Comment on Richardson et al, page 3458
Novel agents have revolutionized the current treatment paradigm for patients with multiple myeloma. Is lenalidomide another thalidomide or does it offer more?
The past 10 years have yielded a plethora of practice-changing treatment options for patients with myeloma. Beginning with the survival benefit associated with high-dose therapy and autologous transplantation,1 current options include the widespread use of thalidomide2 and bortezomib3 for treatment of relapsed and newly diagnosed myeloma. These options have not only improved the magnitude of response but also significantly improved overall survival, advancing the median survival from 2.5 years to between 5 and 7 years for the average patient. With the recent introduction of lenalidomide, these advances will likely continue.
In this issue of Blood, Richardson and colleagues present the results of an important phase 2 clinical trial evaluating 2 different schedules of lenalidomide (30 mg orally once daily vs 15 mg orally twice daily) for the treatment of patients with relapsed/refractory myeloma. The aggregate response rate in this trial of heavily pretreated patients was 25% for the single agent (24% for the once-daily schedule and 29% for the twice-daily schedule), with recent reports from 2 large randomized phase 3 trials suggesting response rates between 55% and 60% with the addition of dexamethasone.4 This is good news for patients and physicians not only because of the single-agent activity but also because many of the treated patients had previously progressed on thalidomide.5
So, were the 2 treatment arms really the same? Despite a similar response rate, toxicity was clearly not the same between the 2 treatment schedules. The original hypothesis behind the twice-daily dosing schedule was that perhaps myelosuppression associated with lenalidomide was related to pharmacokinetics. Though there was a lower Cmin (minimum plasma concentration) for the group randomized to twice-daily therapy, myelosuppression was more prevalent earlier in the course of therapy, and the incidence of treatment-emergent peripheral neuropathy was also higher. Because of these side effects, the twice-daily arm was closed prematurely. “The same” is different, very different.FIG1
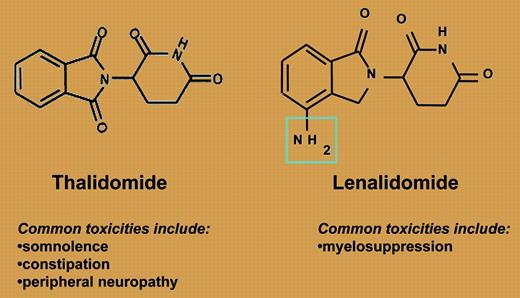
Lenalidomide and thalidomide: structurally simple, functionally different.
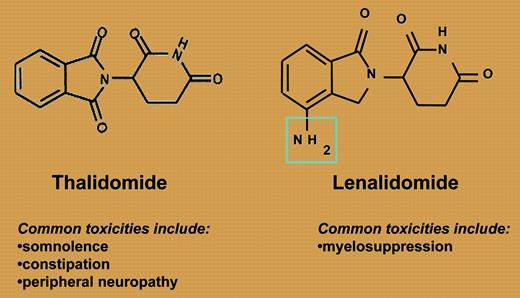
Lenalidomide and thalidomide: structurally simple, functionally different.
So where does this leave us? Thalidomide and lenalidomide are ostensibly in the same class of drugs, as both are immunomodulatory agents (see figure). The efficacy of both agents is enhanced with the addition of dexamethasone, as is the risk of deep vein thrombosis (DVT). However, they are quite different with respect to efficacy and toxicity. Lenalidomide is not associated with the same frequency or severity of commonly seen thalidomide side effects such as neuropathy, constipation, or somnolence. Myelosuppression, the dose-limiting toxicity from phase 1 studies, is a rare side effect with thalidomide. Interestingly, Richardson and colleagues provide the first real insight into the risk factors for developing this complication: previous high-dose therapy and autologous transplantation. Finally, the depth of response is clearly improved with the use of lenalidomide-based therapy, with a complete response (CR)/near complete response (nCR) rate significantly higher than is seen with thalidomide, and is clearly effective among patients who have progressed on thalidomide.
Clearly we have a new weapon in the battle against myeloma and, with the recent Food and Drug Administration (FDA) approval of lenalidomide for the treatment of relapsed myeloma, many patients are already benefiting. The questions yet to be resolved are as follows. How can this agent best be combined with other agents, novel or cytotoxic? What is the optimal anticoagulation prophylaxis, and which patients are at the highest risk for developing myelosuppression? These and many more questions are likely to be answered in the near future. For now, we can be content knowing that caring for our patients with myeloma will not be the same, it will be distinctly different. ▪