Deferoxamine (DFO) therapy has been associated with improved survival of thalassemia patients. However, cardiac disease remains the main cause of death in those patients. In 1995, the oral chelator deferiprone became available for clinical use. We compared the occurrence of cardiac disease in patients treated only with DFO and in those whose therapy was switched to deferiprone during the period of observation, from January 31, 1995, to December 31, 2003. All patients with thalassemia major treated in 7 Italian centers who were born between 1970 and 1993 and who had not experienced a cardiac event prior to January 1995 were included. DFO only was given to 359 patients, and 157 patients received deferiprone for part of the time. A total of 3610 patient-years were observed on DFO and 750 on deferiprone. At baseline, the 2 groups were comparable for age and sex, while ferritin levels were significantly higher in patients switched to deferiprone. Fifty-two cardiac events, including 10 cardiac deaths, occurred during therapy with DFO. No cardiac events occurred during deferiprone therapy or within at least 18 months after the end of it. In the setting of a natural history study, deferiprone therapy was associated with significantly greater cardiac protection than deferoxamine in patients with thalassemia major.
Introduction
Survival and complication-free survival of patients with thalassemia major have significantly improved in recent years as a consequence of better management. However, many transfusion-dependent patients continue to develop progressive accumulation of iron, which is responsible for tissue damage and, eventually, death. During the past 20 years we have been collecting data on survival and complications in a large population of patients followed in 7 Italian Thalassemia Centers. A recent analysis of the database showed a continuous improvement in survival for patients born from 1960 to 1984 that was mirrored by a decrease in cardiac mortality, but it also showed that cardiac disease continues to be responsible for significant disability and that it causes 70% of the deaths in patients chelated with deferoxamine (DFO).1 In 1995, a new oral chelator, deferiprone (1,2-dimethyl-3-hydxoxypyridin-4-one) became available for clinical use. Studies in iron-loaded rat heart cells2 and in gerbils3 had in the past shown the ability of deferiprone to remove iron from myocardial cells at concentrations that can be achieved in the circulation. Recent studies4,5 have suggested that deferiprone provides greater cardiac protection against iron-induced heart disease than does DFO. Using the data available from our 7-center database, we compared development of cardiac disease and survival in patients chelated only with DFO and in patients who had their therapy switched to deferiprone during the study period.
Patients, materials, and methods
Patients
The analysis included all patients treated for thalassemia major at the 7 centers participating in this study who were born between 1970 and 1993 and who on January 31, 1995, were alive, on follow-up, had not undergone bone marrow transplantation, and had not had a cardiac event. This date was chosen because little or no deferiprone was being used in Italy prior to that time. The last follow-up date was December 31, 2003. A number of patients (516) met the study criteria: 359 (70%) received only DFO throughout the study period, while 157 (30%) patients were treated with deferiprone at some point during the study. Of these patients, 13% (68 of 516) were included in a previous analysis.5 This report includes an additional 2 years and 9 months of follow-up data on these patients, and uses a different data analytic approach.
Treatment
All patients included in the study were initially on DFO that was given to all patients when it became available (1975), or after their first year of transfusion. DFO was administered by the intramuscular route until 1980, and then subcutaneously, by means of a pump, in doses varying from 30 to 50 mg/kg per day, 5 to 6 times a week. The first patients to receive deferiprone in our population were treated in 1995 in Ferrara and Torino as part of a trial investigating the safety profile of the oral chelator.6 Starting in 1997, deferiprone was distributed as part of a compassionate use program, under the supervision of the Italian Ministry of Health, to thalassemia major patients older than 6 years. In order to obtain the drug, the patients had to have a serum ferritin level greater than 2000 μg/L or liver iron concentration greater than 4 mg/g liver dry weight and to be either noncompliant or intolerant to DFO.7 This compassionate program continued until the year 2000, when the drug became commercially available in Italy for use in patients for whom DFO therapy was contraindicated or not tolerated. The usual dose of deferiprone was 75 mg/kg body weight, given daily in 3 divided doses. Since this is an epidemiologic, observational study, no data are available regarding compliance with the assigned treatment. In fact, compliance is very difficult to establish in a large population of chronic patients.
Data collection
We collected demographic data, dates of cardiac complications, and, when applicable, date of bone marrow transplantation, date of death, and causes of death. Cardiac complications were defined as cardiac failure or arrhythmias requiring use of inotropic or antiarrhythmic drugs. Serum ferritin levels were obtained at least 3 times a year and the average of these values was entered in the database. Starting in July 2001, some of the patients participated in a trial of the oral chelator ICL670. The date of switch to this drug was also recorded.
Data analysis
The primary approach to analyzing the data was a time-to-event analysis, where an event is a cardiac complication, as defined. Time zero, or study entry, is defined as January 31, 1995, for all patients, in order to be able to compare between the experiences on either treatment from that point forward. This clearly gave a large distribution of times of DFO treatment prior to January 1995, but we did not have data of the start time of DFO. We explored instead the duration since first transfusion.
An observation was considered censored if the patient was cardiac event-free on December 31, 2003, or at last follow-up, or at bone marrow transplantation, or at switch to ICL670. If a death occurred that was clearly a noncardiac cause of death, the observation was also considered censored at time of death for the time to cardiac events analysis. Once a cardiac event occurred, this was defined as a failure event and the observation on the patient terminated for the purpose of this study. Thus, if there was a second cardiac event within the study period, the second event is not included in the analysis. A second analysis included 3 noncardiac deaths as failure events in addition to the cardiac events.
The incidence of cardiac events was calculated by treatment group for each calendar year. The definition of “group” for each year was based on the treatment that the patient was receiving on January 1 of the given year. It was possible to therefore be classified on one treatment on January 1, switch treatments during the year, and have a cardiac event at a later date in the year. In this case, the event would be attributed to the January 1 treatment. However, this interval definition was used in order to apply a consistent definition throughout the data. We note in the results if this circumstance occurred. We calculated incidence rates and exact 95% binomial confidence intervals (CIs) for all incidence estimates. We further constructed for each year a 2 × 2 table for having a cardiac event versus not having an event on each of the 2 treatments, and combined all the annual tables and tested whether the combined odds ratio was 1.0.8
For descriptive purposes only, we also defined 2 treatment groups including either the 359 patients who received DFO only or the 157 patients who received deferiprone at some point in time during the review period. We compared these 2 groups for baseline characteristics to explore whether there was some disposition to provide deferiprone to a particular subset of patients. We also summarized the duration of drug use of either drug using medians and ranges.
Because it was unknown at study entry how many and which patients would receive deferiprone and which would not, it would not have been correct to use the descriptive 2 treatment groups and compare them using a proportional hazards model and a log-rank test. Doing so would mean that we knew in January 1995 who would switch to deferiprone. Moreover, because all those patients who received deferiprone received DFO for part of the study duration, either only before or before and after deferiprone, they cannot be defined as patients receiving deferiprone for the duration of the study. Therefore, we defined a time-varying treatment variable. For each patient, treatment at time “t” was defined as 0 if the treatment was DFO and 1 if the treatment was deferiprone.9 All patients had, therefore, a starting value of 0. The patients who switched to deferiprone had their time-varying treatment variable switched to deferiprone at the time when they changed treatment. Some patients on deferiprone at some point switched back to receiving DFO treatment. Their time-varying treatment variable was changed back to 0 at the time that they switched back to DFO. We fit a Cox regression model with this time-varying treatment variable to test the efficacy of deferiprone. Additional Cox regression models were fit to test for the effect of sex, of birth cohort, and of ferritin level at baseline (using 2500 μg/L as a cutoff).
An additional analysis compared the rates of events using a person-years method. In this approach, the rate of events was compared by calculating the number of events relative to the number of person-years of exposure to either DFO or deferiprone, as appropriate. We then assumed that the cardiac events were generated by a Poisson distribution and tested for equality between a Poisson process underlying cardiac events generated while on DFO and a Poisson process underlying cardiac events generated while on deferiprone. This analysis was performed using StatXact 6.0 (Cytel Software, Cambridge, MA).10
As a secondary endpoint, we compared the rate of deaths from all causes using the same Cox regression model approach with treatment as a time-varying covariate. In this approach, a failure event was death due to any cause and censoring occurred if patient was alive at the end of the follow-up period, or received bone marrow transplantation, or switched to ICL670.
We also compared the rate of change in ferritin levels in the 2 treatment groups using all the patients who had at least 2 annual average ferritin evaluations after study entry for the DFO group or after start of deferiprone for the deferiprone group. We calculated the slopes (rate of change) of ferritin within each patient, and compared the slopes in the 2 treatment groups using a Wilcoxon rank-sum test.
The study was approved by the Ethical Committee of the University of Ferrara, Italy.
Results
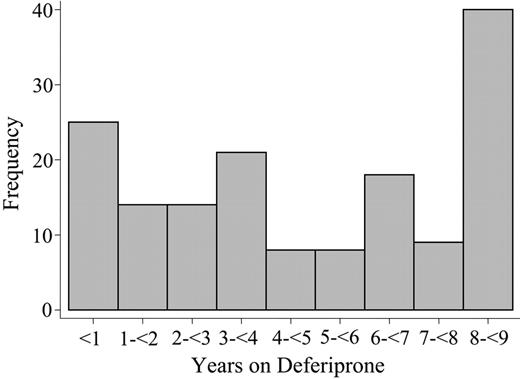
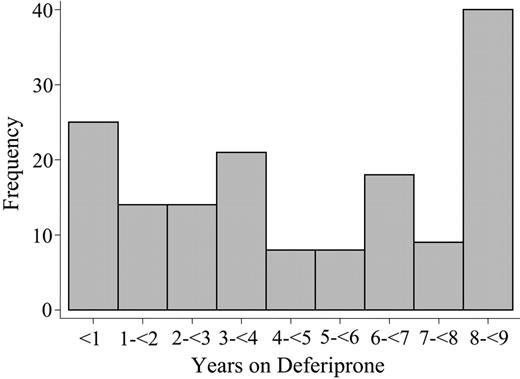
Table 1 shows descriptive statistics of the 2 groups. There were relatively more patients with ferritin levels greater than 2500 μg/L in 1995 among the patients switched to deferiprone than in the group that remained on DFO. All other baseline characteristics are similar. The total study duration was nearly 9 years. All the patients were followed for the entire study duration, except 2 who were lost to follow-up. The median duration of the deferiprone treatment was 4.3 years (range, 0.02-8.9 years), for a total of 750 patient-years. The median time on DFO since January 1995 until switching to deferiprone was 2.0 years (range, 0.06-8.7 years). Figure 1 reports the distribution of duration of deferiprone therapy.
There were 52 cardiac events during the observation period, 10 of which were cardiac deaths. Of the surviving 42 patients, 5 died of cardiac disease within 4 to 47 months after the first cardiac event. All events occurred while on DFO. Six events occurred in patients who had previously received deferiprone, but were on DFO at the time of the event. The time interval between stopping deferiprone treatment and the occurrence of the cardiac event ranged from 1 year and 8 months to 5 years and 4 months. Eight patients were switched to deferiprone after development of a cardiac event.
Table 2 shows the incidence of cardiac events by calendar year and the treatment on January 1 of the given year.
The incidence of cardiac events while on DFO ranged from 0.6 in 1995 to 3.4 in 2003. The confidence intervals for events on deferoxamine are narrow, approximately 2% to 4% wide. The (1-sided) confidence intervals for deferiprone are between 4% and 6% wide, depending on the number of subjects at risk. None of the 52 cardiac events attributed to DFO happened while patients were on deferiprone; thus, the potential of a patient being on DFO on January 1, changing to deferiprone, having a cardiac event, and having the the event attributed to DFO, did not occur.
Eight 2 × 2 tables were created from Table 2, excluding 1995, in which there were no patients on deferiprone at the start of the year; hence, an odds ratio cannot be calculated. The combined odds ratio was significantly different from 1.0 (P < .001). The odds ratio of a cardiac event on DFO versus deferiprone is estimated at infinity, since there were no events on deferiprone, with a lower 95% confidence bound of 2.75.
Duration of deferiprone treatment. The histogram shows the number of patients grouped by years on treatment with deferiprone.
Duration of deferiprone treatment. The histogram shows the number of patients grouped by years on treatment with deferiprone.
There were 9 patients (1.7%, 8 DFO and 1 deferiprone) who underwent bone marrow transplantation and, in performing the Cox regression analysis, their observation of time to cardiac event was censored at the time of the bone marrow transplantation. There were 46 (8.9%, 29 DFO and 17 deferiprone) patients who switched to ICL670, and their observations were censored at the start of the ICL670. Of note, none of the patients who switched to ICL670 had a cardiac event through December 31, 2003; thus, we did not miss any event, either from deferiprone or DFO, because of the censoring at the switch to ICL670. Of the 26 deaths on study, 11 (2.1%) of 516 were noncardiac deaths and were considered censored at the time of death for the time to cardiac event analysis. Finally, there were 2 patients who were lost to follow-up (both on DFO). Therefore, there were a total of 68 (13%) patients whose observations were censored for a reason other than reaching December 31, 2003, without a cardiac event. Because all events in this study occurred while on DFO, a coefficient for a treatment effect, in the Cox regression with treatment as a time-varying covariate, could not be estimated. The hazard of a cardiac event on deferiprone is estimated at 0, and on DFO is more than 0; therefore, the hazard ratio between the 2 treatments is 0, and DFO is a significant risk factor for a cardiac event. In order to estimate the significance, we artificially created one cardiac event on deferiprone, on a low-risk long-term deferiprone-exposure patient, thus maximizing the impact of an event in a single patient on an analysis. In this analysis, the hazard ratio on deferiprone compared with DFO was .09 (CI .012, .66; P = .017). An additional analysis conservatively assumed that a lack of protection of deferiprone from cardiac events may extend up to 2 years beyond the end of deferiprone. This is modeled by keeping the group assignment of deferiprone for an additional 2 years of exposure (unless end of follow-up is reached earlier) for all patients who received deferiprone. Under this assumption, one of the 6 cardiac events that occurred after deferiprone treatment (20 months after end of deferiprone) would be attributed to deferiprone. In this analysis, the hazard ratio on deferiprone compared with DFO was .08 (CI .011, .57; P = .012). When including the known risk factors of sex, age, and ferritin level at baseline in the model in addition to deferiprone, the hazard ratio on deferiprone compared with DFO was .075 (CI .010, .55; P = .011), and all the risk factors were significant (P < .005).
Thus, consistent with the person-years analysis, the hazard of an event on deferiprone is estimated to be less than a tenth of the hazard of an event on DFO. In addition, we performed a Cox regression that included the noncardiac deaths as failure events in addition to the cardiac events (ie, redefining the failure event as cardiac event or death, whichever occurred first). This analysis included the 2 deaths on deferiprone and provided an estimated hazard ratio of a cardiac event or death of .078 (CI .010, .56; P = .011) on deferiprone relative to DFO.
The rate of events on DFO was calculated by adding all the person-years of exposure to DFO, both for the 359 patients who received DFO only and for the 157 patients who received deferiprone, using the years that they were on DFO since January 1995. The 516 patients were exposed to a total of 3610 person-years on DFO (average patient exposure, 7 years), in which 52 events occurred (1.4 events per 100 person-years; CI 1.1, 1.9). The 157 receiving deferiprone were exposed to 750 person-years on deferiprone (average patient exposure, 4.8 years), in which no event occurred (0 events per 100 person-years; CI 0, .0.5). When assuming Poisson processes for both rates and comparing those rates, they are significantly different (P < .002). When excluding the exposure to DFO of the 157 deferiprone patients, and also excluding the 8 events occurring before deferiprone and the 6 events occurring after deferiprone on DFO, results also show significant differences between the process rates (P < .002).
Forty-six (31%) patients discontinued deferiprone as a consequence of clinical or laboratory adverse events. The causes for discontinuation were: increase in ferritin levels or in liver iron concentration (21 patients), arthropathy or arthralgia (10 patients), neutropenia (8 patients), agranulocytosis (1 patient), increased levels of alanine aminotransferase (2 patients), gastric discomfort (2 patients), worsening of renal failure (1 patient), and worsening of hepatic insufficiency in a cirrhotic, hepatitis C virus-positive patient (1 patient). Sixteen (10%) patients discontinued deferiprone for reasons other than adverse events including lack of compliance to the required weekly blood counts (6 patients), end of the deferiprone clinical trial (2 patients), starting on an ICL670 clinical trial (3 patients), fear of long-term adverse events (2 patients), or for unknown reasons (3 patients).
Twenty-six (5%) patients died during the study period, 24 (6.7%) in the DFO group and 2 (1.3%) in the deferiprone-switched group. Of the 24 deaths on DFO, 15 were cardiac-related. Neither death on deferiprone was cardiac related. One patient died in a car crash; the other, of bacterial endocarditis that originated from the indwelling catheter inserted for DFO administration and was still in place. Neutropenia was not present either before or during the infectious process. Using the Cox regression model with a time-varying covariate gave a hazard ratio of .38 (CI 0.9, 1.6) of death on deferiprone (P = .19). The number of events is small; therefore, the study does not have sufficient power to test this question.
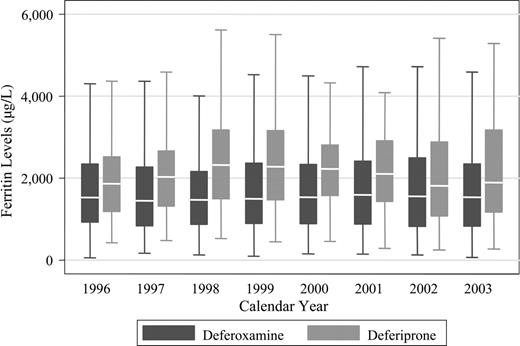
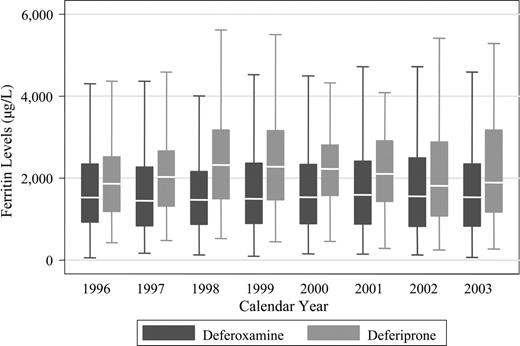
Figure 2 shows the ferritin levels in patients treated with DFO or deferiprone. In general, serum ferritin concentrations were lower in the DFO group than in the deferiprone group at each calendar year. Of the 359 patients who received DFO only, 335 had at least 2 average annual ferritin values after study entry. Of the 157 patients who received deferiprone, 137 had at least 2 average annual ferritin values after starting deferiprone. The median slope of rate of change on DFO was 1.3 μg/L per year, and on deferiprone it was 16 μg/L per year (P = .22). The median intercept for the DFO group was 1386 μg/L and for the deferiprone group it was 2039 μg/L (P < .001). The intercept values are consistent with Table 1, which uses the 1995 baseline values on all patients, and show that ferritin levels are higher in the deferiprone group.
In order to examine whether this cohort was consistent with other cohorts, we examined whether known risk factors for cardiac events were predictive here. Males were 2.5 times more likely to have a cardiac event than females (P = .009). Having a serum ferritin concentration above 2500 μg/L at baseline was associated with a hazard ratio of 3.71 compared with serum ferritin concentration below 2500 μg/L (P < .001) at baseline. Finally, age at entry was a predictive risk factor.
Each increasing year of age was associated with a hazard ratio of 1.17 (P < .001). Overall, these results confirm that this cohort is consistent with other cohorts of thalassemia patients.1
Discussion
Chelation therapy with deferoxamine has been associated with marked decrease in mortality and morbidity in patients with thalassemia major.1,11-13 However, cardiac disease continues to occur and remains the most common cause of death in those patients. Male patients and patients with elevated serum ferritin values appear to be at higher risk of cardiac disease and premature death.1
The results of the current study demonstrate that patients with thalassemia major who switched to deferiprone therapy had a remarkably lower prevalence of cardiac disease and cardiac death than patients chelated with DFO only. No patient developed a cardiac event while on deferiprone. Conversely, 52 (14.5%) of the patients treated with DFO developed a cardiac event. Fifteen of them died of cardiac failure or arrhythmias. Six patients who were on deferiprone for 3 months to 5 years had cardiac events subsequent to the end of deferiprone treatment. One patient had an event 20 months after stopping deferiprone, and the remaining 5 had cardiac events more than 3 years after stopping deferiprone.
Serum ferritin levels in thalassemia major patients treated with deferoxamine or deferiprone. The box stretches from the 25th to 75th percentile. The median is represented by a line across the box. The whiskers extend to the highest and lowest observed values that are lower than and higher than 1.5 times the interquartile range from the third and first quartile values. Outlying values beyond the whisker edges are excluded from the plot.
Serum ferritin levels in thalassemia major patients treated with deferoxamine or deferiprone. The box stretches from the 25th to 75th percentile. The median is represented by a line across the box. The whiskers extend to the highest and lowest observed values that are lower than and higher than 1.5 times the interquartile range from the third and first quartile values. Outlying values beyond the whisker edges are excluded from the plot.
The lower prevalence on deferiprone occurred in spite of a heavier starting iron overload, as judged by baseline serum ferritin concentrations, among the patients on deferiprone. The frequency of adverse events was comparable with previously reported data.6
This study was an epidemiological, natural-history study. An advantage of such a study is that the patients included are more likely to represent the disease population as a whole. However, because there was no randomization, treatment groups may not be comparable. In this study, there may have been a bias against deferiprone, because, at least initially, deferiprone was experimental and was given mainly to patients with higher body iron load as measured by their serum ferritin levels or liver iron concentration. Conversely, since deferiprone was not approved in Italy until the year 2000, some physicians might have been reluctant in prescribing deferiprone to their sicker patients, biasing results in favor of deferiprone. Given that the time of initiating deferiprone shows a fairly uniform distribution over the 9-year interval of the study, neither consideration appears to have strongly biased the results of the study.
There is also some potential for length bias. Length bias occurs when events can only be observed if the observation is long enough for the event to happen. In this study, in order to receive deferiprone, patients would have had to survive long enough to receive it. Thus, the sickest patients, possibly, who had cardiac events, were those who did not have the opportunity to receive deferiprone, and the observations on deferiprone may not have been long enough for cardiac events to occur. Within this study, the average duration of DFO treatment was 7 years per patient and on deferiprone was 4.8 years per patient. Therefore, this bias would favor deferiprone. However, even with this bias, it would be surprising if the bias were strong enough to explain why there were no events with 750 person-years of exposure on more than 150 patients.
The results of this study are consistent with those of Piga et al,5 who conducted a retrospective assessment of survival and occurrence of cardiac disease in all patients with thalassemia major treated for at least 4 years with deferiprone or DFO in a single center. In that study, cardiac disease was significantly more frequent in the DFO-treated than in the deferiprone-treated patients. Also, in a study assessing myocardial siderosis by the magnetic-resonance T2* technique, deferiprone was found to be more effective than DFO in removing myocardial iron and improving heart function.4
The more marked cardioprotective effect of deferiprone could be explained by the fact that this chelator is smaller and more lipophilic than DFO, and therefore it could be more efficient than DFO in accessing intracellular chelatable iron.14 The higher peak serum levels,15 and, possibly, better compliance could also make deferiprone more effective than DFO in removing iron from myocardial cells. Better compliance afforded by the oral route could also contribute to the good results, but the lack of change of the mean ferritin levels in the 2 groups over several years does not point to improved compliance as the mechanism (or as the sole mechanism) for cardioprotection.
The protection afforded by deferiprone, however, is not absolute. Hoffbrand et al16 reported 4 fatalities among 51 iron-overloaded patients switched, because of poor compliance, from DFO to deferiprone. Their cardiac disease may possibly have been too advanced to reverse.16 In the previously mentioned Italian study of deferiprone7 that included 532 patients followed for a mean of 2.27 years (1154 patient-years), there were 11 deaths, 8 of which were due to heart failure or arrhythmias. All the patients who died of cardiac disease were severely iron overloaded (ferritin levels consistently between 2500 and 17 800 μg/L) and had a history of extremely poor compliance to deferoxamine. Two patients died before switching from DFO to deferiprone. In addition, contrary to our patients, they had cardiac disease at the initiation of deferiprone therapy (A. Ceci, personal written communication, July 2005).
In conclusion, this epidemiologic study demonstrated a significant difference in cardiac morbidity and mortality between thalassemia patients treated with deferiprone and those treated with DFO. In contrast to patients treated with DFO, the patients on this study treated with deferiprone did not have cardiac events.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-07-2933.
Supported in part by a grant from the Italian Ministry of the University and Research for projects of National Relevance 2001 (C.B-P.).
C.B.-P., M.D.C., P.D.S., and A.P. initiated and developed the study hypothesis, discussed the core ideas, designed the protocol, and participated in the collection and analysis of data and in writing the paper; M.R.G., G.C.D.V., G.L.F., M.A.R., and R.G. discussed the core ideas and participated in the study design and collection of data; H.Z. and A.C. discussed the core ideas, designed the protocol, and participated in the quality control and analysis of data and in writing the paper. H.Z. also prepared the tables and figures.
C.B-P., A.P., M.D.C., G.L.F., M.R.G., M.A.R., and G.C.D.V. have conducted research studies sponsored by Novartis Pharma. A.P. and M.R.G. have conducted research studies sponsored by Apotex, Inc, and have received support for expenses related to meetings of investigators. C.B.-P., A.P., M.D.C., G.L.F., and M.R.G. have received support for expenses related to meetings by Apotex, Inc; by Chiesi, the Italian distributor of deferiprone; e and by Novartis Inc and honoraria for presentation of results at scientific meetings. Apotex, Inc., provided support for the biostatistical analysis performed by A.C. and H.Z. but had no direct access to the data and no role in the analysis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Prof Adriana Ceci for sharing with us details on the patients reported in Ceci et al.7