (Olympus, Tokyo, Japan) with a 20× objective and a final magnification of 100×. The numeric aperture of the Plan Apochromat lens was 0.70. Images were transferred by JPEG high resolution (24-bit color at 1200×) using 800 software Olympus DP-12 and Olympus DP-12 BW acquisition software. Adobe Photoshop CS version 8 (Adobe Systems, San Jose, CA) was used as word processing software.
Results and discussion
Twenty-five patients with MMM, 17 females and 8 males with a median age of 63 years (range, 39 to 80 years), were studied. Sixteen patients had idiopathic myelofibrosis, 6 patients had MMM secondary to PV, and 3 patients had MMM secondary to ET. Seventeen patients had poor-risk disease, with a Dupriez score of 1 or greater, while the other 8 had low-risk disease. All patients had severe bone marrow fibrosis.
Nineteen patients with myelofibrosis secondary to PH, 17 females and 2 males with a median age of 48 years (range, 27 to 65 years), were also analyzed. Twelve patients had primary PH, and 7 had PH secondary to scleroderma (n = 3), mixed connective tissue disease (n = 2), polymyositis (n = 1), or lupus erythematosus (n = 1). All patients had severe PH with a median pulmonary artery systolic pressure of 90 mm Hg (range, 51 to 141 mm Hg) for a median of 47 months (range, 1 to 270 months). The myelofibrosis was severe in 11 patients, moderate in 5 patients, and mild in the remaining 3 patients. Sixteen patients had anemia with a median hemoglobin (Hb) concentration of 102 g/L (10.2 g/dL) (range, 86 to 124 g/L [8.6 to 12.4 g/dL]); 16 patients had thrombocytopenia with a median platelet count of 58 × 109/L (range, 7 × 109/L to 149 × 109/L); and 14 patients had both.
The number of peripheral-blood CD34 cells was significantly higher in the 25 patients with MMM (1.1%; 95% confidence interval [CI], 0.58% to 1.64%) than in 19 patients with secondary myelofibrosis associated with PH (0.08%; 95% CI, 0.04% to 0.12%; P < .01) and in healthy controls (0.05%; 95% CI, 0.03% to 0.06%; P < .01) (Table 1). There was no significant difference in the CD34+ cell count between patients with PH and healthy controls. Further, on examination of the blood smears, patients with PH were found to lack dacrocytes, erythroid precursors, and myeloid precursors (a leukoerythroblastic picture), and this was the case with 16 patients with moderate to severe bone marrow fibrosis. In contrast, all patients with MMM (either idiopathic myelofibrosis or myelofibrosis secondary to PV and ET) had circulating dacrocytes and some degree of a leukoerythroblastic picture. Clonality studies in patients with an informative marker showed that 15 of 16 women with PH and bone marrow fibrosis were polyclonal, compared with the finding of clonal hematopoiesis in all 14 women with MMM (P < .001, Fisher exact test, 2 tailed).
JAK2 1849G>T somatic mutation was seen in 17 of the 23 patients (74%) with MMM; these patients consisted of 10 of the 15 patients (67%) with idiopathic myelofibrosis and 7 of the 8 patients (88%) with myelofibrosis complicating PV or ET, but this was not seen in any of the 19 patients with myelofibrosis associated with PH (P < .001, Fisher exact test, 2 tailed). Furthermore, both alleles were mutated in 2 of the 15 patients with idiopathic myelofibrosis and 4 of the 8 patients with myelofibrosis after polycythemia vera or essential thrombocythemia. The proportion of patients heterozygous and homozygous for the JAK2 1849G>T mutation was higher in our group than in patients described by others, but this may reflect the fact that the patients referred to us for the management of MMM likely had more advanced disease, because we were evaluating them for possible marrow transplantation.11
Although the cause of the high number of circulating CD34+ cells in the setting of myelofibrosis associated with a myeloproliferative disorder is not known, this study disproves the most readily apparent explanation: the expulsion of bone marrow stem cells from the abnormal marrow microenvironment by the fibrosis. Because we did not find elevated CD34+ counts in patients with PH and bone marrow fibrosis or in patients with PV or ET without myelofibrosis (Table 1), this suggests instead that the MMM hematopoietic precursors (whether idiopathic or developing from PV and ET as a clonal evolution of these myeloproliferative diseases) bearing the clonal somatic mutation(s) are more likely to be released into the bloodstream from the bone marrow because of their decreased adherence.8 We also noted that dacrocytes and a leukoerythroblastic blood morphology are associated with the clonal hematopoiesis typical of myeloproliferative disorders. Similar to our observation in patients with PV and ET made using the same method for determining clonality as we used in the present study,10 we found only clonal hematopoiesis in those women with marrow fibrosis due to MMM, PV, and ET but not in patients with secondary marrow fibrosis (P < .001).5 This is in contrast to reports of polyclonal hematopoiesis in myeloproliferative disorders; however, genomic DNA methylation–based clonality assays were used in these other studies to assess clonality,12 and the data may not be comparable with the approach used here that we submit has sounder biologic basis.
What do the high CD34+ cell counts mean in terms of the pathophysiology and diagnosis of MMM and its differentiation from secondary myelofibrosis? Our data would suggest that a cutoff value for peripheral-blood CD34+ cell counts of more than 0.2% would have correctly identified 21 of our 25 patients with MMM. This is underscored by the fact that 47 of the 48 subjects, consisting of healthy controls, patients with PV or ET without significant myelofibrosis, and patients with myelofibrosis secondary to PH, had a circulating a CD34 count of 0.2% or less. It was earlier suggested that a high circulating CD34+ count may aid in differentiating MMM from other myeloproliferative disorders.6 Our data confirm this observation and extend it to patients with myelofibrosis secondary to PH. This is in contrast to another study conducted in a large cohort of MMM patients7 in which the specificity of the CD34+ cell count in the diagnosis of MMM was questioned. Because all our MMM patients had significant elevation of circulating CD34+ cells, it remains to be established by others if this discrepancy is due to technical factors or if it is from differences in selection of MMM patients, which is a possibility because we have studied only those patients with advanced MMM.11
In this study we also extended our previous observation regarding the secondary myelofibrosis that can occur in patients with PH. In particular we showed that unlike the patients with clonal MMM, the PH patients with polyclonal hematopoiesis do not have elevated circulating CD34+ cell counts or peripheral-blood smears showing dacrocytes or myeloid or erythroid precursors. In addition, none had the acquired somatic mutation, JAK2 1849G>T, seen in most of the patients with MMM whom we have studied.
We therefore conclude that a high circulating CD34+ cell count in patients with myelofibrosis associated with a myeloproliferative disorder is an intrinsic feature of the clonal progenitors in this disease and that this finding is not due to fibrosis-related abnormal marrow architecture.
Prepublished online as Blood First Edition Paper, January 17, 2006; DOI 10.1182/blood-2005-08-3319.
Supported by grants from the National Institutes of Health (R01HL0077-11) and the Ministry of Education of the Czech Republic (MSM 0021620806, J.T.P.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Romelia May for coordinating the study and maintaining the database and thank Betty L Notzon for editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal