In acute myeloid leukemia (AML), two clusters of activating mutations are known in the FMS-like tyrosine kinase-3 (FLT3) gene: FLT3-internal tandem duplications (FLT3-ITDs) in the juxtamembrane (JM) domain in 20% to 25% of patients, and FLT3 point mutations in the tyrosine-kinase domain (FLT3-TKD) in 7% to 10% of patients, respectively. Here, we have characterized a new class of activating point mutations (PMs) that cluster in a 16-amino acid stretch of the juxtamembrane domain of FLT3 (FLT3-JM-PMs). Expression of 4 FLT3-JM-PMs in interleukin-3 (IL-3)-dependent Ba/F3 cells led to factor-independent growth, hyperresponsiveness to FLT3 ligand, and resistance to apoptotic cell death. FLT3-JM-PM receptors were autophosphorylated and showed a higher constitutive dimerization rate compared with the FLT3-wild-type (WT) receptor. As a molecular mechanism, we could show activation of STAT5 and up-regulation of Bcl-x(L) by all FLT3-JM-PMs. The FLT3 inhibitor PKC412 abrogated the factor-independent growth of FLT3-JM-PM-expressing cells. Compared with FLT3-ITD and FLT3-TKD mutants, the FLT3-JM-PMs showed a weaker transforming potential related to lower autophosphorylation of the receptor and its downstream target STAT5.
Mapping of the FLT3-JM-PMs on the crystal structure of FLT3 showed that these mutations reduce the stability of the autoinhibitory JM domain, and provides a structural basis for the transforming capacity of this new class of gain-of-function mutations of FLT3.
Introduction
FMS-like tyrosine kinase-3 (FLT3) has been shown to be mutated in about one-third of patients with acute myeloid leukemia (AML), representing one of the most frequently occurring mutations in this disease.1,2 Until now, two distinct clusters of activating mutations are known: FLT3-internal tandem duplications (FLT3-ITDs) in the juxtamembrane (JM) domain in 20% to 25% of patients, and point mutations (PMs) in the tyrosine-kinase domain (FLT3-TKD) in 7% to 10% of patients.3-9
Recently, the crystal structure of the autoinhibited form of FLT3 was resolved.10 The structure conforms to the prototypical conformation common to other inactive kinases that have a “closed” activation loop, but the remarkable feature is the complete JM domain serving as a critical autoinhibitory loop and interacting with all key features of FLT3. This domain can be divided into three distinct parts: the JM binding motif (JM-B), JM switch motif (JM-S), and the zipper or linker peptide segment (JM-Z). According to that model, the JM-B region is nearly buried in the FLT3 structure. It serves as an autoinhibitory domain, which in an inactive state prevents the N lobe from rotating toward the C lobe of the tyrosine kinase domain (TKD) to generate the activated kinase fold.
The cytoplasmatic juxtamembrane domain is highly conserved between different members of the class III receptor tyrosine kinase (RTK) family. A variety of tumors in animals and humans have been described that harbor activating mutations in the JM domain.11-14 The most frequently occurring activating mutations in AML, FLT3-ITDs, occur primarily in the JM-Z domain. They represent a heterogenous group of mutations, where a fragment of the JM domain, varying in length from 2 to 204 nucleotides (nt), is duplicated and inserted in a direct head-to-tail orientation always maintaining the reading frame.
Recently, we discovered a novel missense point mutation in the JM domain of FLT3 in the AML cell lines Mono-Mac (MM)-1 and MM-6, changing valine with alanine at position 592.15 By performing a LightCycler (Roche, Mannheim, Germany) mutational screening of FLT3 in 785 AML patient samples, we were able to identify two other point mutations: F594L in two AML patients and Y591C in 1 AML patient. In addition, Stirewalt et al16 found additional point mutations in the JM domain of FLT3 (V579A and F590GY591D) in AML patients by using single-stranded conformational polymorphism analyses (polymerase chain reaction [PCR]/SSCPs).
Here, we have studied the functional significance of this new class of activating mutations in patients with AML: PMs that cluster in a 16-aa stretch of the JM domain (FLT3-JM-PMs).
We could clearly demonstrate that FLT3 receptors harboring one of these JM point mutations, when expressed in Ba/F3 cells, induced interleukin-3 (IL-3)-independent growth, increased resistance to apoptosis via up-regulation of Bcl-x(L) and constitutive activation of the receptor and STAT5. Treatment with a specific FLT3 phospho-tyrosine-kinase (PTK) inhibitor, PKC412, was able to block IL-3-independent proliferation and resulted in reduced phosphorylation of STAT5. These data clearly define a third class of activating mutations in AML. Patients with AML harboring these mutations may respond to small-molecule FLT3 inhibitors like PKC412.
Materials and methods
Patient samples
All samples of bone marrow or peripheral blood (with at least 70% circulating blast cells), were obtained at diagnosis and were sent to the leukemia diagnostics laboratory, Munich, Germany. All patients gave informed consent before entering the study. The study design adhered to the principles of the Helsinki Declaration and was approved by the ethics committees of the participating institutions. A total of 785 unselected AML samples were analyzed.
Screening method for mutations in the juxtamembrane domain of FLT3
Mononucleated cells were isolated by standard Ficoll-Hypaque density gradient centrifugation. Nucleic acid isolation and cDNA synthesis was performed as described before.5 Screening for FLT3-V592A mutations was performed using a melting curve-based LightCycler assay with forward primer FLT3JM-F: CAATTTAGGTATGAAAGCCAG, reverse primer FLT3JM-R: TGATCCTAGTACCTT; and hybridization probes FLT3JM-S (sensor) TTCATATTCTCTGAAATCAACGTAGAAGT-FL, and FLT3JM-A (anchor) LC-Red640-TCATTATCTGAGGAGCCGGTCACCT-P. The PCR reaction was carried out in a 20-μL reaction volume with each 0.5 μM forward and reverse primer, 0.75 μM Hyb-Probes (Metabion, Martinsreid, Germany) 4 mM MgCl2, and 2 μL LightCycler-FastStart DNA Master Hyb-Probes (Roche Diagnostics, Mannheim, Germany). LightCycler data were analyzed using LightCycler 3.0 software (Roche Diagnostics) and the second derivative maximum method. Each 20-μL reaction contained 2 μL cDNA, an equivalent of about 3000 cells. Amplification was performed with 40 cycles using 50°C annealing temperature. Final melting curve analysis was started at 35°C up to 85°C with slope of 0.2°C/sec and continuous detection with channel F2/F1.
Reagents and cell lines
Low-passage murine Ba/F3 cells were obtained from the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) and were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 10% WEHI-conditioned medium as a source of murine IL-3 when indicated. PKC412 was kindly provided by Novartis Pharma (Basel, Switzerland).
Cell proliferation of Ba/F3 cells
Cells were seeded at a density of 4 × 104/mL in the presence or absence of IL-3 or FLT3 ligand (Promocell, Heidelberg, Germany) as indicated. Viable cells were counted at indicated time periods in a standard hemacytometer after staining with trypan blue. Figures show mean values and standard deviations from three independent experiments.
Apoptosis analysis
Assessment of apoptotic cells was carried out by annexin V/7-aminoactinomycin D (7-AAD) staining as recommended by the manufacturer (annexin V phycoerythrin [PE] apoptosis detection kit; Becton Dickinson, Heidelberg, Germany) using a FacsCalibur flow cytometer (Becton Dickinson, San Jose, CA). Determination of the DNA content of cell nuclei was performed by propidium iodide (PI) staining as described previously.17
Receptor dimerization experiments, immunoprecipitations, and Western blot analyses
Experiments were performed as described previously.15,18 The following antibodies were used: anti-phospho-STAT5-Tyr694, anti-phospho-AKT-Ser473, anti-AKT, anti-phospho-p44/42 mitogen-activated protein (MAP) kinase (Thr202/Tyr204), anti-p44/42 MAP kinase (all from New England Biolabs, Frankfurt, Germany), anti-STAT5 (sc-835), anti-Bcl-x(L) (sc-8392), anti-FLT3 (S18, sc-480), anti-PY (PY99) (all from Santa Cruz Biotechnology, Heidelberg, Germany), and anti-β-actin (A-5441; Sigma, Munich, Germany).
DNA constructs and vectors
The FLT3-ITD-W51 construct contained a 7-aa duplicated sequence (REYEYDL) inserted between aa 601/602 of human FLT3-WT; the FLT3-ITD-NPOS construct contained a 28-aa duplicated sequence (CSSDNEYFYVDFREYEYDLKWEFPRENL) inserted between aa 611/612 of human FLT3-WT; and the FLT3-ITD-W78 construct contained a 22-aa duplicated sequence (GLVQVTGSSDNEYFYVDFREYE) inserted between aa 598/599 of human FLT3-WT. The FLT3-ITD constructs were kindly provided by Dr G. Gilliland (Howard Hughes Medical Institute and Brigham and Women's Hospital Harvard Institutes of Medicine, Harvard Medical School, Boston, MA). All FLT3 constructs were subcloned in the MSCV-IRES-EYFP retroviral expression vector (kindly provided by R. K. Humphries, The Terry Fox Laboratory, University of British Columbia, Vancouver, BC, Canada). The Bcl-x(L) construct was kindly provided by S. J. Korsmeyer (Howard Hughes Medical Institute, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA).
In vitro mutagenesis
The FLT3-JM point mutations (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D) and the FLT3-TKD point mutations (FLT3-D835Y and FLT3-D835V) were introduced into the FLT3-WT construct using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturers instructions. The correct sequence of all constructs was confirmed by nucleotide sequencing.
Transient transfection of 293T cells
One day before transfection, 293T cells were seeded into 6-well plates at a density of 3 × 105/mL. Transient transfections were then carried out using the calcium-phosphate coprecipitation method with a total of 2 μg plasmid DNA per well. Eighteen hours after transfection, 2 mL fresh medium was added, the cells were allowed to grow for another 30 hours, and the retroviral supernatant was used for transduction of Ba/F3 cells.
Stable transduction of Ba/F3 cells
Ba/F3 cells (2 × 105) were seeded in 1 mL growth medium and subsequently transduced with 200 μL retroviral supernatant in the presence of polybrene (8 μg/mL). The fluorescence-activated cell-sorting (FACS)-Vantage system equipped with a Turbo-Sort device (Becton Dickinson, San Jose, CA) was used to highly purify EGFP/EYFP-positive pool cells 48 hours after transduction. Expression of CD135 was performed as described previously.15
Results
Detection of point mutations in the juxtamembrane domain of FLT3 in patients with AML
By sequencing the complete cDNA of FLT3 in the AML cell lines MM-1 and MM-6, we previously have found a new activating point mutation substituting valine with alanine at position 592 in the functionally important JM domain of FLT3.15 These results encouraged us to perform a screen for point mutations at position 592 in AML patient samples using a melting curve-based LightCycler assay in a total of 785 samples. Two different point mutations were identified in three patients: Y591C in a patient and F594L in two patients. A recently published study by Stirewalt et al16 using PCR/SSCP screened for mutations in FLT3 in exons 14 and 15 in patients with AML. They detected two novel missense point mutations in exon 14, V579A and F590GY591D, as well as V592A in about 2% of patients with AML (Figure 1).16
Localization of point mutations in the JM domain of FLT3 (FLT3-JM-PMs) found in patients with AML. The top panel shows the FLT3-WT juxtamembrane protein sequence from aa 573 to 600. Below are the four point mutants found in our study (*) and the study of Stirewalt et al16 (†) in patients with AML.
Localization of point mutations in the JM domain of FLT3 (FLT3-JM-PMs) found in patients with AML. The top panel shows the FLT3-WT juxtamembrane protein sequence from aa 573 to 600. Below are the four point mutants found in our study (*) and the study of Stirewalt et al16 (†) in patients with AML.
These data show that a third class of mutations, point mutations clustering in the JM domain of FLT3 (FLT3-JM-PMs), exist in patients with AML.
So far, the biologic and clinical relevance of these findings have not yet been investigated. We hypothesized that these mutations, which are located in the functionally important JM region, may play an important role in the pathogenesis of AML.
Point mutations in the JM domain of FLT3 confer IL-3-independent growth to the pro-B-cell line Ba/F3
The acquisition of either ITD or TKD mutations in the FLT3 gene was shown to induce constitutive activation of the receptor- and ligand-independent cell growth in different cell lines.7,8,19-21 To characterize the functional significance of point mutations in the JM domain, we selected four of the described JM point mutations and introduced them into the FLT3-WT cDNA (Figure 1). We stably transduced the pro-B-cell line Ba/F3 with FLT3-V592A, FLT3-V579A, FLT3-F594L, FLT3-F590GY591D, and FLT3-WT. To directly compare these FLT3-JM-PM cell lines with known activating mutations, we also stably transduced three different FLT3-ITD constructs (FLT3-W51, FLT3-NPOS, and FLT3-W78)22 and two different FLT3-TKD constructs (FLT3-D835Y and FLT3-D835V).22 Surface expression levels of FLT3 were confirmed by CD135 antibody staining and FACS analysis (data not shown).
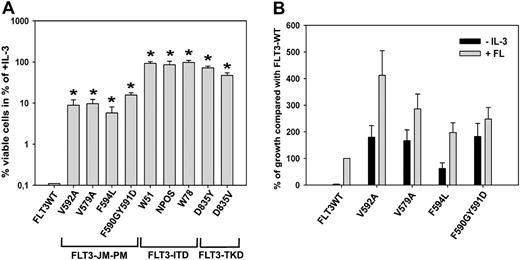
Overexpression of all FLT3-JM-PMs, but not FLT3-WT, induced IL-3-independent growth in Ba/F3 cells (Figure 2A). In detail, the growth rates of FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D were 10%, 11%, 7%, and 18% of the average growth rate of the 3 FLT3-ITD cell lines, respectively. Also, the FLT3-TKD-expressing cells displayed significantly higher growth rates than the FLT3-JM-PM-expressing cells. However, among the 4 different FLT3-JM-PM-expressing cells there was no consistent significant difference in terms of transforming activity. To analyze whether the autonomous growth of FLT3-JM-PMs might be further stimulated by exogenous ligand, all FLT3-JM-PM cell lines were grown in the presence of 50 ng FLT3 ligand (FL)/mL. Viable cells were counted after 72 hours by trypan blue exclusion. The proliferation rates of FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D were 412%, 286%, 197%, and 248% compared with the growth of FLT3-WT cells grown under identical conditions, respectively (Figure 2B).
Taken together, point mutations in the JM domain of FLT3 have transforming potential in Ba/F3 cells, and these mutant receptors confer a signficant proproliferative activity in response to FL.
FLT3-JM-PM-expressing Ba/F3 cell lines are resistant to induction of apoptosis after IL-3 withdrawal
To gain a deeper insight into the phenotype of FLT3-JM-PM-expressing Ba/F3 cells, we analyzed whether they exhibit antiapoptotic activity. Two different assays were performed to identify cells undergoing apoptotic cell death: staining of cells with annexin V and 7-AAD after 48 hours, and staining of nuclei with propidium iodide after 24 hours of growth factor withdrawal. FLT3-WT cells rapidly underwent apoptosis after IL-3 withdrawal in contrast to FLT3-ITD- and FLT3-TKD-expressing cells, which were protected from induction of apoptosis and served as a positive control (Figure 3).
We could clearly demonstrate that the percentage of apoptotic cells in FLT3-V592A-, FLT3-V579A-, FLT3-F594L-, and FLT3-F590GY591D-expressing cell lines grown without IL-3 was significantly lower compared with FLT3-WT after 48 and 24 hours (Figure 3). In the absence of IL-3, the percentage of apoptotic cells in cell lines expressing FLT3-WT was 26% and 80% after 24 and 48 hours, respectively. In contrast, only 11% to 15% (24 hours) and 25% to 49% (48 hours) apoptotic cells were found in FLT3-JM-PM-expressing cells. Addition of IL-3 protected all cell lines from undergoing apoptosis (data not shown).
FLT3-JM-PMs induce IL-3-independent growth in Ba/F3 cells and hyperproliferation in response to FL. (A) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD constructs (W51, NPOS, W78), FLT3-TKD constructs (D835Y, D835V) or one of the FLT3-JM-PM mutants (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D) were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3. Viable cells were counted after 72 hours. The growth of cells in the presence of IL-3 was defined as 100% (control). All FLT3-JM-PM mutants showed a significantly higher proliferation rate compared with FLT3-WT (*P < .05), although not as high as FLT3-ITD or FLT3-TKD. SD is indicated. (B) FLT3-WT and mutant-expressing Ba/F3 cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of human recombinant FL (50 ng/mL). Viable cells were counted after 72 hours by trypan blue exclusion. The cell number of FLT3-WT cells after 72 hours was defined as 100%. SEM is indicated.
FLT3-JM-PMs induce IL-3-independent growth in Ba/F3 cells and hyperproliferation in response to FL. (A) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD constructs (W51, NPOS, W78), FLT3-TKD constructs (D835Y, D835V) or one of the FLT3-JM-PM mutants (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D) were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3. Viable cells were counted after 72 hours. The growth of cells in the presence of IL-3 was defined as 100% (control). All FLT3-JM-PM mutants showed a significantly higher proliferation rate compared with FLT3-WT (*P < .05), although not as high as FLT3-ITD or FLT3-TKD. SD is indicated. (B) FLT3-WT and mutant-expressing Ba/F3 cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of human recombinant FL (50 ng/mL). Viable cells were counted after 72 hours by trypan blue exclusion. The cell number of FLT3-WT cells after 72 hours was defined as 100%. SEM is indicated.
Point mutations in the JM domain of FLT3 induce resistance to apoptosis after IL-3 withdrawal. (A) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD (W51, NPOS, W78), FLT3-TKD (D835Y, D835V) or FLT3-JM-PM (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D) were seeded at a density of 1 × 105 cells/mL and grown for 48 hours in the presence or absence of IL-3. Cells were then analyzed by flow cytometry after staining with annexin V-PE and 7-AAD. All FLT3-JM-PMs showed a significantly lower percentage of apoptotic cells compared with FLT3-WT after IL-3 withdrawal (*P < .05). FLT3-ITDs and FLT3-TKDs protected cells from undergoing apoptosis. (B) Cells were cultured in the presence or absence of IL-3 for 24 hours and analyzed by flow cytometry after staining of nuclei with propidium iodide. FLT3-JM-PMs showed a significantly lower percentage of hypodiploid nuclei compared with FLT3-WT (*P < .05). SD is indicated.
Point mutations in the JM domain of FLT3 induce resistance to apoptosis after IL-3 withdrawal. (A) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD (W51, NPOS, W78), FLT3-TKD (D835Y, D835V) or FLT3-JM-PM (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D) were seeded at a density of 1 × 105 cells/mL and grown for 48 hours in the presence or absence of IL-3. Cells were then analyzed by flow cytometry after staining with annexin V-PE and 7-AAD. All FLT3-JM-PMs showed a significantly lower percentage of apoptotic cells compared with FLT3-WT after IL-3 withdrawal (*P < .05). FLT3-ITDs and FLT3-TKDs protected cells from undergoing apoptosis. (B) Cells were cultured in the presence or absence of IL-3 for 24 hours and analyzed by flow cytometry after staining of nuclei with propidium iodide. FLT3-JM-PMs showed a significantly lower percentage of hypodiploid nuclei compared with FLT3-WT (*P < .05). SD is indicated.
In summary, these data clearly show that the point mutations in JM of FLT3 constitutively activate antiapoptotic signaling pathways of FLT3, although the antiapoptotic activity of these mutants is significantly lower compared with FLT3-ITD and FLT3-TKD mutants.
FLT3-JM-PM receptors are constitutively autophosphorylated on tyrosine residues
We next examined whether the acquisition of point mutations in the JM domain of FLT3 results in constitutive activation of the FLT3 receptor. Cell lysates of unstimulated and FL-stimulated FLT3-JM-PM-expressing Ba/F3 cells as well as FLT3-ITD- and FLT3-TKD-expressing cells were prepared and subjected to immunoprecipitation with FLT3 antibody followed by immunoblotting with anti-phospho-tyrosine antibody. In contrast to the FLT3-WT receptor, all FLT3-JM-PM receptors were constitutively phosphorylated on tyrosine residues (Figure 4A).
Densitometric analysis revealed that the ratios of phosphorylated to total FLT3-JM-PM receptors were 20% for FLT3-V592A-expressing cells, 28% for FLT3-V579-expressing cells, 10% for FLT3-F594L-expressing cells, and 14% for FLT3-F590GY591D-expressing cells, and considerably lower compared with the ratio of phosphorylated FLT3-ITD (42%-60%) and FLT3-TKD (41%-66%) receptors (Figure 4B). We further performed cross-linking experiments to analyze constitutive oligomerization of FLT3-JM-PM receptors. Unstimulated and FL-stimulated cells were incubated in bis(sulfosuccinimidyl)suberate (BS3 ) cross-linking buffer for 30 minutes before cell lysis. In the absence of FLT3 ligand, FLT3-JM-PM receptors showed a higher rate of constitutive oligomerization compared with FLT3-WT receptors (data not shown).
Taken together, the acquisition of FLT3-JM-PMs leads to direct activation of the receptor. FLT3-JM-PMs show increased autophosphorylation and constitutive oligomerization in the absence of FLT3 ligand. The extent of receptor activation is nevertheless weaker compared with FLT3-ITD and FLT3-TKD receptor mutants.
FLT3-JM point mutants induce constitutive STAT5 activation
STAT5 is an important downstream target of the constitutively activated FLT3 receptor and is probably responsible for most of its transforming potential in vitro and in vivo.15,20,23 Activation of STAT5 results in altered expression of several genes regulating cell cycle, apoptosis, and proliferation.24 To investigate the activation of the STAT5 signaling pathway, we prepared crude cell lysates of serum-starved Ba/F3 cells transduced with either FLT3-WT, FLT3-JM-PM (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D) or FLT3-ITD/FLT3-TKD constructs. Lysates were analyzed by immunoblotting with a specific antibody against phospho-STAT5. We could clearly demonstrate that the expression of FLT3-JM-PMs in Ba/F3 cells induces a significantly stronger STAT5 activation compared with FLT3-WT (Figure 5A). The ratio of phosphorylated STAT5 to total STAT5 in FLT3-JM-PM cells ranged from 20% to 35% and was considerably weaker than in FLT3-ITD-expressing cells (49%-61%) and FLT3-TKD-expressing cells (56%-66%) (Figure 5B). We further investigated the FL-independent activation of MAP kinase (MAPK) and the activation of serine/threonine protein kinase AKT as a marker for activation of phosphatidylinositol 3-kinase (PI3K)-dependent pathways. Immunoblotting with phospho-AKT-Ser473-specific antibody revealed a slightly increased basal level of phosphorylation of AKT in FLT3-JM-PM cells compared with FLT3-WT (data not shown). Immunoblotting with phospho-MAPK antibody showed the same level of phosporylation of MAPK in FLT3-JM-PM cells as in FLT3-WT cells (data not shown). Since AKT activation is only slightly increased in FLT3-JM-PM cells and MAPK activation level is not changed compared with FLT3-WT, we conclude that the prominent proliferative signal is mediated by STAT5.
FLT3-JM-PM receptors are constitutively autophosphorylated on tyrosine residues. (A) Lysates of FL-stimulated (100 ng FL /mL for 5 minutes) and unstimulated, serum-starved FLT3-WT-expressing cells, FLT3-JM-PM-expressing cells (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D), and FLT3-W51-expressing cells were subjected to immunoprecipitation with FLT3 antibody followed by immunoblotting with phospho-tyrosine antibody. Blots were stripped and reblotted with FLT3-antibody. (B) FLT3-ITD-expressing cells (W51, NPOS, and W78) and FLT3-TKD-expressing cells (D835Y and D835V) were analyzed as described in panel A and densitometric analysis was performed using TINA 2.0 software to quantify the percentage of phospho-FLT3 of total FLT3.
FLT3-JM-PM receptors are constitutively autophosphorylated on tyrosine residues. (A) Lysates of FL-stimulated (100 ng FL /mL for 5 minutes) and unstimulated, serum-starved FLT3-WT-expressing cells, FLT3-JM-PM-expressing cells (FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D), and FLT3-W51-expressing cells were subjected to immunoprecipitation with FLT3 antibody followed by immunoblotting with phospho-tyrosine antibody. Blots were stripped and reblotted with FLT3-antibody. (B) FLT3-ITD-expressing cells (W51, NPOS, and W78) and FLT3-TKD-expressing cells (D835Y and D835V) were analyzed as described in panel A and densitometric analysis was performed using TINA 2.0 software to quantify the percentage of phospho-FLT3 of total FLT3.
FLT3-JM-PM mutants expressed in Ba/F3 cells show constitutive activation of STAT5 and up-regulation of Bcl-x(L). (A) FLT3-WT, FLT3-W51, FLT3-V592A, FLT3-V579A, FLT3-F594L, FLT3-F590GY591D, or mock-transduced cells were starved for 24 hours in the presence of 0.3% FBS and stimulated with 100 ng FL /mL for 5 minutes. Crude-cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on a nitrocellulose membrane. Blots were incubated with anti-phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody. (B) FLT3-ITD-expressing cells (W51, NPOS, and W78) and FLT3-TKD-expressing cells (D835Y and D835V) were analyzed as described in panel A, and the films were subjected to densitometric analysis to quantify the percentage of phospho-STAT5 in relation to total STAT5 amount in unstimulated cells. (C) Crude-cell lysates were subjected to Western blot analysis using a monoclonal antibody against Bcl-x(L). Bcl-x(L) overexpressed in Ba/F3 cells served as a positive control. Equal protein loading in all lanes was confirmed by immunoblotting using an anti-β-actin antibody.
FLT3-JM-PM mutants expressed in Ba/F3 cells show constitutive activation of STAT5 and up-regulation of Bcl-x(L). (A) FLT3-WT, FLT3-W51, FLT3-V592A, FLT3-V579A, FLT3-F594L, FLT3-F590GY591D, or mock-transduced cells were starved for 24 hours in the presence of 0.3% FBS and stimulated with 100 ng FL /mL for 5 minutes. Crude-cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on a nitrocellulose membrane. Blots were incubated with anti-phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody. (B) FLT3-ITD-expressing cells (W51, NPOS, and W78) and FLT3-TKD-expressing cells (D835Y and D835V) were analyzed as described in panel A, and the films were subjected to densitometric analysis to quantify the percentage of phospho-STAT5 in relation to total STAT5 amount in unstimulated cells. (C) Crude-cell lysates were subjected to Western blot analysis using a monoclonal antibody against Bcl-x(L). Bcl-x(L) overexpressed in Ba/F3 cells served as a positive control. Equal protein loading in all lanes was confirmed by immunoblotting using an anti-β-actin antibody.
As the JM point mutants induce antiapoptotic signaling when expressed in Ba/F3 cells, we hypothesized that this might be due to the up-regulation of an important STAT5 antiapoptotic downstream target, Bcl-x(L). Cell lysates were subjected to Western blot analysis and immunoblotted with specific monoclonal antibody against Bcl-x(L). We found a significant up-regulation of Bcl-x(L) in FLT3-V592A-, FLT3-V579A-, FLT3-F594L-, and FLT3-F590GY591D-expressing cells compared with FLT3-WT (Figure 5C).
Thus, the most important signaling pathway downstream of FLT3, STAT5, is constitutively activated by the FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D receptor mutants and induces up-regulation of the antiapoptotic protein Bcl-x(L).
The FLT3 PTK inhibitor PKC412 induces growth arrest and inhibits tyrosine phosphorylation of STAT5 in FLT3 JM point mutants
PKC412 was inititally developed as a vascular endothelial growth factor receptor (VEGFR) inhibitor and was shown to block the activity of FLT3-WT and mutant FLT3 receptors.25,26 To analyze the inhibitory activity of PKC412 against FLT3-JM-PMs, we treated the FLT3-V592A-, FLT3-V579A-, FLT3-F594L-, and FLT3-F590GY591D-expressing cells with different concentrations of PKC412 up to 50 nM. PKC412 showed a strong growth inhibitory effect on FLT3-V592A-, FLT3-V579A-, FLT3-F594L-, and FLT3-F590GY591D receptor-expressing cells in the absence but not in the presence of IL-3. The inhibitory concentration of 50% (IC50) of PKC412 was significantly lower in FLT3-JM-PM-expressing cells (< 1 nM) and FLT3-D835Y-expressing cells (< 1 nM) compared with the FLT3-W51 mutant (5 nM; Table 1).
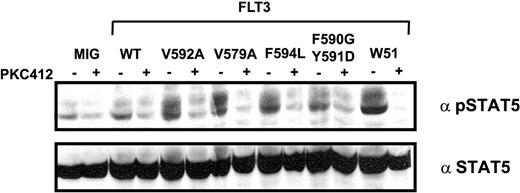
Furthermore, we treated serum-starved FLT3-V592A-, FLT3-V579A-, FLT3-F594L-, FLT3-F590GY591D-, and FLT3-W51-expressing cells for 1 hour with 50 nM PKC412. Immunoblotting with pSTAT5 antibody showed a significant reduction in the amount of phosphorylated STAT5 in FLT3-V592A, FLT3-V579A, FLT3-F594L, FLT3-590G591D, and FLT3-W51, confirming that STAT5 activation is directly mediated by the activated FLT3 receptor (Figure 6).
These data clearly show that the proliferative signal of FLT3-JM-PM receptors can be blocked by PKC412 and that FLT3-JM-PM-expressing cells are considerably more sensitive to this compound than FLT3-ITD-expressing cells.
Discussion
Since 1996, when the first description of activating FLT3 mutations was published, extensive research has been performed to characterize the functional relevance of these mutations in AML.3 Although the FLT3 gene has been studied in detail in AML, we and others were recently able to identify a new class of mutations in patients with AML, the FLT3-JM-PMs.15,16 According to Stirewalt et al,16 who performed a SSCP analysis of the entire JM region, the frequency of these point mutations in the JM region was about 2%. In the present study, we focused mainly on the V592A mutation that we found in MM-1 and MM-6 cell lines. Our LightCycler-based screening assay covers approximately 5 to 6 amino acids surrounding V592A and underestimates the frequency of FLT3-PMs in the JM domain.
PKC412 inhibits autophosphorylation of STAT5 in Ba/F3 cells expressing JM point-mutated FLT3 receptors. Mock cells and cells expressing FLT3-WT, FLT3-W51, FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D were starved for 24 hours and treated with 50 nM PKC412 1 hour before cell lysis. Cell lysates were subjected to Western blot analysis using polyclonal anti-phospho-STAT5 antibody. Blots were stripped and reblotted with polyclonal anti-STAT5 antibody.
PKC412 inhibits autophosphorylation of STAT5 in Ba/F3 cells expressing JM point-mutated FLT3 receptors. Mock cells and cells expressing FLT3-WT, FLT3-W51, FLT3-V592A, FLT3-V579A, FLT3-F594L, and FLT3-F590GY591D were starved for 24 hours and treated with 50 nM PKC412 1 hour before cell lysis. Cell lysates were subjected to Western blot analysis using polyclonal anti-phospho-STAT5 antibody. Blots were stripped and reblotted with polyclonal anti-STAT5 antibody.
Our results show that these JM point mutations in the FLT3 gene induce factor-independent growth and hyperresponsiveness to FL, and have an antiapoptotic activity in hematopoietic cells. The FLT3-JM-PM receptors are autophosphorylated and show a higher constitutive oligomerization rate compared with FLT3-WT receptors. We could show that all FLT3-JM-PMs activate STAT5 and up-regulate Bcl-x-(L). The activation of the receptor and STAT5, respectively, is lower in FLT3-JM-PM-expressing cells compared with FLT3-ITD- and FLT3-TKD-expressing cells, and we propose that these differences are mainly responsible for the weaker phenotype of FLT3-JM-PMs. A selective FLT3 PTK inhibitor, PKC412, was able to abrogate the factor-independent growth and down-regulated the activation of STAT5. Compared with the two known classes of FLT3 mutations (FLT3-ITD and FLT3-TKD), FLT3-JM-PM-expressing cells showed a weaker phenotype in terms of their proliferation rate, antiapoptotic activity, FLT3 receptor activation, and activation of STAT5 (summarized in Table 2).
The reason for the weaker transforming potential might be that the FLT3-JM-PMs, in contrast to FLT3-ITDs, induce only subtle alterations in the protein structure of the autoinhibitory JM domain. All FLT3-JM-PM-expressing cells showed hyperresponsiveness to FLT3 ligand, with a 2 to 4 times higher proliferation rate compared with FL-stimulated FLT3-WT cells. As FLT3 ligand is often coexpressed in primary AML blast cells, this might reflect more precisely the “in vivo” situation. Furthermore, we could demonstrate that a single point mutation in the JM domain is sufficient to constitutively activate the receptor and increase its spontaneous dimerization rate.
STAT5, the most important downstream target of FLT3, is strongly associated with tumor development and progression.27 For example, it directly induces the expression of antiapoptotic proteins, like Bcl-x(L), which play an essential role in resistance against apoptotic cell death.28,29 Bcl-x(L) is overexpressed in a high percentage of blast cells from patients with AML and confers a poor prognosis in these patients.30 Our results clearly show that FLT3-JM-PMs activate STAT5 and induce up-regulation of Bcl-x(L), and suggest that this pathway is critical for the transforming capacity of activated FLT3 mutants.
The success of the small-molecule tyrosine-kinase inhibitor imatinib (Novartis) in the treatment of chronic myelogenous leukemia (CML) has given much encouragment for the development of other molecularly targeted cancer therapeutics. Due to the fact that FLT3 is one of the most frequently mutated genes in AML, it is a promising target for the treatment of AML. Small-molecule FLT3 PTK inhibitors have been developed, and are able to induce apoptosis in cell lines with FLT3-activating mutations and prolong the survival of mice expressing mutant FLT3.31-34 We analyzed the inhibitory activity of PKC412, a compound which is now tested in phase 2 clinical trials in AML, on Ba/F3 cells expressing FLT3-V592A, FLT3-V579A, FLT3-F594L, FLT3-F590GY591D, FLT3-W51, and FLT3-D835Y. Our data clearly show that FLT3-JM-PM-induced cell growth can be inhibited by PKC412. In addition, we were able to show down-regulation of autoactivated STAT5 after treating cells with PKC412, providing direct evidence for the efficacy of this compound to abrogate mitogenic signaling pathways of the receptor. Moreover, the FLT3-JM-PMs are considerably more sensitive to PKC412 than FLT3-ITDs, suggesting that patients with JM point mutations in FLT3 should respond to even lower doses of PKC412.
Our results show that a single amino acid change in the autoinhibitory JM domain is sufficient to activate the transforming potential of FLT3. To structurally analyze the putative effect of the point mutations, we mapped the mutations on the crystal structure of the FLT3 JM domain (Figure 7).10 From the FLT3 crystal structure it is obvious that these point mutations cluster at a core interaction site of the JM domain with the remainder of the molecule. It is therefore likely that these mutations reduce the stability of the JM domain in the autoinhibitory conformation. For instance, F594 and V592 together form a small hydrophobic contact face of the JM domain with the kinase domain. F594L and V592A mutations topologically perturb this hydrophobic contact face, likely resulting in a destabilized interface between JM domain and kinase domain. Likewise, mutations of F590 to glycine and of Y591 to aspartate might destabilize the autoinhibitory conformation of the JM domain by adding more backbone flexibility, but also by removing the stabilizing contacts of the tyrosine side chain with the rest of the molecule. The effect of Y591D on the activity of the molecule is also consistent with the idea that phosphorylation of the tyrosine pair Y589 and Y591 sterically prohibits the observed autoinhibitory conformation of the JM domain. Finally, V579 stabilizes the inserted β strand of JM-B by a hydrophobic interaction with the rest of the molecule. This stabilization is likely reduced by mutating this residue to alanine, which would create a cavity in the molecule. Taken together, the effect of the JM-PMs supports a model in which the JM domain rearranges upon activation of the kinase. This rearrangement could be normally promoted by phosphorylation of Y589 and Y591, but in the case of activating mutations, also by destabilization of the autoinhibitory conformation of the JM domain. In this respect, the mutations might promote tyrosine phosphorylation by rendering the JM domain more accessible for autophosphorylation.
Structural mapping of JM point mutations. (Left) Ribbon model of the crystal structure of the FLT3 kinase domain (Protein Data Bank [PDB] accession no. 1RJB) with green activation loop and yellow JM domain. The positions of internal tandem duplications (ITDs) in JM-Z, leading to FLT3 activation, are indicated. *Some ITDs are found in the tyrosine kinase domain and are not indicated. (Right) Close view of the mutation sites in the JM domain (yellow). The structure is shown as a ribbon backbone, with side chains shown as color-coded sticks. Point mutations identified in this study are depicted in red and are annotated. The clustering and location of the point mutations suggest that they reduce the stability of the observed inhibitory conformation of the JM domain. Structural analysis of FLT3-JM-PMs was done with PyMol (DeLano Scientific, San Carlos, CA).
Structural mapping of JM point mutations. (Left) Ribbon model of the crystal structure of the FLT3 kinase domain (Protein Data Bank [PDB] accession no. 1RJB) with green activation loop and yellow JM domain. The positions of internal tandem duplications (ITDs) in JM-Z, leading to FLT3 activation, are indicated. *Some ITDs are found in the tyrosine kinase domain and are not indicated. (Right) Close view of the mutation sites in the JM domain (yellow). The structure is shown as a ribbon backbone, with side chains shown as color-coded sticks. Point mutations identified in this study are depicted in red and are annotated. The clustering and location of the point mutations suggest that they reduce the stability of the observed inhibitory conformation of the JM domain. Structural analysis of FLT3-JM-PMs was done with PyMol (DeLano Scientific, San Carlos, CA).
The FLT3-ITDs, which are primarily located in the JM-Z region, confer a strong transforming potential to hematopoietic cell lines, but the mechanisms by which these duplicated sequences of FLT3-ITD mutants change the conformation of the FLT3 protein structure remain unknown. It was proposed that the increased length of the JM-Z offsets the position of JM-S, likely disturbing the ideal orientation of JM-S to position JM-B in its binding site.10 It is difficult to say what effect a length increase in JM-Z has on the overall integrity of the protein structure, but the close location to JM-B suggests that these mutations might completely prevent formation of an autoinhibitory state. From a structural point of view, the presence of additional residues in JM-Z could easily prevent the formation of structurally observed folds of JM-S in the autoinhibitory state. Although FLT3-ITDs can involve the same amino acids in the JM domain as those affected by point mutations, they probably alter the protein conformation in a different way. Compared with FLT3-ITDs, the point mutations represent a much more subtle change in the structural chemistry of this region and might still allow formation of an autoinhibitory complex, albeit with reduced stability. A full (FLT3-ITD) versus partial (FLT3-JM-PM) interference with the autoinhibitory state could explain the much stronger phenotype of ITD mutations compared with point mutations.
The cytoplasmatic juxtamembrane domain is highly conserved between different members of class III receptor tyrosine kinases. A variety of malignancies have been described which harbor activating mutations in the juxtamembrane domain of KIT.11-14,35-37 In 10% to 20% of all gastrointestinal tumors (GISTs), gain-of-function single amino acid substitutions of the JM region were reported, and two possible hotspot regions, comprising codons 550 to 560 and 567 to 576, were suggested.38,39 Among others, one frequently affected position is KIT-V560. This residue is homologous to V579 in FLT3 that was found to be mutated in one AML patient (FLT3-V579A). Furthermore the second region, aa 567 to 576 in KIT, is partially homologous to FLT3 and comprises all other 3 reported point mutations (FLT3-V592A, FLT3-F590GY591D, and FLT3-F594L). The clustering of these mutations in the JM domains of both KIT and FLT3 supports their specific biological significance. Further confirmation on the functional relevance of the JM domain was provided by 2 investigators who performed a detailed mutational screening.40,41 Ma et al40 analyzed the region from M552 to I563 in the JM of the KIT receptor, and Irusta et al41 analyzed the region from R529 to W561 in JM of platelet-derived growth factor receptor β (PDGFRβ). Single amino acids were mutated to alanine, and the constitutive tyrosine phosphorylation of the receptor was analyzed by both groups. The V560A mutation in KIT, homologous to V579A in FLT3, as well as V550A mutation in PDGFRβ, homologous to V592A in FLT3, resulted in autophosphorylation of the receptor. Mutation of I537A and D551A in PDGFRβ, corresponding to FLT3 positions V579 and F594, displayed a strong and weak autophosphorylation, respectively. These results clearly show that point mutations targeting this region lead to destabilization of the autoinhibitory JM conformation and finally to activation of the receptor.
In conclusion, we were able to define a new class of activating FLT3 mutations, point mutations that cluster in the JM region. The FLT3-JM-PMs confer a transforming potential to hematopoietic cells in vitro that was weaker than that of FLT3-ITD and FLT3-TKD mutant receptors. Our data suggest that patients carrying FLT3-JM-PMs might respond to treatment with selective FLT3 inhibitors.
Prepublished online as Blood First Edition Paper, January 12, 2006; DOI 10.1182/blood-2005-06-2596.
Supported by a grant from the Deutsche Forschungsgemeinschaft (DFG Sp556/3-1) and the Deutsche Krebshilfe (10-1997-Sp2).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Stefan Bohlander and Rob Chapman for critical reading of the manuscript.





![Figure 7. Structural mapping of JM point mutations. (Left) Ribbon model of the crystal structure of the FLT3 kinase domain (Protein Data Bank [PDB] accession no. 1RJB) with green activation loop and yellow JM domain. The positions of internal tandem duplications (ITDs) in JM-Z, leading to FLT3 activation, are indicated. *Some ITDs are found in the tyrosine kinase domain and are not indicated. (Right) Close view of the mutation sites in the JM domain (yellow). The structure is shown as a ribbon backbone, with side chains shown as color-coded sticks. Point mutations identified in this study are depicted in red and are annotated. The clustering and location of the point mutations suggest that they reduce the stability of the observed inhibitory conformation of the JM domain. Structural analysis of FLT3-JM-PMs was done with PyMol (DeLano Scientific, San Carlos, CA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/9/10.1182_blood-2005-06-2596/2/m_zh80090695120007.jpeg?Expires=1767820554&Signature=mvV8yS0oABxFxnQNbcp2HDXON5VLRmFTGZrX92DzrB65Y5iT3RDT6HBCfzsZCWhy9lSLvKsNs1lVQfpn6hlk~jOO6TFKUP-UW93Z35RnAOizjSzfO58BpwdF10mi5eLRwo3fNRhbS2GSVJYaYE-SYnJBMz6EUQ8LJs0LshM5RJ3CO-hg~XS1cO8APCrALvW~M9741O-C7h1I0Xh0yRF7Ts68QUWnla0BuGgdYCR6lTFX~WbCrGPIkwkXKtNCUS7NNfhjGdpM0BzdMsmDxqLEc7yl5gCUXgfKPHiC9iY9hByOqQNd5f6D6CSRbPapDsBe7-SbQcsiP4RfrC~ULRE9IQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
