Acute graft-versus-host disease (GVHD) is a major cause of morbidity and mortality in patients undergoing allogeneic bone marrow transplantation (BMT) for the treatment of leukemia and other immunogenetic disorders. The use of tolerogenic dendritic cells (DCs) that induce the generation/activation of regulatory T (Tr) cells for the treatment of acute GVHD following allogeneic BMT has been recently established. Therefore, the identification of factors that contribute to the development of tolerogenic DCs is highly relevant. We report on the use of the known immunosuppressive neuropeptide, the vasoactive intestinal peptide (VIP), as a new approach to induce tolerogenic DCs with the capacity to prevent acute GVHD. DCs differentiated in the presence of VIP impair allogeneic haplotype-specific responses of donor CD4+ cells in mice given transplants by inducing the generation of Tr cells in the graft. VIP-induced tolerogenic DCs did not abrogate the graft-versus-leukemia response presumably by not affecting the cytotoxicity of transplanted T cells against the leukemic cells. Therefore, the inclusion of VIP-induced tolerogenic DCs in future therapeutic regimens may minimize the dependence on nonspecific immunosuppressive drugs used currently as antirejection therapy, and facilitate the successful transplantation from mismatched donors, by reducing the deleterious consequences of acute GVHD and extending the applicability of BMT.
Introduction
Allogeneic bone marrow transplantation (BMT) is the treatment of choice in many hematopoietic malignancies. Following high-dose chemotherapy or irradiation, the host is reconstituted with bone marrow cells, and the donor T cells are responsible for the graft-versus-tumor (GVT) effects that eliminate the remaining malignant cells in the host. However, the same donor T cells initiate a graft-versus-host reaction. In fact, acute graft-versus-host disease (GVHD) is a major cause of morbidity and mortality in patients undergoing allogeneic BMT.1 Therefore, a desirable therapy will eliminate GVHD, without affecting the GVT response. Most therapeutic approaches designed to reduce acute GVHD have focused on the development of immunosuppressive agents and the ex vivo removal of donor T cells from the BM graft.2 However, removal of these T cells before grafting was shown to lead to transplant failure, sustained immunosuppression, and leukemia relapse.3,4
Dendritic cells (DCs) are a heterogeneous population of antigen-presenting cells (APCs) that contribute to innate immunity and initiate the adaptive immune response.5 In addition to their classical role as sentinels of the immune system, DCs also play an important role in immune homeostasis by inducing and maintaining tolerance.6 Although the tolerogenic mechanisms are not entirely understood, the maturation/activation state of DCs might be the control point for the induction of peripheral tolerance through the generation/activation of regulatory T (Tr) cells. Thus, whereas mature DCs (mDCs) are potent APCs enhancing T-cell immunity, immature DCs (iDCs) are involved in the induction of peripheral T-cell tolerance under steady-state conditions.5-9 In mice, tolerogenic DCs have been shown to prevent lethal GVHD in lethally irradiated hosts reconstituted with allogeneic BM while maintaining the GVT response.7,10-12 This emphasizes the need to develop tolerogenic DCs with a strong potential to induce Tr cells.
Vasoactive intestinal peptide (VIP), a neuropeptide released from the innervation and by Th2 cells in response to antigen stimulation and under inflammatory/autoimmune conditions, is a potent immunosuppressive agent that affects both innate and adaptive immunity.13,14 Recent studies in mice15 and human cells (see the accompanying paper by Gonzalez-Rey et al,16 beginning on page 3632) show that the presence of VIP during the early phases of DC differentiation leads to the generation of regulatory/tolerogenic DCs with the capacity to induce Tr cells and to inhibit the progression of autoimmune disorders. In this study, we investigated whether the VIP-induced regulatory/tolerogenic DCs reduce the deleterious consequences of acute GVHD following allogeneic BMT while maintaining the graft-versus-leukemia (GVL) response.
Materials and methods
Animals
C57Bl/6 (B6) (H-2b), Balb/c (H-2d), DBA/2 (H2q), [B6 × DBA/2] F1 (H2bxd) 5- to 9-week-old female mice were obtained from Iffa Credo (L'Arbresle, France) and Jackson Laboratories (Bar Harbor, ME). All animal protocols were approved by the Committee on Use and Care of Laboratory Animals at Rutgers University and CSIC.
Cell preparation
BM-DCs were generated as previously described.15 Briefly, BM cells (2 × 106) obtained from Balb/c (H-2d), C57Bl/6 (H-2b), or DBA/1 (H2q) mice were incubated in complete medium (RPMI 1640 supplemented with 100 U/mL penicillin/streptomycin, 2 mM l-glutamine, 50 mM 2-mercapto-ethanol, and 10% heat-inactivated fetal calf serum) containing 20 ng/mL GM-CSF (PreproTech, Rocky Hill, NJ) in the presence or absence of VIP (10-8 M; Calbiochem, San Diego, CA). At day 6, nonadherent cells were collected (routinely containing 80%-90% CD11c+ cells) and stimulated for 48 hours with LPS (1 μg/mL) to induce activation/maturation.
Allogeneic T cells, naive CD4+CD62L+ cells, and CD8+ cells were purified from spleen mononuclear cells obtained from normal mice (H-2b or H-2d) by positive immunomagnetic selection following the manufacturer's instructions (magnetic-activated cell sorting [MACS], Miltenyi Biotec, Auburn, CA). Purified naive CD4 and CD8 T cells (5 × 105) were exposed to allogeneic DCcontrols or DCVIPs (105). After 3 days of culture, CD4 and CD8 T cells were recovered by immuodepletion of CD11c+ DCs, rested for 3 days in complete medium supplemented with IL-2 (20 U/mL), and used as potential CD4Tr and CD8Tr cells in mice given transplants as indicated in “Models for acute GVHD and GVT response.”
Models for acute GVHD and GVT response
Allogeneic transplantation was performed by a single intravenous injection of T cell-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS; 1.5 × 107 cells/mouse) isolated from C57Bl/6 (H-2b) into recipient Balb/c (H-2d) mice lethally irradiated (10 Gy total body irradiation [TBI] from a 200-Kv x-ray source). In addition, GVHD was induced in irradiated recipient C57Bl/6 mice by allogeneic transplantation of Balb/c BMS (1.5 × 107 cells/mouse). GVHD was also induced in [B6 × DBA/2] F1 (H-2b/d) recipients by transplantation of BMS (1.5 × 107 cells/mouse) isolated from C57Bl/6 (H-2b). GVT was induced by injecting intravenously A20 leukemic cells (derived from Balb/c mice, 104 cells) or P815 mastocytoma cells (derived from DBA/2 mice, 104 cells) into irradiated Balb/c mice at the time of BMS transplantation (H-2b BMS, supplemented with different numbers of spleen mononuclear cells: 1.5 × 106, 5 × 105, or 1 × 105 cells). Recipients received single or repetitive intravenous injections of different numbers of host-matched or host-mismatched DCcontrols or DCVIPs (H-2b, H-2d, or H-2q), 2 or 5 days (or both) after transplantation. Alternatively, recipients received different numbers of Trcontrols or TrVIP cells (H-2b or H-2d) at the time of BMS transplantation. Recipients were monitored once every day from the day of transplantation until they died naturally of GVHD or tumor burden to determine survival time and body weight. Tumor growth/elimination was assessed by the presence of A20 cells in blood detected by coexpression of B220 and H-2Kd and by their large size using flow cytometry. In other experiments, serum and spleen cells were harvested at different times following transplantation. For mice bearing P815 tumor cells the liver and spleen were weighed at the time of death or 60 days after transplantation.
Where mentioned, the transplant recipients received intravenous injections of anti-CD25 monoclonal antibody (mAb), neutralizing anti-IL-10 polyclonal antibody (Ab), neutralizing anti-TGFβ mAb, or preimmune rat IgG (500 μg Ab/mouse) every other day up to 15 days after transplantation.11 To trace the injected cells in vivo, DCcontrols and DCVIPs were labeled with 5,6-carboxy-succimidyl-fluoresceine-ester (CFSE; Molecular Probes, Eugene, OR) and injected intravenously, and their presence was determined in spleen by flow cytometry.
In vivo priming and characterization of grafted T cells in mice given transplants
Following transplantation of H-2d recipients with H-2b BM plus BMS cells, we isolated splenic I-KbCD4+ and I-KbCD8+ T cells by immunomagnetic selection as described.11 Briefly, spleen mononuclear cells obtained 5 days after transplantation were depleted of recipient cells by incubation with anti-I-Kd mAb followed by goat anti-mouse IgG mAb-conjugated immunomagnetic beads. Donor-derived CD4 and CD8 T cells were selected from the isolated donor-I-Kb cells by using anti-CD8 mAb or anti-CD4 mAb (BD PharMingen, San Diego, CA), followed by sheep anti-rat IgG mAb-conjugated immunomagnetic beads. The T-cell preparations were typically more than 98% pure as indicated by flow cytometry. Donor I-KbCD4+ cells (5 × 105) were cultured with medium alone (none) or with mature DCs (H-2d) (5 × 104) in the presence or absence of IL-2 (20 U/mL), and the proliferative response was determined by using a cell proliferation assay (BrdU) from Roche Diagnostics (Mannheim, Germany). Cytokine contents in the culture supernatants were determined by specific sandwich enzyme-linked immunosorbent assays (ELISAs) using capture/biotinylated-detection Abs from BD PharMingen. Donor I-KbCD8+ cells were assayed for cytotoxicity against tumor cells as described in “Cytotoxicity assay.”
Flow cytometry
Cells were incubated with various PerCP-, FITC-, and PE-labeled mAbs (BD PharMingen) diluted at optimal concentration for immunostaining, fixed in 1% paraformaldehyde, and analyzed on a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA). We used isotype-matched Abs as controls and IgG block (Sigma, St Louis, MO) to avoid nonspecific binding to Fc-receptors. For analysis of intracellular CTLA4, cells were stained first for surface CD4 with PerCP-anti-CD4, fixed with Cytofix/Cytoperm solution (BD PharMingen), incubated with PE-anti-CTLA4 mAb diluted in 0.5% saponin, and analyzed by flow cytometry. For intracellular cytokine analysis isolated CD4 T cells (106 cells/mL) were stimulated with PMA (1 ng/mL) plus ionomycin (20 ng/mL) for 8 hours. Monensin (1.33 μmol/mL) was added for the last 4 hours of culture. Cells were stained with PerCP-anti-CD4 mAbs for 30 minutes at 4°C, washed, fixed/saponin permeabilized with Cytofix/Cytoperm, and stained with 0.5 μg/sample of FITC- and PE-conjugated anti-IL-2-, anti-IFN-γ-, anti-IL-4-, or anti-IL-10-specific mAbs for 45 minutes at 4°C. Cells were analyzed by flow cytometry.
Cytotoxicity assay
In vivo primed CD8 T cells were cultured with Na251CrO4-labeled (100 μCi [3.7 MBq]/106 cells, NEN Life Science Products, Boston, MA) P815, EL4, or A20 cells (104) for 4 hours at various effector-to-target cell ratios (E/T ratios). The radioactivity released in the supernatants was measured, and the percent specific lysis was calculated.11
Statistical analysis
Differences in survival of treatment groups were analyzed using the log-rank test. Differences in proliferation and cytokine production by cultures, serum cytokine levels, and percentage of cells were analyzed using the 2-tailed Student t test. A P value below .01 was considered significant.
Results
DCVIPs protect from acute GVHD
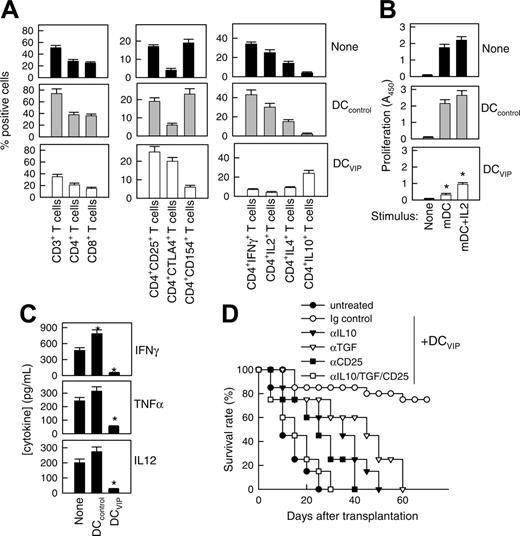
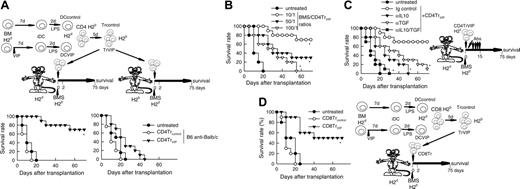
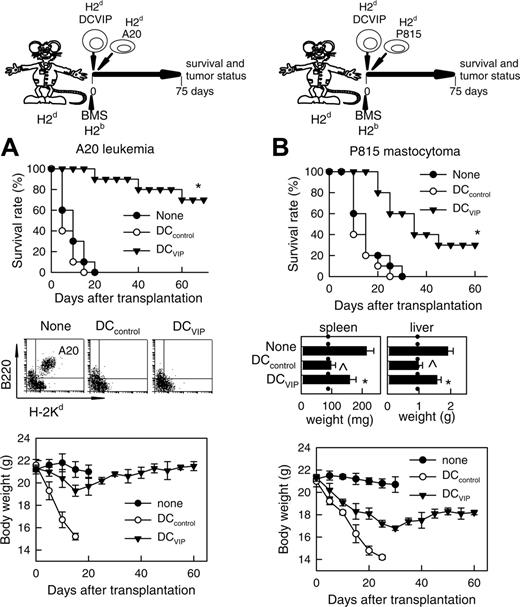
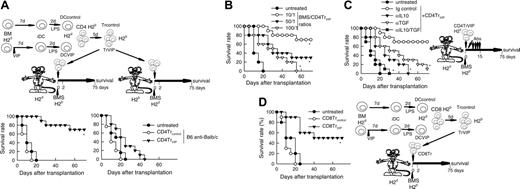
We reported recently that the presence of VIP in the early differentiation stages of murine BM-derived DCs and of human monocyte-derived DCs results in the generation of DCs with a regulatory/tolerogenic phenotype.15,16 DCs differentiated in the presence of VIP (DCVIPs) are CD11cloCD45RBhiCD4-CD8-B220-, do not up-regulate CD40, CD80, and CD86, and do not express TNF-α and IL-12 following LPS or other inflammatory stimulation (not shown). In contrast to DCcontrols, DCVIPs secreted high amounts of IL-10 and exhibited a very poor stimulatory activity for allogeneic CD4 T cells (not shown). In addition, murine CD4 T cells primed with allogeneic DCVIPs exhibit a Tr1-like phenotype, characterized by IL-10 and TGF-β, but not IL-2 and IFN-γ production, and efficiently suppress the proliferative response of syngeneic responder CD4 T cells cocultured with allogeneic mDCs.15,17 Therefore, we examined the potential therapeutic effect of DCVIPs in acute GVHD following allogeneic BMT. We transplanted either T cell-depleted BM cells or T-depleted BMS from C57Bl/6 (H-2b) mice into lethally irradiated Balb/c recipients (H-2d). Following transplantation, we injected DCcontrols or DCVIPs generated from Balb/c BM cells. Mice given T cell-depleted BM appeared healthy, and 100% of the animals survived for at least 75 days (not shown). Mice that received BMS developed severe signs of GVHD, including weight loss, reduced mobility, hunched posture, diarrhea, and ruffled fur, and died within 25 days (Figure 1A). Whereas treatment with DCcontrols enhanced GVHD lethality, the administration of host major histocompatibility complex (MHC)-matched DCVIPs (H2d) protected from lethal GVHD, and more than 70% of the mice survived for more than 75 days (Figure 1A). The therapeutic effect was dose-dependent for recipients receiving a single injection with DCVIPs, and repetitive injections of lower doses of DCVIPs (2 and 5 days after transplantation) enhanced the survival rate (Figure 1B). In contrast to the therapeutic effect of the host MHC-matched DCVIPs, the injection of host-mismatched DCVIPs following BMT failed to protect from acute GVHD (Figure 1C). The window of opportunity for DCVIPs administration (2 × 106 cells/mouse) was apparently short, however, because delaying this injection further until day 5 after BMT reduced the therapeutic effect (10% survival). Higher levels of protection (60% survival) were obtained with 6 × 106 DCVIPs administered on day 5 (not shown). The therapeutic window of DCVIPs was significantly increased with a haploidentical model of GVHD, a model closer to human applications. In this model, lethally irradiated [B6 × DBA/2] F1 (H-2bxd) mice were given transplants of B6 BMS (H-2b) and treated with DCcontrols or DCVIPs at different times after BMT. DCVIPs were more efficient preventing lethality in this model than in a full H-2 mismatch transplant and showed significant therapeutic effect when administered 10 days after BMT (Figure 1D).
DCs differentiated with VIP protect from acute GVHD. (A-B) GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of T-depleted BM cells plus spleen mononuclear cells (BMS) from B6 (H-2b) mice. After transplantation, recipients were given injections of medium (untreated) or BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Untreated mice (•) or mice treated with 2 × 106 DCcontrols (○) or DCVIPs (▾) 2 days after transplantation (12 mice/group). *P < .01, versus untreated recipients. (B) Untreated mice (•) or DCVIPs-treated mice (with 2 × 106 cells, ○;2 × 105, ▾; 2 × 104, ▿, at day 2 after transplantation; or with 2 × 105, ▪; 2 × 104, □, at days 2 and 5 after transplantation; 10-12 mice/group). P < .01 untreated recipients versus any other group. (C) GVHD was induced in recipient B6 (H-2b) mice by allogeneic transplantation of BMS Balb/c (H-2d). Two days after transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCVIPs from different donors (H-2b ○, H-2d ▾, H-2q ▿; 10-12 mice/group). *P < .01 versus untreated recipients. (D) GVHD was induced in a haploidentical model in recipient [B6 × DBA/2] F1 (H-2b/d) by allogeneic transplantation of BMS B6 (H-2b). After transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCcontrols (H-2d, ○) or DCVIPs DBA/2 (H-2d) at day 0 (▾), day 2 (▿) or day 10 (▪) (10-12 mice/group). *P < .01 versus untreated recipients.
DCs differentiated with VIP protect from acute GVHD. (A-B) GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of T-depleted BM cells plus spleen mononuclear cells (BMS) from B6 (H-2b) mice. After transplantation, recipients were given injections of medium (untreated) or BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Untreated mice (•) or mice treated with 2 × 106 DCcontrols (○) or DCVIPs (▾) 2 days after transplantation (12 mice/group). *P < .01, versus untreated recipients. (B) Untreated mice (•) or DCVIPs-treated mice (with 2 × 106 cells, ○;2 × 105, ▾; 2 × 104, ▿, at day 2 after transplantation; or with 2 × 105, ▪; 2 × 104, □, at days 2 and 5 after transplantation; 10-12 mice/group). P < .01 untreated recipients versus any other group. (C) GVHD was induced in recipient B6 (H-2b) mice by allogeneic transplantation of BMS Balb/c (H-2d). Two days after transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCVIPs from different donors (H-2b ○, H-2d ▾, H-2q ▿; 10-12 mice/group). *P < .01 versus untreated recipients. (D) GVHD was induced in a haploidentical model in recipient [B6 × DBA/2] F1 (H-2b/d) by allogeneic transplantation of BMS B6 (H-2b). After transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCcontrols (H-2d, ○) or DCVIPs DBA/2 (H-2d) at day 0 (▾), day 2 (▿) or day 10 (▪) (10-12 mice/group). *P < .01 versus untreated recipients.
DCVIPs impair allogeneic antigen-specific responses of donor CD4+ T cells in mice that have received transplants by inducing the generation of Tr cells in the graft
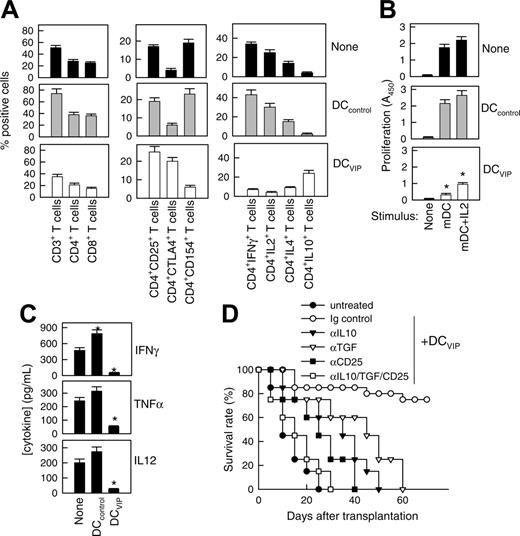
A hallmark of acute GVHD is the expansion of alloreactive T cells. Disease progression is characterized by the differentiation of alloreactive CD4+ and CD8+ T cells into effector cells leading to tissue damage, recruitment of additional inflammatory cells, and further cytokine unbalance.2-4 We therefore investigated whether DCVIPs regulate the differentiation of GVHD-causing alloreactive T-effector cells in the grafted mice. First we examined the subpopulations of transplanted I-Kb T cells and their ability to produce cytokines in DCcontrols or DCVIPs-treated recipients (H-2d) (Figure 2A). Inoculation of DCVIPs decreased the number of CD3+, CD4+, and CD8+ I-Kb donor-derived T cells, reduced the percentage of activated IL-2/IFN-γ-producing CD154+ (CD40L) Th1 cells, and increased the number of regulatory IL-10-producing CTLA4+ T cells in the I-Kb CD4+ T-cell population (Figure 2A). I-Kb CD4+ T cells obtained from untreated or DCcontrols-treated mice that had received transplants responded vigorously to allogeneic mDCs (H-2d). In contrast, I-KbCD4+ T cells from DCVIPs-treated recipients were hyporesponsive, and the addition of IL-2 partially restored this response (Figure 2B). We further examined the serum levels of inflammatory cytokines. DCVIPs treatment reduced the levels of the proinflammatory cytokines IFN-γ, TNF-α, and IL-12 in the serum of grafted mice (Figure 2C). These data indicate that the treatment of mice given transplants with DCVIPs reduced the number/activation of transplanted Th1 cells, the inflammatory response against the recipient tissue, and the subsequent GVHD lethality, and could be involved in the generation of Tr cells. This correlates with the fact that DCVIPs induce in vitro the generation of IL-10/TGF-β-producing regulatory CD4+CD25+CTLA4+ cells.15,17 Therefore, we further examined the role of Tr cells in the therapeutic effect of DCVIPs on acute GVHD. In vivo blockade experiments showed that treatment with anti-CD25, anti-IL-10, or anti-TGF-β Abs significantly decreased survival rates, and treatment with all 3 Abs abrogated the survival effect exerted by DCVIPs (Figure 2D).
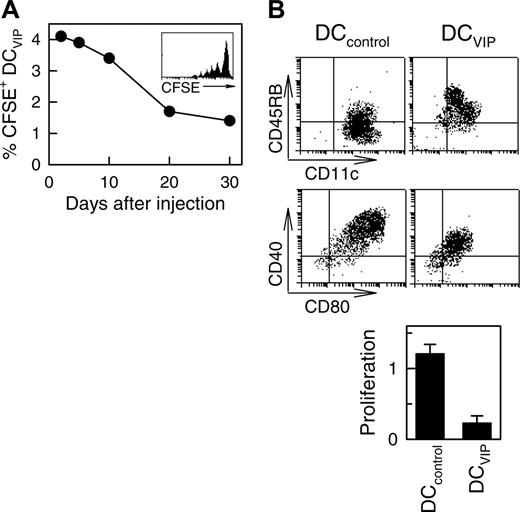
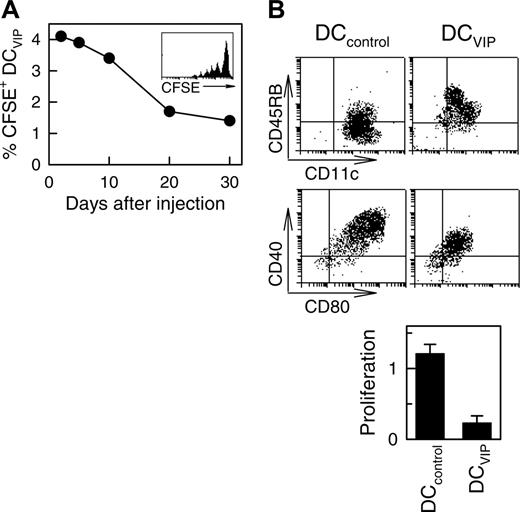
To better understand the half-life, stability, and trafficking of the infused DCVIPs, we injected CFSE-labeled DCs into mice given transplants. As previously described for other tolerogenic DCs,11 we detected the inoculated DCVIPs in the spleen of the recipients the next day after transplantation, with a half-life of about 17 days and some degree of proliferation (Figure 3A). In addition, DCVIPs showed little or no change in phenotype and in the capacity to induce anergic T cells following in vivo infusion (Figure 3B). These results indicate that in vivo DCVIPs remain viable and tolerogenic even in inflammatory conditions and that the long-term persistence of host MHC-matched DCVIPs might be crucial for their therapeutic efficacy. Importantly, regulation of GVHD by DCVIPs did not affect the establishment of lasting donor chimerism because donor I-KbCD4+ and I-KbCD8+ T cells are detected in spleens of recipient mice at the conclusion of the experiment at day 80 (not shown).
DCVIPs impair allogeneic antigen-specific responses of donor CD4+ T cells in transplanted mice by inducing the generation of Tr cells in the graft. GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. Two days after transplantation, recipients were given injections of medium (none) or BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Donor I-Kb cells isolated from recipient spleens 5 days after transplantation were assayed for phenotype and cytokine profile by flow cytometry. Data are expressed as percentage positive cells (n = 8). P < .01 untreated and DCcontrols recipients versus DCVIPs group for any T-cell population. (B) Donor I-KbCD4+ cells isolated from recipient spleens were cultured with medium alone (none) or with mature DC (H-2d) in the presence or absence of IL-2 at a T-cell/DC ratio of 10:1, and the proliferative response was determined (n = 8). *P < .01 versus untreated recipients. (C) Cytokine concentrations in sera obtained 5 days after transplantation were assayed by ELISA (n = 8). *P < .01 versus untreated recipients. (D) Untreated recipients (•) or recipients that were given injections of DCVIPs and treated with control immunoglobulin (○), anti-IL-10 (▾), anti-TGF-β (▿), or anti-CD25 mAbs (▪), or a combination of all 3 mAbs (□; 12 mice/group). P < .01, control immunoglobulin-treated DCVIPs recipients versus any other group.
DCVIPs impair allogeneic antigen-specific responses of donor CD4+ T cells in transplanted mice by inducing the generation of Tr cells in the graft. GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. Two days after transplantation, recipients were given injections of medium (none) or BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Donor I-Kb cells isolated from recipient spleens 5 days after transplantation were assayed for phenotype and cytokine profile by flow cytometry. Data are expressed as percentage positive cells (n = 8). P < .01 untreated and DCcontrols recipients versus DCVIPs group for any T-cell population. (B) Donor I-KbCD4+ cells isolated from recipient spleens were cultured with medium alone (none) or with mature DC (H-2d) in the presence or absence of IL-2 at a T-cell/DC ratio of 10:1, and the proliferative response was determined (n = 8). *P < .01 versus untreated recipients. (C) Cytokine concentrations in sera obtained 5 days after transplantation were assayed by ELISA (n = 8). *P < .01 versus untreated recipients. (D) Untreated recipients (•) or recipients that were given injections of DCVIPs and treated with control immunoglobulin (○), anti-IL-10 (▾), anti-TGF-β (▿), or anti-CD25 mAbs (▪), or a combination of all 3 mAbs (□; 12 mice/group). P < .01, control immunoglobulin-treated DCVIPs recipients versus any other group.
GVL activity is maintained in the presence of DCVIPs
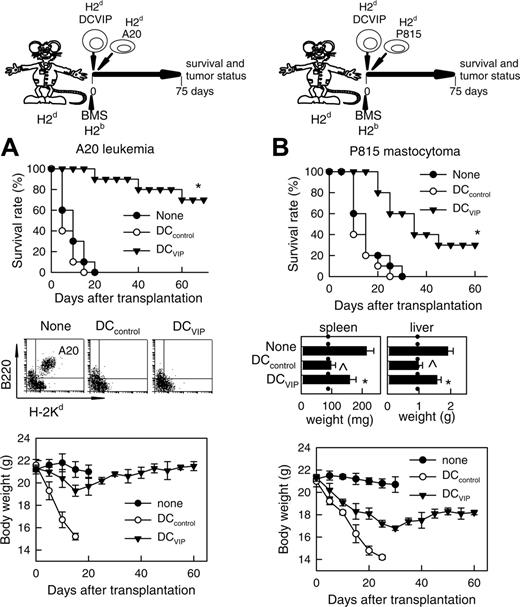
The current preclinical immunosuppressive therapies for the prevention of GVHD fail to control the balance between the anti-GVHD effect and the beneficial GVT activity of the allogeneic BM transplants. We therefore tested whether the GVT effects in the recipients could be maintained in the presence of DCVIPs. Balb/c mice (H-2d) receiving leukemic (H-2d) A20 cells died within 20 days from leukemia, as attested by the presence of leukemic cells in the blood (Figure 4A). Similarly, Balb/c mice (H-2d) receiving P815 mastocytoma (H-2d) cells died within 30 days, as attested by the presence of growing tumors at the site of injection and hepatosplenomegaly (Figure 4B). Mice given transplants with BMS (H-2b) together with A20 or P815 cells, and treated with or without DCcontrols (H-2d), died with clinical signs characteristic of GVHD and marked body weight loss, although leukemic cells could not be detected in blood and developing tumors at the site of injection and hepatosplenomegaly were not observed, attesting to an efficient GVT effect (Figure 4). In contrast, most of the leukemia-bearing mice given transplants with DCVIPs were still alive on day 70 (Figure 4A). Compared to the 2 control groups, the presence of DCVIPs protected mice from lethal GVHD, whereas the GVL activity was maintained. Indeed, leukemic cells could not be detected in these mice, except for one animal that died at day 40. A20-bearing mice given transplants with BMS and with host-mismatched DCVIPs succumbed to GVHD, whereas GVT response was maintained intact (not shown), indicating the requirement for the haplotype-specificity of DCVIPs. Treatment of leukemia-bearing mice with host-matched DCVIPs and no BMS or with T cell-depleted BM cells did not affect tumor growth (not shown), indicating that graft allogeneic T cells are responsible for GVT effect. Interestingly, although acute GVHD was completely prevented, DCVIPs were less efficient in maintaining GVT response in mastocytoma-bearing mice compared to A20 leukemia, as assessed by a significant hepatosplenomegaly and body weight loss in the P815 recipients (Figure 4B). However, GVT maintenance by DCVIPs was not exclusive for A20 cells because a similar GVT response was observed in a BCL1 lymphoma model (not shown).
Treatment with DCVIPs does not abrogate the cytotoxicity of transplanted T cells against leukemic cells
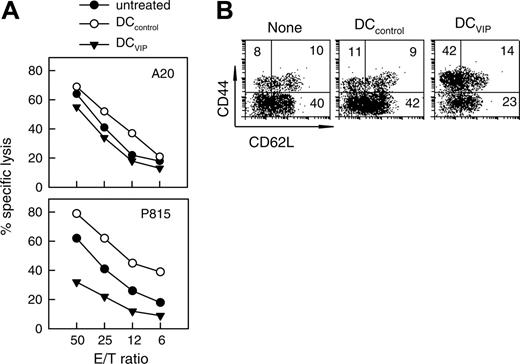
To investigate the mechanisms through which DCVIPs prevent GVHD in mice receiving transplants while maintaining an effective GVT response, we first determined whether the DCVIPs treatment affects the cytotoxicity of transplanted T cells against tumor cells. I-KbCD8+ T cells isolated from DCVIPs-treated recipients exhibit potent lytic activity against A20 cells (H-2d), similar to the CD8 T cells obtained from untreated or DCcontrols-treated recipients (Figure 5A). In contrast, I-KbCD8+ T cells isolated from DCVIPs-treated recipients showed reduced cytotoxic activity against the mastocytoma P815 cells (H-2d) (Figure 5A). In addition, there was no lytic activity of the I-KbCD8+ T cells against EL4 cells (H-2b), indicating their H-2d-specific cytotoxicity (not shown).
Long-term survival of DCVIPs in transplanted mice. Balb/c (H-2d) mice received transplants of BMS from B6 (H-2b) mice. CFSE-labeled DCVIPs were injected 2 days after transplantation. (A) The presence of CFSE-labeled DCVIPs in recipient spleens was determined at the indicated times by flow cytometry (n = 3). Inset: Histogram represents CFSE-labeling profile at day 5 after injection. (B) Ten days after injection, spleen CFSE-labeled DCVIPs were sorted, stimulated with LPS (1 μg/mL) for 24 hours, and analyzed for CD11c, CD40, CD45RB, and CD80 expression by flow cytometry. Sorted CFSE-labeled DCVIPs (104 cells) were incubated with allogeneic T cells (5 × 104) from B6 mice and proliferation was determined (n = 3). LPS-matured DCs (DCcontrols) were used as controls.
Long-term survival of DCVIPs in transplanted mice. Balb/c (H-2d) mice received transplants of BMS from B6 (H-2b) mice. CFSE-labeled DCVIPs were injected 2 days after transplantation. (A) The presence of CFSE-labeled DCVIPs in recipient spleens was determined at the indicated times by flow cytometry (n = 3). Inset: Histogram represents CFSE-labeling profile at day 5 after injection. (B) Ten days after injection, spleen CFSE-labeled DCVIPs were sorted, stimulated with LPS (1 μg/mL) for 24 hours, and analyzed for CD11c, CD40, CD45RB, and CD80 expression by flow cytometry. Sorted CFSE-labeled DCVIPs (104 cells) were incubated with allogeneic T cells (5 × 104) from B6 mice and proliferation was determined (n = 3). LPS-matured DCs (DCcontrols) were used as controls.
GVT response is maintained in the presence of DCVIPs. GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. GVT effect was induced by injecting A20 leukemic (H-2d) cells (A) or P815 mastocytoma (H-2d) cells (B) into recipient mice at time of BMS transplantation. Recipients were treated with DCcontrols (○) or DCVIPs (▾) obtained from Balb/c (H-2d) mice at time of transplantation. Mice given injections of A20 or P815 cells alone were used as controls (none, •). Recipients were monitored for survival and body weight. Liver and spleen weights were determined from representative recipients at time of death or 60 days after transplantation (dotted vertical lines correspond to spleen and liver weights of normal Balb/c mice). Leukemia growth/elimination was assessed by the presence of A20 cells in blood detected by coexpression of B220 and H-2Kd and by size (5-10 mice/group). *P < .01 versus untreated recipients. P < .01 versus DCVIPs group.
GVT response is maintained in the presence of DCVIPs. GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. GVT effect was induced by injecting A20 leukemic (H-2d) cells (A) or P815 mastocytoma (H-2d) cells (B) into recipient mice at time of BMS transplantation. Recipients were treated with DCcontrols (○) or DCVIPs (▾) obtained from Balb/c (H-2d) mice at time of transplantation. Mice given injections of A20 or P815 cells alone were used as controls (none, •). Recipients were monitored for survival and body weight. Liver and spleen weights were determined from representative recipients at time of death or 60 days after transplantation (dotted vertical lines correspond to spleen and liver weights of normal Balb/c mice). Leukemia growth/elimination was assessed by the presence of A20 cells in blood detected by coexpression of B220 and H-2Kd and by size (5-10 mice/group). *P < .01 versus untreated recipients. P < .01 versus DCVIPs group.
Recently it has been reported that DC-activated CD8+CD44high T cells are poor inducers of acute GVHD, whereas CD8+CD44lowCD62Llow T cells are extremely potent. We investigated whether DCVIPs induce the generation of CD8+CD44high or CD44low T cells. The donor I-KbCD8+ T cells isolated from mice that received trasnplants treated with DCcontrols showed a high percentage of CD44low cells similar to untreated animals (Figure 5B). Treatment with DCVIPs significantly increased the number of CD44highCD62Llow cells in the grafted CD8+ T cells (Figure 5B). Therefore, DCVIPs could be inducing/activating CD8+CD44highCD62Llow T cells in the graft that fail to generate GVHD while maintaining effective GVT activity.
DCVIPs-induced Tr cells prevent acute GVHD while maintaining GVL response
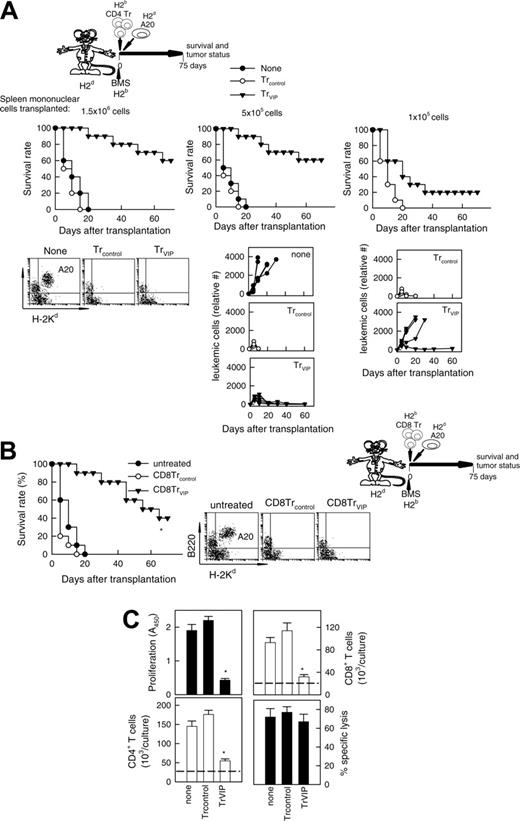
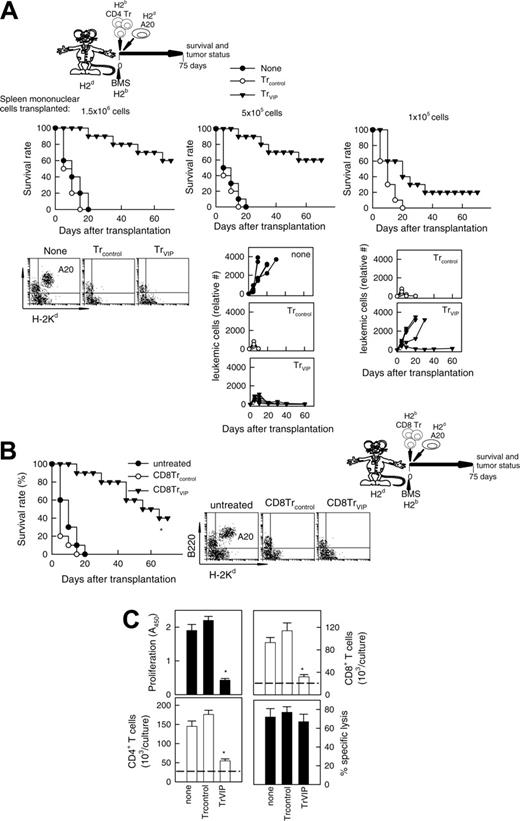
In certain circumstances, the successful suppression of an alloreactive response might require high numbers of Tr cells, and the in vivo administration of DCVIPs might not be sufficient for a complete and rapid suppression. Therefore, we decided to generate DCVIPs-induced Tr cells in vitro and to determine their suppressive capacity in vivo both in a model of BMT. We generated CD4TrVIPs through the stimulation of H-2b CD4 T cells with H-2d DCVIPs. CD4Trcontrols were generated in the same manner with DCcontrols. Administration of CD4TrVIPs, but not CD4Trcontrols, to H-2d mice given transplants with H-2b allogeneic BMS significantly increased survival by preventing GVHD (Figure 6A left panel). The effect was haplotype-specific because mice (H-2b) given transplants with BMS (H-2d) and treated with CD4TrVIPs (H-2b) generated with DCVIPs (H-2d) succumbed to GVHD (Figure 6A right panel). The therapeutic effect of CD4TrVIPs was dose-dependent (Figure 6B) and mediated mainly through TGF-β and IL-10 because since in vivo administration of anti-IL-10 or anti-TGF-β Abs abrogated the protective effect (Figure 6C). These findings suggest that DCVIPs induce haplotype-specific CD4Tr cells, which suppress the deleterious antihost activity of the grafted alloreactive T cells.
We have recently demonstrated that VIP-differentiated human tolerogenic DCs generate CD8Tr cells, which regulate the function of allogeneic Th1 cells.16 Therefore, the generation of regulatory CD8 T cells could also be involved in the DCVIPs prevention of GVHD. We investigated the effect of murine CD8 T cells (H-2b) exposed in vitro to allogeneic DCcontrols or DCVIPs (H-2d) in the prevention of GVHD. Whereas CD8Trcontrols did not show any beneficial effect, the treatment of BMS (H-2b) transplanted mice (H-2d) with CD8TrVIPs (H-2b) significantly prevented lethal GVHD (Figure 6D). Similar to CD4TrVIPs, this effect was haplotype-specific because CD8TrVIPs (H-2b) showed little or no effect (not shown).
We investigated next whether DCVIPs-induced CD4 and CD8 Tr cells can prevent GVHD while maintaining GVT response. The tumor-bearing control group receiving neither irradiation nor undergoing allogeneic BMT died from progressive leukemia (Figure 7A none). After cotransplantation of BMS and CD4Trcontrols, all animals died within 20 days from severe GVHD without signs of tumor relapse before their GVHD death (Figure 7A). In contrast, when leukemic-bearing mice were given cotransplants with allogeneic BMS together with CD4TrVIPs, recipients were protected from GVHD, and none of them had a relapse from A20 leukemia (Figure 7A left and middle panels). Similar results were obtained in the BCL1 model (not shown). Transplanted allogeneic T cells are responsible for both GVHD and GVT effect. In the absence of T cells (T-depleted BM transplants) there is no GVHD but no elimination of tumor cells either, even in the presence of CD4TrVIPs (not shown). Also, if the graft contains a limited number of allogeneic T cells, CD4 TrVIPs suppress GVHD but tumor relapses occur in most animals (Figure 7A, right panel). CD8 TrVIPs behave similar to CD4TrVIPs (Figure 7B).
Treatment with DCVIPs does not abrogate cytotoxicity of transplanted T cells against leukemic cells. GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. Two days after transplantation, recipients were given injections of medium (none) or with BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Donor I-KbCD8+ cells isolated from recipient spleens (untreated, •; DCcontrols-treated, ○; DCVIPs-treated, ▾) were subjected to cytotoxicity assays against leukemia A20 (H-2d) and mastocytoma P815 (H-2d) cells (n = 8). (B) Donor I-KbCD8+ cells isolated from recipient spleens were analyzed for CD44 and CD62L expression by flow cytometry. Numbers represent the percentage of cells in each quadrant. Result is representative of 8 identical experiments.
Treatment with DCVIPs does not abrogate cytotoxicity of transplanted T cells against leukemic cells. GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. Two days after transplantation, recipients were given injections of medium (none) or with BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Donor I-KbCD8+ cells isolated from recipient spleens (untreated, •; DCcontrols-treated, ○; DCVIPs-treated, ▾) were subjected to cytotoxicity assays against leukemia A20 (H-2d) and mastocytoma P815 (H-2d) cells (n = 8). (B) Donor I-KbCD8+ cells isolated from recipient spleens were analyzed for CD44 and CD62L expression by flow cytometry. Numbers represent the percentage of cells in each quadrant. Result is representative of 8 identical experiments.
Tr cells generated with DCVIPs prevent acute GVHD. DCs were generated from mouse BM cells in the absence (DCcontrols) or presence (DCVIPs) of VIP and activated with LPS to induce DC maturation. CD4 and CD8 T cells (H-2b) were exposed to allogeneic (H-2d)DCcontrols or DCVIPs to generate potential Tr cells (Trcontrols and TrVIPs). (A) Balb/c mice (H-2d) received transplants of T-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from B6 (H-2b) mice (left panel) and B6 mice (H-2b) received transplants of T cell-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from Balb/c (H-2d) mice (right panel). Medium (untreated, •), CD4Trcontrols (○) or CD4TrVIPs (▾) (H-2b) were injected (1.5 × 106 cells/mouse) in both recipients 2 days after allogeneic transplantation, and survival was monitored (10 animals/group). *P < .01 versus untreated recipients. (B) The effect of CD4TrVIPs is dose-dependent. Balb/c mice (H-2d) received transplants of T-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from B6 (H-2b) mice and CD4TrVIPs (H-2b) were injected in recipients together with transplantation at different numbers: 1.5 × 106 cells, BMS/TrVIPs ratio of 10:1 (○); 3 × 105 cells, BMS/TrVIPs ratio 50:1 (▾); or 1.5 × 105 cells, BMS/TrVIPs ratio 100:1 (▿). Untreated control mice (•, n = 10). *P < .01 versus untreated recipients. (C) Balb/c mice that were (•) or that had received injections of CD4TrVIPs and been treated with control immunoglobulin (○), anti-IL-10 (▾), anti-TGF-β (▿), or anti-IL-10 plus anti-TGFβ (▪) antibodies after BMS B6 transplantation (n = 10). *P < .01 versus control immunoglobulin-treated CD4TrVIPs recipients. (D) Balb/c mice (H-2d) were given transplants of T cell-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from B6 (H-2b) mice. Recipients were treated with medium (untreated, •), CD8Trcontrols (○) or CD8TrVIPs (▾) (5 × 106 cells) at time of allogeneic transplantation and survival was monitored (5 animals/group). *P < .01 versus untreated recipients.
Tr cells generated with DCVIPs prevent acute GVHD. DCs were generated from mouse BM cells in the absence (DCcontrols) or presence (DCVIPs) of VIP and activated with LPS to induce DC maturation. CD4 and CD8 T cells (H-2b) were exposed to allogeneic (H-2d)DCcontrols or DCVIPs to generate potential Tr cells (Trcontrols and TrVIPs). (A) Balb/c mice (H-2d) received transplants of T-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from B6 (H-2b) mice (left panel) and B6 mice (H-2b) received transplants of T cell-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from Balb/c (H-2d) mice (right panel). Medium (untreated, •), CD4Trcontrols (○) or CD4TrVIPs (▾) (H-2b) were injected (1.5 × 106 cells/mouse) in both recipients 2 days after allogeneic transplantation, and survival was monitored (10 animals/group). *P < .01 versus untreated recipients. (B) The effect of CD4TrVIPs is dose-dependent. Balb/c mice (H-2d) received transplants of T-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from B6 (H-2b) mice and CD4TrVIPs (H-2b) were injected in recipients together with transplantation at different numbers: 1.5 × 106 cells, BMS/TrVIPs ratio of 10:1 (○); 3 × 105 cells, BMS/TrVIPs ratio 50:1 (▾); or 1.5 × 105 cells, BMS/TrVIPs ratio 100:1 (▿). Untreated control mice (•, n = 10). *P < .01 versus untreated recipients. (C) Balb/c mice that were (•) or that had received injections of CD4TrVIPs and been treated with control immunoglobulin (○), anti-IL-10 (▾), anti-TGF-β (▿), or anti-IL-10 plus anti-TGFβ (▪) antibodies after BMS B6 transplantation (n = 10). *P < .01 versus control immunoglobulin-treated CD4TrVIPs recipients. (D) Balb/c mice (H-2d) were given transplants of T cell-depleted BM cells supplemented with 1.5 × 106 spleen mononuclear cells (BMS, 1.5 × 107 cells/mouse) from B6 (H-2b) mice. Recipients were treated with medium (untreated, •), CD8Trcontrols (○) or CD8TrVIPs (▾) (5 × 106 cells) at time of allogeneic transplantation and survival was monitored (5 animals/group). *P < .01 versus untreated recipients.
These results suggest that CD4/CD8 TrVIPs inhibit haplotype-matched mature T cells present in the BM graft from initiating an alloreactive response against the host, probably by suppressing the massive expansion of alloreactive T cells, thereby permitting the cotransplantation of sufficient numbers of T cells for tumor eradication. We investigated next whether CD4TrVIPs affect CD4/CD8 cell expansion and CD8 lytic activity. In contrast to CD4Trcontrols, CD4TrVIPs inhibit the proliferation of T cells in response to allogeneic mDCs, affecting the expansion of both effector CD4 and CD8 T cells (Figure 7C). However, CD4TrVIPs did not suppress the cytotoxic activity of the CD8 T cells against tumor cells (Figure 7C).
Discussion
Allogeneic BMT is the treatment of choice for many hematologic malignancies and primary immunodeficiencies. GVHD is a life-threatening and frequent complication of allogeneic BMT, due to mature donor T cells present in the transplant. It has been proposed recently to use tolerogenic DCs as a therapeutic strategy to limit the pathologic effect of donor-alloreactive T cells.7,10 Although underlying mechanisms are not fully elucidated, the capacity to induce Tr cells is an important property of tolerogenic/regulatory DCs.6-12 In this study, we established a novel immunotherapeutic approach using the neuropeptide VIP to induce tolerogenic DCs that prevent acute GVHD while maintaining GVT effect. We previously showed that the VIP presence during the early stages of DC differentiation from human blood monocytes or from mouse BM cells leads to the generation of DCs that cannot mature following inflammatory stimuli. The DCVIPs exhibit a tolerogenic phenotype characterized by low expression of costimulatory molecules, low production of proinflammatory cytokines, and increased production of IL-10.15-17 The stimulation of T cells with allogeneic DCVIPs induced CD4 and CD8 T cells that display the typical properties of Tr1-like cells, including a characteristic cytokine profile (high IL-10 and TGF-β, and little or no IFN-γ, IL-2, or IL-4), intrinsic low proliferative capacity, and suppression of the antigen-specific proliferation/activation of other CD4 T cells. The present study shows that the treatment of recipients of allogeneic BM transplants with DCVIPs prevents lethal GVHD, even when administered after disease onset. Sequential administration of DCVIPs enhances their therapeutic efficacy. A hallmark of acute GVHD is the alloreactive T-cell expansion in the inflammatory environment. DCVIPs retain their capacity to induce Tr in vivo under inflammatory conditions, including autoimmune diseases,15 allograft rejection and acute GVHD. The in vivo efficacy of DCVIPs depends on their compatibility with the host MHC antigens. We found that DCVIPs directly suppress not only the effector functions of in vivo primed allogeneic CD4 T cells but also their responsiveness to in vitro restimulation. Therefore, the mechanism responsible for the DCVIPs therapeutic effect in acute GVHD could involve the induction of tolerant Tr cells as well as direct suppression of effector T cells in vivo. DCVIPs treatment of mice receiving BM transplants reduced the number of alloreactive CD4+CD154+ Th1 effectors, whereas increasing the levels of CD4+CD25+CTLA4+IL-10+ Tr1-like cells within the grafted T-cell population. CTLA4, TGF-β, and IL-10 are characteristic markers of Tr cells and mediators of their regulatory functions.18-24 The fact that in vivo TGF-β/IL-10 blockade and CD25+ cell deletion reversed the therapeutic effect of DCVIPs in GVHD confirmed the partial involvement of newly generated Tr cells. In agreement with this hypothesis, DCVIPs in vitro generated TrVIPs express high levels of CTLA4, IL-10, and membrane-bound TGF-β.15,17 In addition, our study demonstrates that treatment with TrVIPs abrogates acute GVHD in a haplotype- and TGF-β/IL-10-dependent manner. GVHD was prevented only when the administered TrVIPs were of the same haplotype as the BMT cells.
GVL effect is maintained in the presence of TrVIPs. (A) Balb/c mice (H-2d) were given transplants of T cell-depleted BM cells (1.5 × 107) supplemented with different numbers (1.5 × 106 cells, left panels; 5 × 105 cells, middle panels; 1 × 105 cells, right panels) of spleen mononuclear cells from B6 (H-2b) mice. GVL effect was induced by injecting A20 leukemic cells (H-2d) into recipients at time of BMS transplantation. Recipients were treated at time of transplantation with different numbers of CD4Trcontrols (○) or CD4TrVIPs (▾) (H-2b) (1.5 × 106 cells, left panels; 5 × 105 cells, middle panels; 1 × 105 cells, right panels) generated in the presence of DCcontrols or DCVIPs (H-2d). Mice that received injections of A20 cells alone were used as controls (none, •). Survival was monitored and tumor growth/elimination was assessed by the presence of A20 cells in blood detected by coexpression of B220 and H-2Kd and by size (6-10 animals/group). P < .01 untreated recipients versus any TrVIPs group. (B) GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. GVT effect was induced by injecting A20 leukemic (H-2d) cells into recipients mice at time of BMS transplantation. At time of transplantation, recipients were treated with CD8Trcontrols (○) or CD8TrVIPs (▾) generated by coculture with DCcontrols or DCVIPs. Mice that received injections of A20 cells alone were used as controls (untreated, •). Recipients were monitored for survival and leukemia growth/elimination (5-10 mice/group). *P < .01 versus untreated recipients. (C) TrVIPs suppress CD4 and CD8 T-cell expansion but not cytotoxicity activity of CD8 T cells against leukemic cells. T cells (105) isolated from B6 (H-2b) mice were cultured with allogeneic mDCs (104) from Balb/c (H-2d) mice in the absence (none) or presence of CD4Trcontrols or CD4TrVIPs (H-2b) (3 × 104) generated in the presence of DCcontrols or DCVIPs from Balb/c mice. After 5 days, the proliferative response and absolute cell number of CD4 and CD8 T cells in cultures were determined by flow cytometry. CD4 and CD8 T-cell numbers were the same in all the treatment groups at initiation of culture (dashed lines). CD8 T cells (5 × 104) were reisolated by flow cytometry from the cultures and tested for A20 tumor cytolysis at an E/T ratio of 10:1. Each result is the mean ± SD of 4 experiments performed in duplicate. *P < .01 versus untreated recipients.
GVL effect is maintained in the presence of TrVIPs. (A) Balb/c mice (H-2d) were given transplants of T cell-depleted BM cells (1.5 × 107) supplemented with different numbers (1.5 × 106 cells, left panels; 5 × 105 cells, middle panels; 1 × 105 cells, right panels) of spleen mononuclear cells from B6 (H-2b) mice. GVL effect was induced by injecting A20 leukemic cells (H-2d) into recipients at time of BMS transplantation. Recipients were treated at time of transplantation with different numbers of CD4Trcontrols (○) or CD4TrVIPs (▾) (H-2b) (1.5 × 106 cells, left panels; 5 × 105 cells, middle panels; 1 × 105 cells, right panels) generated in the presence of DCcontrols or DCVIPs (H-2d). Mice that received injections of A20 cells alone were used as controls (none, •). Survival was monitored and tumor growth/elimination was assessed by the presence of A20 cells in blood detected by coexpression of B220 and H-2Kd and by size (6-10 animals/group). P < .01 untreated recipients versus any TrVIPs group. (B) GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of BMS from B6 (H-2b) mice. GVT effect was induced by injecting A20 leukemic (H-2d) cells into recipients mice at time of BMS transplantation. At time of transplantation, recipients were treated with CD8Trcontrols (○) or CD8TrVIPs (▾) generated by coculture with DCcontrols or DCVIPs. Mice that received injections of A20 cells alone were used as controls (untreated, •). Recipients were monitored for survival and leukemia growth/elimination (5-10 mice/group). *P < .01 versus untreated recipients. (C) TrVIPs suppress CD4 and CD8 T-cell expansion but not cytotoxicity activity of CD8 T cells against leukemic cells. T cells (105) isolated from B6 (H-2b) mice were cultured with allogeneic mDCs (104) from Balb/c (H-2d) mice in the absence (none) or presence of CD4Trcontrols or CD4TrVIPs (H-2b) (3 × 104) generated in the presence of DCcontrols or DCVIPs from Balb/c mice. After 5 days, the proliferative response and absolute cell number of CD4 and CD8 T cells in cultures were determined by flow cytometry. CD4 and CD8 T-cell numbers were the same in all the treatment groups at initiation of culture (dashed lines). CD8 T cells (5 × 104) were reisolated by flow cytometry from the cultures and tested for A20 tumor cytolysis at an E/T ratio of 10:1. Each result is the mean ± SD of 4 experiments performed in duplicate. *P < .01 versus untreated recipients.
Achieving reduced GVHD lethality without sacrificing a high level of donor engraftment or an effective GVT response underscores the importance of being able to control the progression of antihost-specific T cells following transplantation. Recent publications demonstrated that the use of tolerogenic DCs or of freshly isolated Tr cells allows the control of GVHD without affecting the GVT activity against leukemic cells and lymphomas.10,11,25-27 Our results show that both host-matched DCVIPs and host-mismatched TrVIPs did not interfere with long-lasting BM engraftment, preventing lethal GVHD while permitting GVT responses. GVHD progression seems to be rather due to an excessive donor T-cell expansion and inflammatory cytokine production, a process controlled by Tr, whereas GVT activity is mediated mainly by transplanted CD8 through perforin-dependent cytotoxicity, which is not controlled by Tr cells.25 This suggests that allogeneic DCVIPs are tolerogenic primarily for CD4 T cells. Several findings support this hypothesis. Donor CD8 T cells isolated from DCVIPs-treated recipients maintain cytotoxicity against leukemic cells. In addition, TrVIPs generated with allogeneic DCVIPs reduced the expansion of CD8 T cells without affecting their lytic activity against tumor cells. As expected, GVT activity in TrVIPs-treated recipients required transplantation of sufficient numbers of alloreactive T cells containing CD8+ T cells. DCVIPs induced the expansion/activation of graft CD8+CD44highCD62Llow T cells, which represent effector/memory T cells that are defective in mediating acute GVHD but retain GVT activity.28 Preliminary experiments show that DCVIPs induce memory CD8+CD44high T cells from naive CD8+CD44low T cells in vitro, arguing against an expansion of the already existing CD8+CD44high T cells by DCVIPs (M.D., E.G.-R., A.C., unpublished results, May 2005). Finally, DCVIPs are able to induce allogeneic regulatory CD8 T cells capable of preventing acute GVHD while permitting tumor eradication.
Despite the involvement of these redundant mechanisms, the maintenance of the GVT effect by DCVIPs or TrVIPs depends of the tumor model. Whereas GVT activity was maintained for tumors such as A20 leukemia, which infiltrates BM, and BCL1 lymphoma, which primarily invades the liver and spleen, DCVIPs did not maintain the GVT response against P815 mastocytoma. Similar results have been obtained by others for the same tumor model,.25,26 although Sato and colleagues were able to generate regulatory DCs with the capacity to maintain GVT response against P815 cells.11 Because the GVT activity against P815 mastocytoma strongly depends on alloreactive donor T cells,29 the TrVIPs-mediated decrease in donor T-cell alloreactivity could explain the abrogation of GVT against P815 cells. Indeed, CD8+ T cells isolated from transplanted mice treated with DCVIPs showed decreased cytotoxicity against P815, but not A20 cells.
Immunosuppressive therapies, traditionally focused on lymphocytes, have been revolutionized by targeting the development and key functions of DCs, and the generation of “designer” DCs using specific cytokines, or immunologic and pharmacologic reagents, such as vitamin D3, IL-10, TGF-β, glucocorticoids, and N-acetyl-l-cysteine, alone or in combinations, has become the focus of new therapies.30 Our data demonstrate that VIP is a very efficient agent for the induction of regulatory DCs, and we propose that VIP addition to cocktails of immunomodulatory agents will increase their efficiency. The possibility of generating tolerogenic DCVIPs opens new therapeutic perspectives for the treatment of allogeneic BMT. In vitro pulsing of tolerogenic DCVIPs with alloantigens, followed by in vivo administration, leads to the differentiation of haplotype-specific Tr cells in the graft. Therefore, the inclusion of tolerogenic DCVIPs in future therapeutic regimens may minimize the dependence on nonspecific immunosuppressive drugs used currently as antirejection therapy, facilitate the successful transplantation from mismatched donors, reduce the deleterious consequences of acute GVHD, and extend the applicability of BMT.
Prepublished online as Blood First Edition Paper, January 17, 2006; DOI 10.1182/blood-2005-11-4495.
Supported by grants from the Spanish Ministry of Health (PI04/0674; M.D.), the National Institutes of Health (2RO1A047325; D.G. and M.D.), the Ramon Areces Foundation (M.D.), and the fellowships from Junta de Andalucia (M.D. and E.G.-R.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. DCs differentiated with VIP protect from acute GVHD. (A-B) GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of T-depleted BM cells plus spleen mononuclear cells (BMS) from B6 (H-2b) mice. After transplantation, recipients were given injections of medium (untreated) or BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Untreated mice (•) or mice treated with 2 × 106 DCcontrols (○) or DCVIPs (▾) 2 days after transplantation (12 mice/group). *P < .01, versus untreated recipients. (B) Untreated mice (•) or DCVIPs-treated mice (with 2 × 106 cells, ○;2 × 105, ▾; 2 × 104, ▿, at day 2 after transplantation; or with 2 × 105, ▪; 2 × 104, □, at days 2 and 5 after transplantation; 10-12 mice/group). P < .01 untreated recipients versus any other group. (C) GVHD was induced in recipient B6 (H-2b) mice by allogeneic transplantation of BMS Balb/c (H-2d). Two days after transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCVIPs from different donors (H-2b ○, H-2d ▾, H-2q ▿; 10-12 mice/group). *P < .01 versus untreated recipients. (D) GVHD was induced in a haploidentical model in recipient [B6 × DBA/2] F1 (H-2b/d) by allogeneic transplantation of BMS B6 (H-2b). After transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCcontrols (H-2d, ○) or DCVIPs DBA/2 (H-2d) at day 0 (▾), day 2 (▿) or day 10 (▪) (10-12 mice/group). *P < .01 versus untreated recipients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/9/10.1182_blood-2005-11-4495/2/m_zh80090694930001.jpeg?Expires=1771024002&Signature=yHZo0RyUcDr4hP76eHg~NyltldTabQ8wMpJR5Gybj23UmQdtrVIFCeFV6unZ12OOuuyfryOQ6ns6-5rlaALeVa0SycaxhN0312~n8IAItwZwaayO7L5LRcd6kOAv7-W7X13X6oYCxvRPuaD9~JtofQXjgjQFg8gU3F2NecMsHpoGIWrsuOCt1ZfX1KnmjBjg~wDc3j9X732Vrz1NcpZQ-LMgx9DAxsAOGlnE7V~WTc~8lHPW3uiuSf0fZ4PMHegpk8j~Flf5kUxpE-c~4FMYgBmjxPVSnT6iwFkx9~UeqvTJg1Hkka1Eb5sfOtUS7XnXg2z2A11tGYOhXk1TGFRMrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. DCs differentiated with VIP protect from acute GVHD. (A-B) GVHD was induced in recipient Balb/c (H-2d) mice by allogeneic transplantation of T-depleted BM cells plus spleen mononuclear cells (BMS) from B6 (H-2b) mice. After transplantation, recipients were given injections of medium (untreated) or BM-DCs from Balb/c (H-2d) generated in the absence (DCcontrols) or presence (DCVIPs) of VIP. (A) Untreated mice (•) or mice treated with 2 × 106 DCcontrols (○) or DCVIPs (▾) 2 days after transplantation (12 mice/group). *P < .01, versus untreated recipients. (B) Untreated mice (•) or DCVIPs-treated mice (with 2 × 106 cells, ○;2 × 105, ▾; 2 × 104, ▿, at day 2 after transplantation; or with 2 × 105, ▪; 2 × 104, □, at days 2 and 5 after transplantation; 10-12 mice/group). P < .01 untreated recipients versus any other group. (C) GVHD was induced in recipient B6 (H-2b) mice by allogeneic transplantation of BMS Balb/c (H-2d). Two days after transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCVIPs from different donors (H-2b ○, H-2d ▾, H-2q ▿; 10-12 mice/group). *P < .01 versus untreated recipients. (D) GVHD was induced in a haploidentical model in recipient [B6 × DBA/2] F1 (H-2b/d) by allogeneic transplantation of BMS B6 (H-2b). After transplantation, recipients were given injections of medium (untreated, •) or 2 × 106 DCcontrols (H-2d, ○) or DCVIPs DBA/2 (H-2d) at day 0 (▾), day 2 (▿) or day 10 (▪) (10-12 mice/group). *P < .01 versus untreated recipients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/9/10.1182_blood-2005-11-4495/2/m_zh80090694930001.jpeg?Expires=1771024003&Signature=cJhPLV4f3-~BmZGVZUJ-~OpDziYDawHXUUafHn6u2HBdQhc1N-z1IIJvma5j8OtnkhXa7aV2z5wYvUMFEZqmypD~gkXylmwXsi3bSX-ptqS8QLE7SjNHYfdkeTGh3pYEZ3~r4NB9CHpOilEUJdjofMsxlmfKTKBhkyiEYKfgP89mdwWyJKQnetoXgF7lL-p98qGGjzXXRzOVqVmvpLLSqyOZNqtsWlYsaROWrRUKgZoDvqq4CKF2hW7z~foh0FzrkVmmUCnipOAxyl3gPl-MHXuNSmt8RtKk5sbEk7eoraHoKuzlSF5KWLqkIQu4ET1CNexnUn34QpriU96ZRht6GQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)