We read with great interest the paper of Popat and colleagues, who, examining an impressive case series of patients with myelofibrosis (MF) associated with myeloproliferative disorders (MPDs), showed that these patients have higher levels of peripheral blood CD34+ cells than patients with MF secondary to severe pulmonary hypertension (PH).1 On the basis of their data, the authors suggest that the increased levels of circulating CD34+ cells that are observed in patients with a stem cell defect are not due to displacement from the marrow microenvironment, because the phenomenon cannot be observed when MF of equivalent severity is secondary to another condition, such as PH.
However, we should like to comment on the authors' results by offering some additional interpretations on the mechanisms that contribute to the phenomenon they have described. It is well known that PH either primary or secondary to parenchymal or connective tissue diseases is strongly characterized by altered vascular biology and endothelial dysfunction. Another emerging concept is that circulating CD34+ progenitors include subsets of endothelial-committed cells involved in endothelial homeostasis and vascular repair.2
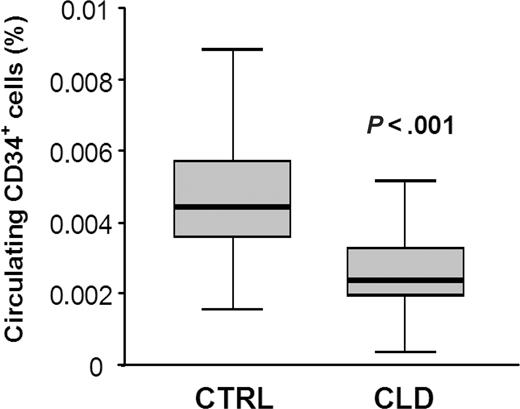
We have recently evaluated the presence of circulating CD34+ cells in patients with PH secondary to diffuse lung diseases. Our data indicate that severe lung diseases, which are associated to secondary PH, including idiopathic pulmonary fibrosis and lung fibrosis associated with connective tissue disorders, are characterized by reduced levels of circulating CD34+ cells (Figure 1). Moreover, a number of data indicate that alterations of the pulmonary vasculature may depend upon reduced or defective marrow-derived progenitor cells.3-7 Therefore, when MF develops in patients with PH, it may be suggested that the increase in peripheral CD34+ cells is masked by the reduced CD34+ cell count that is observed at basal conditions in these subjects. This may explain why the authors found no difference in CD34+ cells between patients with PH-induced MF and control subjects. If this was the case, we suggest that reduction of peripheral CD34+ cells could reflect homing of progenitor cells to the lungs, where they may contribute to the vascular lesions.8 An easy approach to confirm this hypothesis could be to evaluate the presence and/or localization of CD34+ cells in lung biopsy samples obtained from patients with MF secondary to severe PH.
An additional, though not necessarily alternative hypothesis, is that PH is associated with certain degrees of marrow fibrosis, which in turn reduces the ability of bone marrow to provide progenitor cells for the maintaining of vascular homeostasis in the pulmonary circulation, ultimately leading to the most severe forms of PH. Therefore, MF not only may cause anemia and thrombocytopenia, but also may impair the regenerative capacity of the pulmonary endothelium hit by the primary cause (environmental, immunologic, genetic, or other), and ultimately favoring vascular remodeling. In conclusion, we believe that the authors should further investigate a crucial question: whether MF precedes or follows the development of PH (ie, is it the consequence or the cause of the pulmonary complication?).
Levels of circulating CD34+ cells in control subjects and in patients with chronic lung disease. Peripheral blood CD34+ cells were significantly lower in patients with idiopathic pulmonary fibrosis (n = 8) and lung fibrosis associated with connective tissue disorders (n = 7) on long-lasting oxygen therapy than age- and sex-matched control subjects free from clinical and instrumental signs of pulmonary and hematologic diseases (n = 45): mean ± SEM 0.051% ± 0.0024% versus 0.026% ± 0.003% of circulating leukocytes, respectively (P < .001). CTRL indicates controls; CLD, chronic lung diseases.
Levels of circulating CD34+ cells in control subjects and in patients with chronic lung disease. Peripheral blood CD34+ cells were significantly lower in patients with idiopathic pulmonary fibrosis (n = 8) and lung fibrosis associated with connective tissue disorders (n = 7) on long-lasting oxygen therapy than age- and sex-matched control subjects free from clinical and instrumental signs of pulmonary and hematologic diseases (n = 45): mean ± SEM 0.051% ± 0.0024% versus 0.026% ± 0.003% of circulating leukocytes, respectively (P < .001). CTRL indicates controls; CLD, chronic lung diseases.
Elevated circulating CD34+ cells in primary myelofibrosis are not due to increased circulating endothelial progenitors
We appreciate that Dr Fadini and colleagues have been complementary about our recent paper on the differential value of elevated CD34+ cells in primary and secondary myelofibrosis. They have raised an interesting possibility that the normal levels of circulating CD34+ cells in secondary myelofibrosis associated with pulmonary hypertension may be due to a decrease in the absolute level of circulating endothelial progenitors in peripheral blood. Clearly, endothelial progenitors are present among circulating CD34+ cells. While this is an interesting theoretical possibility, it is not supported by available data. Until recently, quantitative information about these cells was not available; however, 2 recent papers1,2 demonstrate that endothelial progenitors represent a minority of circulating CD34+ cells (0.0005% of leukocytes),1 and even when the proportion of endothelial progenitors in circulation is elevated as in primary myelofibrosis,2 it is still less than those of hematopoietic progenitors. The rarity of these cells in circulation was also a subject of recent review in this journal (Ingram et al3 and D. Ingram, personal oral communication, May 2006).
Thus, we must conclude that the quantitative elevation of circulating CD34 cells, as well as other markers we published (ie, clonality, the JAK-2 mutation, and presence and absence of dacrocytes in peripheral blood), are important laboratory parameters that are invaluable in differential diagnosis of primary and secondary myelofibrosis.
Supported by Myeloproliferative Disease (MPD) Consortium grant 1P01CA108671-.1A2.
Correspondence: Josef T. Prchal, University of Utah School of Medicine, Department of Hematology, 30 N 1900 E, Room 4C416, Salt Lake City, UT 84112; e-mail: josef.prchal@hsc.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal