Dendritic cells (DCs) derive from CD34+ cells or monocytes and stimulate alloimmune responses in transplantation. We hypothesized that the interaction between CD34+ cells and allogeneic T cells would influence the function of hematopoietic stem cells (HSCs). Cord blood (CB) CD34+ cells proliferated briskly in response to allogeneic, but not autologous, T cells when mixed with irradiated T cells for 6 days in vitro. This proliferation was significantly inhibited by an anti-HLA class II monoclonal antibody (mAb), by an anti-TNFα mAb, or by CTLA4-Ig. Allogeneic T cells induced the differentiation of CD34+ progenitors into cells with the morphology of dendritic monocytic precursors and characterized by the expression of HLA-DR, CD86, CD40, CD14, and CD11c, due to an endogenous release of TNFα. Cotransplantation of CD34+ cells with allogeneic T cells into nonobese diabetic-severe combined immunodeficiency (NOD/SCID) mice resulted in a greater engraftment of myeloid CD1c+ dendritic cells compared with cotransplantation with autologous T cells. In vitro, CD34+ cell-derived antigen-presenting cells (APCs) were functionally capable of both direct and indirect presentation of alloantigens. Based on these findings, we hypothesize that in HSC transplantation the initial cross talk between allogeneic T cells and CD34+ cells may result in the increased generation of APCs that can present host alloantigens and possibly contribute to the development of graft-versus-host disease. (Blood. 2006;108:203-208)
Introduction
CD34+ cell populations in human bone marrow and peripheral blood can act as antigen-presenting cells (APCs) and activate allogeneic T cells in vitro due to the presence of committed myeloid progenitors that constitutively express HLA and adhesion and costimulatory molecules.1,2 These latter molecules are upregulated on CD34+ cells upon binding to T cells or after addition of tumor necrosis factor-α (TNFα) for 24 hours to the culture.2,3 Similarly, ICOS-L expression on CD34+ cells can be induced by TNFα and can act as an alternative costimulatory pathway on stem cells.4 Moreover, the expression of costimulatory molecules identifies a subpopulation of CD34+ progenitors that differentiate into dendritic cells (DCs) in liquid culture.3,5 CD34+ cells from cord blood (CB) also generate myeloid DCs in response to granulocyte-macrophage colony-stimulating factor (GM-CSF) and TNFα in vitro6,7 and allow the engraftment of functional DCs in nonobese diabetic-severe combined immunodeficiency (NOD/SCID) mice.8
After hematopoietic stem cell (HSC) transplantation, allogeneic donor T cells respond to host APCs and initiate graft-versus-host disease (GVHD) but also facilitate the engraftment of donor CD34+ cells.9,10 Experiments in NOD/SCID mice suggested an important role of CD8+ T cells in facilitating the migration, homing, and engraftment of CD34+ cells through alteration of signaling via the chemokine receptor CXCR4.11 However, while T cells can enhance the stimulatory activity of DCs,12 the effect of donor T cells on the APC function of CD34+ cells is not known. Multiple clinical studies have shown direct correlations between CD34+ cell dose13-16 and the development of GVHD. Mielcarek et al17 have hypothesized that donor CD34+ APCs, or APCs derived from CD34+ cells, may contribute to the development of GVHD by indirect presentation of host alloantigen.
In this study, we show that allogeneic T cells can affect the function of CD34+ cells by inducing the proliferation and rapid differentiation of a subset of progenitors into monocytic-dendritic precursors capable of both direct and indirect alloantigen presentation. Cotransplantation of CD34+ cells and allogeneic T cells into NOD/SCID mice resulted in a better engraftment of human CD45+ cells with dendritic phenotype than cotransplantation with autologous T cells. Among the mechanisms used by T cells to stimulate CD34+ cell proliferation and differentiation, we describe here both the ligation of cellular receptors and the release of cytokines, such as GM-CSF and TNFα.
Materials and methods
Flow cytometry
Flow cytometric analysis was performed on samples of peripheral blood (PB) and CB cells. The following fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or peridin chlorophyll protein (PerCP)-conjugated monoclonal antibodies (mAbs) were employed: CD34, HLA-DR, CD14, CD45, CD86, CD40, CD11c, CD33, CD1a, and CD19 from Becton Dickinson (San Jose, CA) and CD1c (BDCA-1) from Miltenyi Biotec (Auburn, CA). Appropriate isotype controls were also used. Stained cells were washed twice in phosphate-buffered saline (PBS) and sample acquisition and analysis was performed within 2 hours on a FACSCalibur (Becton Dickinson).
Cell separation
CB mononuclear cells (MNCs) were obtained by centrifugation over Ficoll/Hypaque (Amersham Biosciences, Piscataway, NJ) gradients. Lightdensity cells were washed twice in PBS (Cambrex, Walkersville, MD) with 1% bovine serum albumin (BSA; Sigma Chemical, St Louis, MO), and CD34+ cells were purified by MidiMACS high-gradient magnetic separation column (Miltenyi Biotec). To assess the purity, aliquots of isolated CD34+ cells were restained with an FITC-conjugated anti-CD34 mAb (Becton Dickinson). PB or CB T cells were immunomagnetically purified from MNCs on a MidiMACS column by negative selection of T cells by using a Pan T Cell Isolation Kit II (Miltenyi Biotec). This kit uses a cocktail of biotin-conjugated mAbs against CD14, CD16, CD19, CD36, CD56, CD123, and glycophorin A as a primary labeling reagent and antibiotin mAbs conjugated to microbeads as a secondary reagent. The unlabeled cells that passed through the column were then stained with an anti-CD3-FITC mAb (Becton Dickinson) as a check for purity. Isolated CD34+ or CD3+ T-cell samples were acquired on a FACS Calibur (Becton Dickinson) and analyzed using Cell Quest software (Becton Dickinson) and showed, on average, 95% cell purity. In selected experiments, CB MNCs were enriched in monocytes by cell adherence, and then monocyte-derived DCs (Mo-DCs) were generated.18 Briefly, MNCs were allowed to adhere to 6-well plates. After 2 hours at 37°C, the nonadherent cells were removed and the adherent cells were detached by gently scraping and PBS containing 0.5 mM EDTA. The adherent fractions were then cultured in RPMI-10% human serum (HS) supplemented with 1000 U/mL GM-CSF (Amgen, Thousand Oaks, CA) and 1000 U/mL interleukin 4 (IL-4; Pharmingen Becton Dickinson) for 6 days. Coexpression of HLA-DR, CD86, and CD1a was analyzed to check the differentiation of DCs.
Primary MLC
CD34+ cells were freshly isolated or cocultured for 6 days with irradiated T cells (see the next paragraph), washed twice, irradiated (3000 cGy), and then tested as stimulators (Ss) in a primary mixed-lymphocyte culture (MLC). Irradiated autologous peripheral blood MNCs were used as negative control. Cells were resuspended in medium containing RPMI-1640 (Cambrex), 25 mM Hepes (Cambrex), 100 U/mL penicillin (Cambrex), 100 μg/mL streptomycin (Cambrex), and 10% AB HS (Hyclone, Logan, UT) inactivated at 56°C for 30 minutes. Purified T-cell responders (Rs; 5 × 104) were mixed with stimulators at a 1:2 stimulator-responder (S/R) ratio in round-bottomed 96-well plates for 6 days at 37°C in a 5% CO2 humidified atmosphere. Where indicated, anti-GM-CSF and/or anti-TNFα antibodies (Pharmingen BD, San Diego, CA) were used in cell culture at 350 ng/mL and 10 μg/mL, respectively, to block cytokine activity. Cells were pulsed with 1 μCi/well (0.037 MBq/well) 3H-thymidine for 18 hours before harvest on day 6. Stimulation index (SI) was calculated for each individual experiment as follows: SI = cpm (T-cell responders + stimulators)/cpm (T-cell responders), where cpm indicates counts per minute.
Stem-cell proliferation and differentiation
Freshly isolated CB CD34+ cells were cultured with irradiated (3000 cGy) autologous CB or allogeneic peripheral blood T cells at a 1:2 ratio, or in medium alone, in round-bottomed 96-well plates for 6 days at 37°C in a 5% CO2 humidified atmosphere. No exogenous cytokines were added. Proliferation was measured by pulsing the cells with 1 μCi/well (0.037 MBq/well) 3H-thymidine for 18 hours before harvest on day 6. Cells were analyzed by flow cytometry for the expression of CD34, CD86, CD40, CD1a, CD11c, HLA-DR, and CD14. A morphologic analysis was performed by cytospins stained with May-Grünwald-Giemsa. Dead cells were removed prior to staining by centrifugation over Ficoll/Hypaque gradients. In selected experiments, anti-GM-CSF and/or anti-TNFα mAbs (Pharmingen BD), recombinant CTLA4-IgG4m fusion molecule (CTLA4-Ig; kindly provided by Dr Richard Boismenu, Repligen, Waltham, MA; 50 μg/mL), or exogenous recombinant GM-TNF (1 ng/mL) and TNFα (100 pg/mL) were added to the culture with CD34+ cells and irradiated T cells.
CFU-C assay
Purified CD34+ CB cells were plated at 1 × 103 cells/plate in duplicate cultures containing 1 mL IMDM with 1% methylcellulose, 30% FBS, 2 mM l-glutamine, 10-4 M 2-mercaptoethanol, and the following cytokines: 3 U/mL recombinant human erythropoietin (rh-Epo), 50 ng/mL rh stem cell factor (rh-SCF), 20 ng/mL rh-GM-CSF, 20 ng/mL rh-IL-3, 20 ng/mL rh-IL-6, and rh granulocyte colony-stimulating factor (rh-G-CSF; METHOCULT GF H4435; Stem Cell Technologies, Vancouver, BC, Canada). In selected experiments, recombinant CTLA4-IgG4m fusion molecule (CTLA4-Ig; kindly provided by Dr Richard Boismenu, Repligen) was added to the cultures at 50 μg/mL. Colony-forming cells (CFU-Cs), including granulocyte-macrophage CFUs (CFU-GMs), erythroid progenitors (erythroid burst-forming units [BFU-Es]), and CFU-Mix, were scored after 14 days of incubation in 35-mm tissue-culture dishes at 37°C in a fully humidified 5% CO2 atmosphere.
Indirect antigen presentation
Allogeneic PB or autologous CB MNCs were incubated in 10% AB HS-RPMI at 37°C in a 5% CO2 humidified atmosphere for 16 hours with a microbial alkaloid, staurosporin (Sigma), at a 5-mM concentration in order to induce the apoptotic death of the cells.19 After washing 3 times, the cells were evaluated by flow cytometry using an annexin V-FITC/propidium iodide (PI)-PE staining. The proportion of apoptotic cells, including early apoptotic (annexin V+PI-) and late apoptotic (annexin V+PI+) cells, was always greater than 95%.
After incubation with irradiated allogeneic T cells, CD34+ cells were washed twice and incubated for 48 hours with apoptotic allogeneic or autologous cells at a 1:2 ratio or with medium alone. In control experiments, CB Mo-DCs were also incubated with apoptotic allogeneic cells at the same ratio. Cells were irradiated (3000 cGy) and mixed with autologous CB MNCs at a 1:2 ratio in round-bottomed 96-well plates for 6 days at 37°C in a5%CO2 humidified atmosphere. Autologous cord blood or allogeneic PB apoptotic cells alone were mixed with responders as controls. Proliferation was measured by 3H-thymidine incorporation assay, as described above.
Transplantation
NOD/SCID mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and were maintained as previously described.20 Human CB-purified CD34+ cells (2 × 105 /animal) were mixed with autologous CB- or allogeneic PB-selected T cells (5 × 105 /animal) and then infused intravenously into separate cohorts of sublethally irradiated (300 cGy) NOD/SCID mice. Control mice were injected with CD34+ cells alone, autologous T cells alone, or allogeneic T cells alone. Six weeks after transplantation the animals were killed and femurs and tibias were flushed with Hanks balanced salt solution (HBSS; Biowhittaker, Walkersville, MD) with 2% FBS (Hyclone Laboratories) and 0.02% sodium azide. Cells were counted and preincubated with 1 mg/mL human gamma globulin in staining to block human Fc receptors. Murine Fc receptors were blocked by a second incubation of the cells in 2.4G2 (an anti-mouse Fc receptor mAb). Marrow cells were analyzed by flow cytometry to detect engraftment of various human lineages as previously described.21 In particular, human myeloid dendritic cells were characterized as CD45+CD1a+ or CD45+ CD1c(BDCA1)+CD19- cells.
Statistical analysis
Statistical analysis was performed by using the t test or analysis of variance (ANOVA) test, when more than 2 series of data were compared.
Results
Allogeneic T cells induce CD34+ cell proliferation through soluble factors
We have previously demonstrated that allogeneic T cells proliferate briskly and produce proinflammatory cytokines in response to purified CD34+ cells.22 To test the hypothesis that activation of allogeneic T cells could directly stimulate CD34+ cell proliferation, we evaluated the proliferation of isolated CB CD34+ cells after stimulation with either irradiated allogeneic peripheral blood or autologous cord blood T cells. In 4 separate experiments, CD34+ cells proliferated when cultured with irradiated allogeneic peripheral blood T cells but not with autologous cord blood T cells (Figure 1A). Addition of anti-HLA class II but not anti-class I mAbs significantly reduced proliferation (P = .04; Figure 1B). Addition of anti-TNFα mAb also significantly inhibited CD34+ cell proliferation (P = .01; Figure 1C). We next hypothesized that blockade of T-cell costimulation would prevent the proliferation of cocultured CD34+ cells. As shown in Figure 2A, the addition of CTLA4-Ig, which is known to inhibit T-cell responses by blocking the B7:CD28 costimulatory pathway, prevented allogeneic T-cell stimulation of CD34+ cell proliferation (P = .05). The inhibition of CD34+ cell proliferation induced by CTLA4-Ig was reversed when GM-CSF and TNFα were exogenously added to the culture, suggesting that costimulatory blockade could prevent CD34+ cell proliferation by inhibiting the release of cytokines from allogeneic T cells. In order to rule out the possibility of residual T-cell proliferation, we performed additional experiments in which both CD34+ cells and allogeneic T cells were irradiated. No proliferation was detected, demonstrating that CD34+ cells were responsible for all of the proliferation observed (data not shown). To rule out a direct toxic effect of CTLA4-Ig on CD34+ progenitors, we performed clonogenic assays. As shown in Figure 2B, CTLA4-Ig did not affect the number of either CFU-Cs or CFU-GMs. These findings show that allogeneic T cells stimulate the expansion of CD34+ progenitors through both cellular signaling and release of soluble factors.
CD34+ cell proliferation stimulated by allogeneic T cells. Freshly isolated CB CD34+ cells were mixed with or without 5 × 104 irradiated autologous T cells (auto-T cells) or HLA-mismatched PB T cells (allo-T cells) at 1:2 S/R ratio, or medium, and cell proliferation was measured by 3H-Thymidine incorporation assay after 6 days of culture. Results are represented as the mean cpm ± SD of 3 separate experiments. (A) CD34+ cell proliferation was greater after coculture with allogeneic T cells than with autologous T cells or with medium alone (P = .02). (B) When anti-HLA class I (anti-class I) or anti-HLA class II (anti-class II) mAbs were added to the culture, only anti-class II reduced proliferation. Results are represented as the mean cpm ± SD of 3 separate experiments. Only the anti-HLA class II antibody reduced the CD34+ cell proliferation significantly (P = .04). (C) Addition of anti-TNFα (anti-TNF) and anti-GM-CSF (anti-GM) mAbs, or anti-TNFα mAb alone (P = .01), reduced proliferation in 3 separate experiments. *P < .05.
CD34+ cell proliferation stimulated by allogeneic T cells. Freshly isolated CB CD34+ cells were mixed with or without 5 × 104 irradiated autologous T cells (auto-T cells) or HLA-mismatched PB T cells (allo-T cells) at 1:2 S/R ratio, or medium, and cell proliferation was measured by 3H-Thymidine incorporation assay after 6 days of culture. Results are represented as the mean cpm ± SD of 3 separate experiments. (A) CD34+ cell proliferation was greater after coculture with allogeneic T cells than with autologous T cells or with medium alone (P = .02). (B) When anti-HLA class I (anti-class I) or anti-HLA class II (anti-class II) mAbs were added to the culture, only anti-class II reduced proliferation. Results are represented as the mean cpm ± SD of 3 separate experiments. Only the anti-HLA class II antibody reduced the CD34+ cell proliferation significantly (P = .04). (C) Addition of anti-TNFα (anti-TNF) and anti-GM-CSF (anti-GM) mAbs, or anti-TNFα mAb alone (P = .01), reduced proliferation in 3 separate experiments. *P < .05.
CTLA4-Ig prevents CD34+ cell proliferation upon stimulation with allogeneic T cells. (A) Proliferation of purified CD34+ cells was assessed after 6 days in culture with medium alone or with irradiated allogeneic T cells (irrad T-cells) and CTLA4-IgG4m (CTLA4-Ig), with or without the addition of exogenous cytokines (GM-CSF/TNFα). CTLA4-Ig significantly inhibited CD34+ cell proliferation (P = .05). Results are represented as the mean cpm ± SD of 3 separate experiments. (B) The number of CFU-Cs and CFU-GMs generated from CD34+ cells in clonogenic assays was not affected by addition of CTLA4-Ig. The results are represented as mean colonies ± SD in 3 separate experiments. *P = .05.
CTLA4-Ig prevents CD34+ cell proliferation upon stimulation with allogeneic T cells. (A) Proliferation of purified CD34+ cells was assessed after 6 days in culture with medium alone or with irradiated allogeneic T cells (irrad T-cells) and CTLA4-IgG4m (CTLA4-Ig), with or without the addition of exogenous cytokines (GM-CSF/TNFα). CTLA4-Ig significantly inhibited CD34+ cell proliferation (P = .05). Results are represented as the mean cpm ± SD of 3 separate experiments. (B) The number of CFU-Cs and CFU-GMs generated from CD34+ cells in clonogenic assays was not affected by addition of CTLA4-Ig. The results are represented as mean colonies ± SD in 3 separate experiments. *P = .05.
Morphologic and immunophenotypic characteristics of CD34+ cells stimulated by allogeneic T cells. (A) Freshly isolated CB CD34+ cells were mixed with irradiated autologous CB (AUTOLOGOUS) or allogeneic blood (ALLOGENEIC) T cells at a 1:2 S/R ratio, or with medium alone (MEDIUM), for 6 days and then were analyzed for CD86 and CD1a expression by flow cytometry. The proportion of positive cells is shown in each quadrant. This result is representative of 3 separate experiments. (B) Cytospins of freshly isolated CB CD34+ cells (left), or CB CD34+ cells that had been in culture with allogeneic T cells for 6 days and then separated on ficoll-gradient (right), were stained with May-Grünwald-Giemsa. Images were visualized under an Axioskop 2 microscope equipped with a 63 ×/1.4 oil-immersion objective lens (Zeiss, Thornwood, NY). Images were captured with a Zeiss Axiocam camera and Zeiss Axiovision software version 3.1, and processed with Adobe Photoshop 5.5 software (Adobe Systems, San Jose, CA). (C) The phenotypic expression of markers such as HLA-DR, CD86, CD40, CD14, and CD11c was evaluated in CD34+ cell-derived APCs obtained after culture with allogeneic T cells. The proportion of positive cells is shown in each quadrant. The results are representative of 3 separate experiments.
Morphologic and immunophenotypic characteristics of CD34+ cells stimulated by allogeneic T cells. (A) Freshly isolated CB CD34+ cells were mixed with irradiated autologous CB (AUTOLOGOUS) or allogeneic blood (ALLOGENEIC) T cells at a 1:2 S/R ratio, or with medium alone (MEDIUM), for 6 days and then were analyzed for CD86 and CD1a expression by flow cytometry. The proportion of positive cells is shown in each quadrant. This result is representative of 3 separate experiments. (B) Cytospins of freshly isolated CB CD34+ cells (left), or CB CD34+ cells that had been in culture with allogeneic T cells for 6 days and then separated on ficoll-gradient (right), were stained with May-Grünwald-Giemsa. Images were visualized under an Axioskop 2 microscope equipped with a 63 ×/1.4 oil-immersion objective lens (Zeiss, Thornwood, NY). Images were captured with a Zeiss Axiocam camera and Zeiss Axiovision software version 3.1, and processed with Adobe Photoshop 5.5 software (Adobe Systems, San Jose, CA). (C) The phenotypic expression of markers such as HLA-DR, CD86, CD40, CD14, and CD11c was evaluated in CD34+ cell-derived APCs obtained after culture with allogeneic T cells. The proportion of positive cells is shown in each quadrant. The results are representative of 3 separate experiments.
Allogeneic T cells induce the rapid differentiation of CB CD34+ cells
We next evaluated whether allogeneic T cells stimulated the APC capacity of CD34+ cells in the same model. CD34+ cells were analyzed for CD34, CD86, and CD1a expression both prior to culture and after stimulation in culture with allogeneic T cells, autologous T cells, or medium alone (Figure 3A). At initiation of culture, less than 2% CD34+ cells expressed CD86, CD40, and CD14 (data not shown). In 3 separate experiments, stimulation with allogeneic T cells increased the proportion of CD86+ cells more than 10-fold, to 21% ± 4%, compared with stimulation with autologous T cells or medium (Figure 3A). Morphologic examination of CD34+ cells cultured with allogeneic T cells showed large cells, often with monocytoid/dendritic characteristics (Figure 3B). Consistent with this morphology, between 12% and 25% of these cells coexpressed several markers typical of professional APCs, such as CD86, HLA-DR, CD40, CD14, and CD11c (Figure 3C). Thus, both the morphology of the cells and the coexpression of HLA-DR, costimulatory molecules, and CD14 or CD11c antigens demonstrate that a subset of CD34+ cells can rapidly differentiate into DC monocytic precursors when cultured with allogeneic T cells.
Differentiation of CD34+ cells upon culture with allogeneic T cells is mediated by endogenous GM-CSF and TNFα. Purified CD34+ cells were cultured with irradiated allogeneic T cells alone (control) or in the presence of anti-GM-CSF (anti-GM-CSF), anti-TNFα (anti-TNFα) mAb, or both (anti-GM-CSF anti-TNFα), then analyzed by flow cytometry for the expression of CD86 and CD11c. Markers were set in each histogram based upon isotype controls in order to calculate the percentage of positive cells.
Differentiation of CD34+ cells upon culture with allogeneic T cells is mediated by endogenous GM-CSF and TNFα. Purified CD34+ cells were cultured with irradiated allogeneic T cells alone (control) or in the presence of anti-GM-CSF (anti-GM-CSF), anti-TNFα (anti-TNFα) mAb, or both (anti-GM-CSF anti-TNFα), then analyzed by flow cytometry for the expression of CD86 and CD11c. Markers were set in each histogram based upon isotype controls in order to calculate the percentage of positive cells.
Co-infusion of allogeneic T cells increases the engraftment of myeloid dendritic cells after CD34+ cell transplantation in NOD/SCID mice. Engraftment of human myeloid DCs was compared in the marrow of NOD/SCID mice 6 weeks after cotransplantation of CB CD34+ cells with allogeneic (allo) blood T cells (n = 5) or autologous (auto) CB T cells (n = 4). All mice showed evidence of huCD45+ cells, which was not significantly different between mice also receiving allogeneic versus autologous T cells. More CD19-BDCA1+ myeloid dendritic cells (A) were observed in huCD45+ cells from mice that received cotransplants of allogeneic blood T cells (Allo) or autologous CB T cells (Auto). A trend toward more CD1a+ immature DCs (B) was also detected. Dots represent individual animals; bars, mean values.
Co-infusion of allogeneic T cells increases the engraftment of myeloid dendritic cells after CD34+ cell transplantation in NOD/SCID mice. Engraftment of human myeloid DCs was compared in the marrow of NOD/SCID mice 6 weeks after cotransplantation of CB CD34+ cells with allogeneic (allo) blood T cells (n = 5) or autologous (auto) CB T cells (n = 4). All mice showed evidence of huCD45+ cells, which was not significantly different between mice also receiving allogeneic versus autologous T cells. More CD19-BDCA1+ myeloid dendritic cells (A) were observed in huCD45+ cells from mice that received cotransplants of allogeneic blood T cells (Allo) or autologous CB T cells (Auto). A trend toward more CD1a+ immature DCs (B) was also detected. Dots represent individual animals; bars, mean values.
Potent allo-antigen (allo-Ag)-presenting function of CD34-derived APCs induced by allogeneic T cells. Purified CD34+ cells, either freshly isolated (□), after culture with irradiated allogeneic T cells for 6 days (Irrad Allo-T cells, ▪), or with irradiated allogeneic T cells and anti-GM-CSF plus anti-TNFα blocking antibodies (blocking MAbs, ▦), were irradiated and mixed with blood T-cell responders from the same allogeneic donor at a 1:2 ratio in MLC. T-cell alloreactivity was measured by 3H-Thymidine incorporation assay after 6 days of culture. Results are the mean SI ± SD of 4 separate experiments. *P = .05.
Potent allo-antigen (allo-Ag)-presenting function of CD34-derived APCs induced by allogeneic T cells. Purified CD34+ cells, either freshly isolated (□), after culture with irradiated allogeneic T cells for 6 days (Irrad Allo-T cells, ▪), or with irradiated allogeneic T cells and anti-GM-CSF plus anti-TNFα blocking antibodies (blocking MAbs, ▦), were irradiated and mixed with blood T-cell responders from the same allogeneic donor at a 1:2 ratio in MLC. T-cell alloreactivity was measured by 3H-Thymidine incorporation assay after 6 days of culture. Results are the mean SI ± SD of 4 separate experiments. *P = .05.
Endogenous GM-CSF and TNFα induce CD34+ cell differentiation
We next evaluated whether CD34+ cell differentiation induced by allogeneic T cells depends on endogenous soluble factors, such as GM-CSF and TNFα. Addition of anti-GM-CSF and/or anti-TNFα mAbs to culture of CD34+ cells and allogeneic T cells largely inhibited CD34+ cell differentiation (Figure 4). Blockade of both cytokines almost completely prevented the generation of CD86+ cells and CD11c+ cells, suggesting that the endogenous release of cytokines stimulated the differentiation of CD34+ cells in these cultures.
Allogeneic blood T cells help differentiate CD34+ cell-derived DCs in vivo
We next tested whether allogeneic PB T cells would stimulate differentiation of CD34+ cells in vivo. We cotransplanted CD34+ cells together with autologous CB T cells or allogeneic PB T cells. All the mice receiving autologous T cells (n = 4) or allogeneic T cells (n = 5) were engrafted and no clinical sign of GVHD due to xenogeneic responses was detected in any animal. The overall proportion of human CD45+ (huCD45+) marrow cells in these 2 groups was 2.4 ± 1.1 and 1.4 ± 0.3 (P = not significant [NS]), respectively. In mice that received a transplant with CD34+ cells and allogeneic T cells there was a greater marrow engraftment of human CD1c+ (BDCA1+CD19-) myeloid circulating dendritic cells (P = .03) but only a trend for greater proportions of immature DCs (CD1a+; P = .1; Figure 5). Other huCD45+ cell populations analyzed in mice that received a transplant with CD34+ cells and allogeneic T cells versus autologous T cells included CD14+ cells (5% ± 3% vs 8% ± 4%), CD34+ cells (2.5% ± 1.4% vs 5.4% ± 3%), and CD19+ cells (50% ± 7% vs 71% ± 35%), and none of the differences were statistically significant.
T-cell-induced CD34+ cell-derived APCs are capable of direct and indirect alloantigen presentation
We next evaluated whether CD34+ cells stimulated by allogeneic T cells would function as APCs in vitro. CD34+ cells were used as stimulators in an MLC either after culture with irradiated allogeneic T cells or after fresh isolation by immunomagnetic beads. In 4 separate experiments, CD34 cells that had differentiated into APCs after stimulation with T cells (CD34-APCs) induced a significantly greater allogeneic T-cell response than unstimulated CD34+ cells or CD34+ cells stimulated by T cells in the presence of antibodies to GM-CSF and TNFα (P = .05; Figure 6). Thus, CD34-APCs that had been stimulated by alloreactive T cells could directly present alloantigen. We next hypothesized that CD34-APCs can also indirectly present alloantigen to autologous T cells. In order to test this hypothesis, we first used a microbial alkaloid, staurosporin, to induce the apoptosis in MNCs from autologous CB or from allogeneic PB. CD34-APCs were then incubated with media or with apoptotic cells as a source of alloantigen for 48 hours. Cells were then washed, irradiated, and tested as stimulators for autologous CB T-cell responders in a primary MLC. In 4 separate experiments, the autologous CB T-cell response to CD34-APCs loaded with allogeneic apoptotic cells was significantly greater than the response to CD34-APC-loaded autologous apoptotic cells (P = .02; Figure 7). Importantly, allogeneic apoptotic cells alone did not stimulate the T-cell proliferation, demonstrating the requirement for APCs. In addition, indirect presentation of alloantigens by autologous DCs obtained from CB MNCs after liquid culture with GM-CSF and IL-4 was comparable to that of CD34-APCs. These findings demonstrate that the CD34+ cell population that differentiates upon alloreactive T-cell contact can also induce efficient responses from autologous T cells by capturing and presenting alloantigens from apoptotic cells.
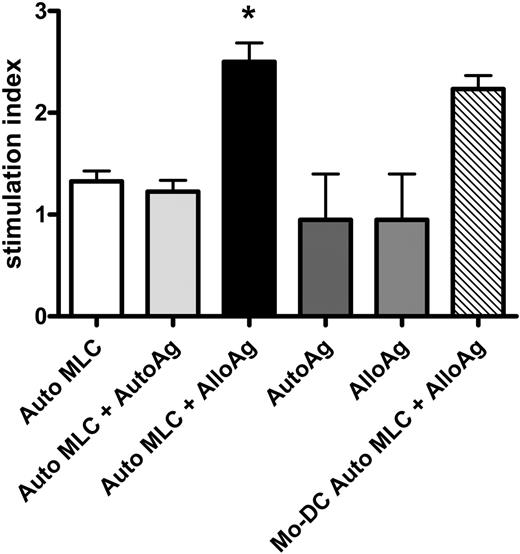
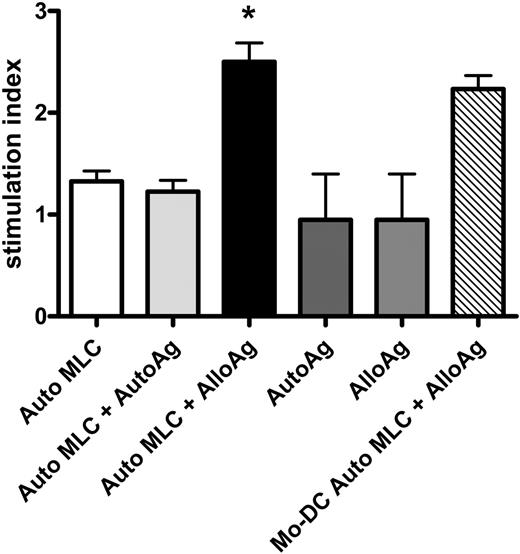
Indirect alloAg presentation by CD34+ cell-derived APCs induced upon contact with allogeneic T cells. CD34-APCs obtained after culture with irradiated allogeneic T cells were incubated for 48 hours with autologous (Auto MLC + AutoAg) or allogeneic apoptotic cells (Auto MLC + AlloAg) or without apoptotic cells (Auto MLC; see “Indirect antigen presentation”). CB Mo-DCs were incubated with allogeneic apoptotic cells (Mo-DC Auto MLC + AlloAg). CD34-APCs or Mo-DCs were then irradiated and cultured with autologous CB responders in primary MLC at a 1:2 ratio. Autologous (AutoAg) or allogeneic (AlloAg) apoptotic cells in the absence of irradiated APCs were also tested as stimulators. T-cell proliferation was measured by 3H-Thymidine incorporation assay. The CB T-cell response to alloantigen indirectly presented by autologous CD34-APCs was significantly greater (P = .02) than the response to autoantigen or to either apoptotic cell population alone. Results are the mean SI ± SD of 4 separate experiments. *P < .05.
Indirect alloAg presentation by CD34+ cell-derived APCs induced upon contact with allogeneic T cells. CD34-APCs obtained after culture with irradiated allogeneic T cells were incubated for 48 hours with autologous (Auto MLC + AutoAg) or allogeneic apoptotic cells (Auto MLC + AlloAg) or without apoptotic cells (Auto MLC; see “Indirect antigen presentation”). CB Mo-DCs were incubated with allogeneic apoptotic cells (Mo-DC Auto MLC + AlloAg). CD34-APCs or Mo-DCs were then irradiated and cultured with autologous CB responders in primary MLC at a 1:2 ratio. Autologous (AutoAg) or allogeneic (AlloAg) apoptotic cells in the absence of irradiated APCs were also tested as stimulators. T-cell proliferation was measured by 3H-Thymidine incorporation assay. The CB T-cell response to alloantigen indirectly presented by autologous CD34-APCs was significantly greater (P = .02) than the response to autoantigen or to either apoptotic cell population alone. Results are the mean SI ± SD of 4 separate experiments. *P < .05.
Discussion
In this study we demonstrate that allogeneic T cells induce the proliferation and differentiation of human CD34+ cells into functional APCs capable of direct and indirect alloantigen presentation. We observed that the interaction between CD34+ HSCs and T cells directly affected hematopoietic progenitor fate. Because CD34+ cells can stimulate an allogeneic T-helper 1 (Th1) response in vitro,22 we evaluated whether T cells can drive the differentiation of CD34+ cells into functional APCs. Multiple cytokines, including GM-CSF and TNFα, are capable of inducing the differentiation of hematopoietic progenitors into mature DCs in liquid culture.6,23,24 Using a coculture of irradiated allogeneic T cells and unirradiated CD34+ cells, we demonstrated an increased proliferation of CD34+ cells and a rapid differentiation of a fraction of CD34+ cells into large cells with monocytic/dendritic cell precursor characteristics that express HLA-DR, CD86, CD40, and CD11c, consistent with DC monocytic precursors. Importantly, these morphologic and phenotypic changes in CD34+ cells were associated with a 4- to 5-fold greater capacity to stimulate T-cell alloresponses. Neutralization of TNFα abrogated both proliferation and differentiation of CD34+ cells, whereas neutralization of GM-CSF had only an additive inhibitory effect in differentiation of the progenitors. These results suggest that these cytokines are among those rapidly released upon CD34+ cell: allogeneic T-cell interaction. CD4+ T cells are likely to be the principal mediators of this effect, since blockade of HLA class II molecules significantly reduced CD34+ cell proliferation. However, it is probable that other cytokines were released in this setting, since previous studies suggested that exogenous TNFα stimulates CD34+ cell proliferation only indirectly.25,26 Interestingly, blockade of B7 ligands on CD34+ cells also inhibited CD34+ cell proliferation. Since we have previously demonstrated that CTLA4-Ig prevents T-cell alloreactivity to CD34+ cells, this result may confirm the need for costimulatory signals in order for allogeneic T cells to release soluble factors. Alternatively, CTLA4-Ig may act by signaling to CD34+ cells directly.
These in vitro data were consistent with in vivo results in NOD/SCID mice, where cotransplantation of CD34+ cells and allogeneic blood T cells resulted in a greater relative engraftment of circulating human myeloid DCs, identified by CD1c (BDCA1) expression,27,28 compared with cotransplantation of CD34+ cells with autologous T cells. Because the overall engraftment of huCD45+ cells was slightly lower in mice receiving allogeneic T cells (1.4% vs 2.4%), we cannot exclude the possibility that CD34+ cells could also be the target of allogeneic T-cell responses, but the small number of animals may preclude a definitive conclusion on this point.
Since APCs of both donor and recipient trigger T-cell responses that occur in allogeneic HSC transplantation, CD34+ cells that differentiate into APCs after encountering allogeneic T cells may later contribute to the activation of those T cells. Host T cells capable of interacting with donor CD34+ cells as well as professional APCs still circulate at the time of transplantation, despite myeloablative conditioning regimens.29 Thus, donor APCs that rapidly differentiate from CD34+ cells could stimulate persisting host T cells to reject the graft (direct presentation). Donor APCs might also have a role in the development or maintenance of GVHD by stimulating an antihost response through indirect presentation of host alloantigen to donor T cells. Such antihost responses have recently been demonstrated in models of allogeneic HSC transplantation, where after engraftment APCs that were 100% donor caused chronic GVHD by indirect presentation of alloantigen,30-32 or through induction of autoimmunity.33 Our results suggest that the generation of donor CD34+ cell-derived APCs is likely to start early after allogeneic transplantation. These cells might initially expand and differentiate into APCs due to host T-cell response against any type of donor APCs, including also a fraction of CD34+ cells. These new CD34-derived APCs could then indirectly present host alloantigens to donor T cells. Activated T cells would be capable of recruiting new DC progenitors from the expanding pool of engrafting CD34+ cells, creating an amplification loop where reciprocal activation perpetuates the T-cell response to host alloantigens, which persist in nonhematopoietic tissues.
The magnitude and importance of such an amplification loop to the physiology of allogeneic bone marrow transplantation is yet to be determined. Interactions between donor T cells and host APCs also generate cytokines that can drive the differentiation of CD34+ cells into functional APCs, and the scale of that alloreaction in all likelihood will be greater than that occurring between host T cells and donor CD34+ cells. But this amplification loop could be of significant proportion in transplantations using nonmyeloablative chemoradiotherapy because CD34+ cells of the host that persist after conditioning may differentiate into APCs following interactions with donor T cells, enhancing the antihost response by direct presentation of alloantigen.34,35 These interactions could help explain recent clinical data showing that the overall rate of acute GVHD in allogeneic nonmyeloablative stem cell transplantation is delayed but not significantly reduced compared with myeloablative transplantations.36,37
The chronic nature of the interactions between CD34-APCs and T cells in amplifying an antihost response suggests strategies for preventing and treating GVHD that are not usually hypothesized in animal models where the onset of disease is rapid. In particular, neutralization of TNFα may be considered not only because it is a direct mediator of target organ damage38,39 but also for its role in the generation of mature DCs, as shown in this study. Future experiments will address the ability of TNFα blockade to prevent the proliferation and differentiation of CD34-APCs at the time of transplantation. Other approaches, such as blockade of B7 costimulatory pathway, could be exploited not only to induce T-cell unresponsiveness but also to reduce the number of CD34-APCs generated. Such strategies, aimed at reducing T-cell responses by preventing the development of CD34-derived APCs, may eventually also prove useful to prevent graft rejection in solid organ transplantation and to intervene in autoimmune disorders.
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-11-4330.
Supported in part by University of Illinois at Chicago funding (D.R.) and National Institutes of Health (NIH) grant PO1 CA 39542. J.L.M.F. is the recipient of a Doris Duke Distinguished Clinical Scientist award, and P.R. is the recipient of a Doris Duke Clinical Scientist Development Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Richard Boismenu (Repligen, Waltham, MA) for providing the CTLA4-IgG4m. Ed Bruno is acknowledged for technical support.