Abstract
Activating mutations of FMS-like tyrosine kinase 3 (FLT3) are present in approximately one third of patients with acute myeloid leukemia (AML) and are associated with adverse prognosis. The important role played by FLT3 in the survival and proliferation of blasts, and its overexpression in most patients with AML, make FLT3 an attractive therapeutic target. We undertook a phase 2 trial of the FLT3-selective tyrosine kinase inhibitor lestaurtinib (CEP701) used as monotherapy in untreated older patients with AML not considered fit for intensive chemotherapy, irrespective of FLT3 mutation status. Lestaurtinib was administered orally for 8 weeks, initially at a dose of 60 mg twice daily, escalating to 80 mg twice daily, and was generally well tolerated. Clinical activity, manifest as transient reductions in bone marrow and peripheral-blood blasts or longer periods of transfusion independence, was seen in 3 (60%) of 5 patients with mutated FLT3 and 5 (23%) of 22 evaluable wild-type FLT3 patients. Laboratory data demonstrated that clinical responses occurred where the presence of sustained FLT3-inhibitory drug levels were combined with in vitro cytotoxic sensitivity of blasts to lestaurtinib. Further evaluation of this compound, in combination with cytotoxic chemotherapy or other targeted agents, is warranted in both FLT3 mutant and wild-type patients.
Introduction
It is generally considered that the treatment of acute myeloid leukemia (AML) with conventional cytotoxic agents has reached its limits, with long-term survival still being achieved in less than 50% of cases. Increased understanding of the heterogeneous cytogenetic and molecular mechanisms underlying AML suggests that novel agents targeting individual molecular lesions used either alone, in combination, or as an adjunct to conventional chemotherapy, hold promise for future improvements. The drive for a targeted therapeutic approach has been given momentum by the remarkable success of the tyrosine kinase inhibitor imatinib mesylate in chronic myeloid leukemia (CML),1 although, given its greater molecular heterogeneity, it is unlikely that any single targeted agent will achieve the same universal impact in AML.
The type III receptor tyrosine kinase FMS-like tyrosine kinase 3 (FLT3) is a potentially attractive therapeutic target in AML. FLT3 plays an important role in the development of normal multipotent stem cells and B cells2,3 and is overexpressed in most patients with AML.4-6 Activating mutations of FLT3, including internal tandem duplication (ITD) mutations within the juxtamembrane region7 and single-base point mutations within the tyrosine kinase domain of the receptor (TKD-PMs)8,9 are among the commonest molecular lesions so far described in AML, with a combined prevalence of 31%.10 Both types of mutation cause autophosphorylation of the receptor, leading to activation of downstream signaling pathways involved in regulation of transcription, proliferation, and apoptosis.11-13 The overexpressed wild-type (WT) FLT3 receptor may also be activated in AML cells by endogenous coexpression of the FLT3 ligand.14,15 FLT3 in both its mutated and WT configurations may thus contribute significantly to leukemogenesis, although for AML to occur, at least 1 further molecular event that is inhibitory to cell differentiation is likely to be required.16,17 The presence of a FLT3 ITD mutation is independently predictive of increased relapse risk and reduced overall survival.18-20
There is a strong clinical rationale for the therapeutic targeting of activated FLT3 in AML and a number of FLT3-selective small-molecule tyrosine kinase inhibitors have been developed in recent years. Preclinical studies have demonstrated that inhibition of FLT3 is cytotoxic to cell lines and primary AML cells harboring FLT3 mutations.21-25 Early clinical studies have generally been restricted to patients with FLT3 mutations in the context of relapsed or refractory disease. In these phase 1 and 2 trials, FLT3 inhibitors have largely been well tolerated, with clinical responses being limited to transient reductions in peripheral-blood and bone marrow blasts.26-30
Lestaurtinib (CEP701) is an orally bioavailable indolocarbazole alkaloid compound that is synthetically derived from the bacterial fermentation product K-252a. The compound is FLT3 selective, inhibiting phosphorylation of FLT3 with an IC50 of 2 to 3 nM, but that of other class III receptor tyrosine kinases with IC50s in excess of 500 nM. Lestaurtinib is cytotoxic to human AML cell lines expressing both mutant and WT FLT3 and prolongs survival in a mouse model of FLT3/ITD leukemia.21 The drug has been shown to have a low toxicity profile in animal studies, in healthy human volunteers, and in cancer patients31 where dose-limiting toxicities were reported in 1 patient at 80 mg twice daily (grade 3 nausea) and a further patient at 120 mg twice daily (grade 3 hypotension). In a recently published phase 1/2 clinical trial, lestaurtinib was used at doses of 40 to 80 mg twice daily as single-agent salvage therapy for 17 patients with relapsed, refractory, or poor-risk AML harboring FLT3-activating mutations. Transient, limited clinical responses were seen in 5 patients and correlated with evidence of sustained in vivo FLT3 inhibition.28
We undertook a phase 2 trial to determine the clinical effects of lestaurtinib monotherapy in a population of previously untreated older patients with AML who were not considered fit for a conventional intensive chemotherapeutic approach. Our preclinical studies of lestaurtinib32,33 show successful in vitro FLT3 inhibition and induction of cytotoxicity in primary blasts obtained both from patients harboring activating mutations of FLT3 and those expressing only WT FLT3; on this basis, the presence of a FLT3 mutation was not a prerequisite for trial entry. As an adjunct to the trial, correlative laboratory studies were performed to assess the relationship between clinical response to lestaurtinib and in vivo inhibition of FLT3, FLT3-expression level, and in vitro cytotoxic response to the drug.
Patients, materials, and methods
Patient selection
Patients were eligible for the study if they had untreated (de novo or secondary) AML as defined by the World Health Organization (WHO) classification34 and were considered unfit for intensive chemotherapy. This included all patients over the age of 70 years, as well as patients aged 60 to 70 years with an Eastern Cooperative Oncology Group (ECOG) performance score greater than 2 or a history of cardiac disease. A stable white blood cell count (WCC) of less than 30 × 109/L for 7 days was required before starting lestaurtinib treatment. Patients with a WCC greater than 30 × 109/L were able to receive hydroxyurea (hydroxycarbamide) for up to 21 days to reduce the WCC to less than 10 × 109/L before commencing lestaurtinib. Trial entry was not restricted on the basis of FLT3 mutation status. Exclusion criteria included acute promyelocytic leukemia, inadequate renal or hepatic function (creatinine, bilirubin, alanine aminotransferase [ALT], or aspartate aminotransferase [AST] levels greater than 2 times the upper limit of normal), active gastrointestinal ulceration or bleeding, and HIV positivity. Written, informed consent was obtained from all patients before study enrollment. The study was approved by the Wales Multicentre Research Ethics Committee and the Local Research Ethics Committee at each participating institution and conducted in accordance with the Declaration of Helsinki.
Study design
The study was a multicenter, open-label phase 2 trial of single-agent lestaurtinib. The primary endpoint was the determination of the response rate of older patients with previously untreated AML. Secondary endpoints were the assessment of drug safety and tolerability, response duration and the relationship of clinical response to FLT3 mutation status, FLT3-expression level, and inhibition of FLT3 phosphorylation. Patients were recruited using a Simon 2-stage, minimax design, allowing early closure if none of the first 18 patients achieved a clinically significant response.35
Lestaurtinib was supplied by Cephalon (West Chester, PA) as a clear yellow oral solution in amber glass vials and administered following dilution in fruit juice. Patients received lestaurtinib for a period of 56 days, initially at a dose of 60 mg twice daily, with escalation to 80 mg twice daily from day 29 in the absence of significant drug-related toxicity. Dose reduction to 40 mg twice daily was permitted at the discretion of the treating physician in the presence of unacceptable toxicity, or when the study drug was restarted following recovery from grades 3/4 nonhematologic toxicity. Following the completion of treatment, responding patients had the opportunity of continuing lestaurtinib until evidence of disease progression.
Assessment of toxicity and response
Patients were monitored at least weekly for the duration of the study. Safety assessments included evaluation of adverse events and vital signs, routine hematologic and biochemical tests, clinical review, and physical examination. Toxicity was graded according to the National Cancer Institute (NCI) Common Toxicity Criteria version 2.0. Response to treatment was assessed by weekly peripheral-blood examination, and bone marrow examinations on study days 14, 28, and 56. Standard criteria were used to define complete remission (CR) and partial remission (PR), respectively, as a decrease in marrow blasts to less than 5% (CR), and a more than 50% decrease in marrow blasts to 5% to 25% (PR) in a marrow of sufficient cellularity, with a peripheral neutrophil count of more than 1 × 109/L and a platelet count of more than 100 × 109/L, where these changes were maintained for at least 4 weeks.36,37 Bone marrow response (BMR) was defined as a reduction in marrow blasts by more than 50% from diagnosis without hematologic recovery, and hematologic response (HR) as disappearance of blasts from the peripheral blood not attributable to hydroxyurea therapy. No response (NR) and progressive disease (PD) were defined respectively as no evidence of improvement in blood or bone marrow, and evidence of increased blasts in bone marrow or peripheral blood.
FLT3 mutation analysis
Bone marrow mononuclear cells (BM MNCs) were obtained from samples taken at trial entry (day 0) by Ficoll-Hypaque centrifugation (Sigma Aldrich, Gillingham, United Kingdom). mRNA was extracted using Trizol and Eppendorf Phase Lock Gel tubes (Fisher Scientific, Loughborough, United Kingdom). FLT3-ITD reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using the forward primer 5′-GCA AAT TAG GTA TGA AAG CCA GC-3′ and the reverse primer 5′-CTT TCA GCA TTT TGA CGG CAA CC-3′. FLT3 TKD-PMs were detected using the forward primer 5′-CCG CCA GGA ACG TGT TTG-3′ and the reverse primer 5′-CAC AGT AAT ATT CCA TAT GAC CAG ATA TC-3′ followed by EcoRV or HinfI digestion. PCR products were analyzed and allelic ratios calculated using DNA500 chips on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) Individual mutations were sequenced using the BigDye protocol (Applied Biosystems, Foster City, CA).
FLT3-expression analysis
For serial assessment of cell-surface FLT3 expression, BM MNCs obtained on trial days 0, 14, 28, and 56 were stained with a PE-conjugated anti-CD135 antibody (Beckman Coulter, London, United Kingdom) and analyzed by flow cytometry. Blasts were identified by CD45/side-scatter gating, and FLT3 protein expression was quantified as a test-to-control ratio of molecules of equivalent soluble fluorochrome (MESF) values. FLT3 RNA expression was quantified in BM MNCs obtained at trial entry relative to the level of the stably expressed housekeeping gene S14, measured by a real-time PCR fluorescent detection method using the DNA-specific dye SYBR Green I. Primer sequences were the same as those used for FLT3-ITD mutation analysis. The results were analyzed by LightCycler Relative Quantification Software (Roche, Mannheim, Germany).
Assays of in vivo and ex vivo FLT3 phosphorylation
In vivo inhibition of FLT3 signaling was assessed by comparison of FLT3 phosphorylation in lysed BM MNCs obtained at day 0 with that seen in samples obtained on trial days 14, 28, and 56. Cells were washed 3 times in ice-cold phosphate-buffered saline (PBS) then lysed by resuspension in lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 1% Igepal (Sigma Aldrich), 10% glycerol, 10 mM EDTA, 20 mM NaF, and 3 mM NaVO4) plus complete protease inhibitors (MiniComplete EDTA free; Roche) for 30 minutes at 4°C followed by centrifugation at 23 500g. Clarified protein lysate (500 μg) was incubated overnight with anti–FLT3 S18 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) prior to the addition of protein A agarose beads (Upstate Biotechnology, Lake Placid, NY) for 2 hours. Following electrophoresis using NuPage precast 4% to 12% Bis-Tris gels (Invitrogen, Paisley, United Kingdom) and transfer to PVDF membranes (Invitrogen), immunoblotting was performed with anti–phosphotyrosine 4G10 antibody (Upstate Biotechnology) to measure phosphorylated FLT3. The extract was then stripped and reprobed with anti-FLT3 antibody to measure total FLT3 protein. Blots were visualized by chemiluminescence (ECL Advance; Amersham Biosciences, Piscataway, NJ), with the resultant images being analyzed using a UVIDoc Acquisition Unit and UVISoft Image Analysis Software (UVITec, Cambridge, United Kingdom).
The plasma inhibitory activity (PIA) for FLT3 from patients treated with lestaurtinib was assessed using a surrogate ex vivo bioassay as previously described.28 Briefly, a clonal cell line expressing constitutively phosphorylated FLT3 was created by transfecting TF-1 cells (human AML M6, lacking FLT3 protein expression; ATCC, Rockville, MD), with a FLT3 ITD mutation isolated from a patient with AML. The resulting TF/ITD cells showed dose-dependent FLT3 dephosphorylation in response to increasing concentrations of lestaurtinib. Plasma was obtained from trial patients at day 0 and immediately prior to morning drug dosing on trial days 14, 28, and 56 and stored at –20°C. Frozen samples were thawed and clarified by centrifugation at 23 500g for 2 minutes. For each timepoint, 4 × 106 TF/ITD cells were incubated with 1mL plasma at 37°C for 2 hours, then washed twice with ice-cold PBS, lysed, and analyzed by sequential immunoblotting with antiphosphotyrosine and anti-FLT3 antibodies. TF/ITD-cell FLT3-phosphorylation levels were quantified by densitometric analysis of scanned immunoblot images as a surrogate measure of FLT3 inhibition in patients at different trial timepoints.
In vitro cytotoxicity assay
In vitro cytotoxic response to lestaurtinib was assessed in all day-0 bone marrow samples where adequate numbers of cells were obtained. BM MNCs were isolated by density gradient centrifugation and cryopreserved in 10% dimethyl sulfoxide (DMSO). After rapid thawing and repeat density gradient centrifugation to remove dead or dying cells, cells were cultured in RPMI/10% fetal bovine serum containing a range of concentrations of lestaurtinib. After 72 hours, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium (MTS) assay was performed according to the manufacturer's instructions (Promega, Madison, WI) to measure survival of lestaurtinib-treated cells relative to that of control cells exposed to equal DMSO concentrations.
Results
Patients
Between September 2003 and September 2004, 29 eligible patients (13 women, 16 men) with previously untreated AML were enrolled at 12 centers. Patient characteristics are summarized in Tables 1 and 2. The median age was 73 years (range, 67-82 years). Four patients aged younger than 70 years were recruited, with inclusion criteria in these cases being an ECOG performance score of 3 (patient 8) and significant accompanying cardiac disease (patients 6, 20, and 25). Five patients harbored activating mutations of FLT3: patients 8 and 15 had FLT3 ITD mutations of 30 and 33 base pairs, accounting for 13% and 34% of total FLT3 RNA, while TKD-PMs were detected in 3 patients, including 1 I836 deletion mutation (patient 5), 1 D835Y mutation (patient 19), and 1 previously undescribed D835V, I836F, M837P 3-codon triple-point mutation (patient 25).38 Five patients were treated with hydroxyurea for a median of 10 days (range, 6-20 days) before commencing lestaurtinib; none of these patients received lestaurtinib and hydroxyurea simultaneously.
Toxicity
Lestaurtinib was generally well tolerated. Commonly observed toxicities included mild nausea (8 patients), emesis (5 patients), constipation (5 patients), diarrhea (6 patients), and elevations in alkaline phosphatase concentration (13 patients). In 2 patients (patients 12 and 16), lestaurtinib was withdrawn after 11 and 8 days, respectively, following the development of NCI grade 2 nausea and vomiting. The dose of lestaurtinib was increased to 80 mg twice daily in 19 (66%) patients, with grades 3 to 4 nausea (2 patients) and diarrhea (2 patients) being seen at the higher dose. Dose reduction to 40 mg was performed in only 1 patient (patient 20), at the discretion of the treating physician, following the development of grade 2 rectal bleeding in the setting of grade 4 thrombocytopenia. Lestaurtinib was discontinued at day 17 in 1 patient (patient 15) who developed grade 4 fatigue at a dose of 60 mg twice daily. One patient suffered a fatal intracerebral hemorrhage in the setting of grade 4 thrombocytopenia on day 36 while taking lestaurtinib at a dose of 80 mg twice daily. The frequency and grading of adverse effects are summarized in Table 3.
Clinical response
Clinical responses were evaluable in 27 of 29 patients. Clinical activity was evident in 8 (30%) patients, including 3 (60%) of 5 FLT3 mutant patients and 5 (23%) of 22 evaluable FLT3 WT patients, the difference in response rates between mutation groups not reaching statistical significance. Responses are summarized in Table 4 and detailed individually in Table 2.
No patient attained CR or PR by standard criteria.36;37 One patient (patient 6) achieved a more than 50% reduction in bone marrow blasts with normalization of peripheral counts, but this response was maintained for only 14 days. In patient 28 a reduction in bone marrow blasts to less than 5% was seen, but this was accompanied by persistent thrombocytopenia and bone marrow hypocellularity.
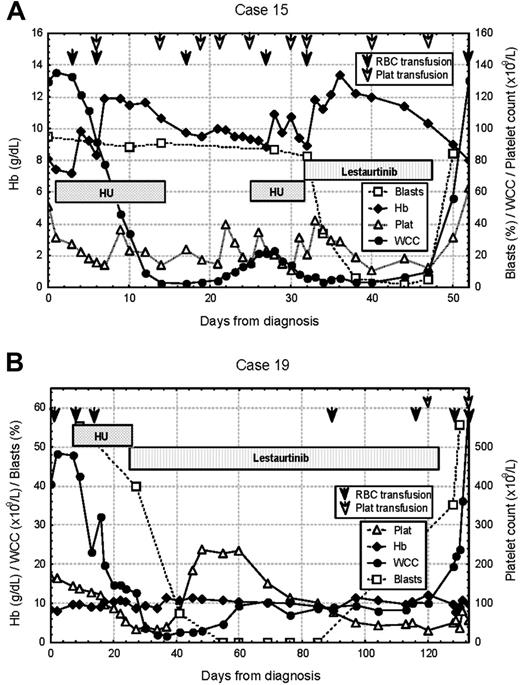
The majority of clinical responses were of short duration (median time to progression, 25 days; range, 14-270 days). Figure 1 illustrates typical hematologic responses seen in 2 patients with activating mutations of FLT3. Both of these patients were initially cytoreduced with hydroxyurea before commencing lestaurtinib and achieved complete clearance of blasts from the peripheral blood after the study drug was introduced. Patient 15 progressed rapidly after lestaurtinib was withdrawn due to toxicity, while patient 19 achieved transfusion independence for 2 months prior to disease progression. In such cases it is difficult to fully exclude the influence of hydroxyurea from the initial clinical response, this being an unavoidable consequence of the ethical requirement to use a nonexperimental agent for cytoreduction to protect patients with high presenting WCC against the possibility of nonresponse. A similar overall response rate to lestaurtinib was seen, however, in patients that did not require preliminary hydroxyurea therapy, with clinical activity evident in 6 (26%) of 23 evaluable patients.
All of the clinical responses to lestaurtinib that were seen during the 56-day dosing period occurred at the starting dose of 60 mg twice daily. No patient responded to 80 mg lestaurtinib who previously failed to respond to 60 mg, although 1 patient, patient 6, made transient bone marrow responses to both 60 mg and 80 mg lestaurtinib, with disease progression occurring within 14 days on both occasions.
Serial blood counts obtained in 2 clinical responders to lestaurtinib. (A) Patient 15, a 74-year-old woman with an ITD mutation, presented with a WCC of 140.7 × 109/L, and was initially cytoreduced with hydroxyurea (HU). HR was achieved on 60 mg of lestaurtinib, with complete clearance of peripheral-blood blasts. There was rapid disease progression after lestaurtinib was withdrawn at day 17 due to the onset of grade 4 fatigue. (B) Patient 19, a 74-year-old man with a D835Y point mutation, presented with a WCC of 42.4 × 109/L and was initially cytoreduced with hydroxyurea. HR was achieved on 60 mg of lestaurtinib, with peripheral-blood blast clearance, transient normalization of neutrophil and platelet counts, and transfusion independence lasting 2 months before disease progression.
Serial blood counts obtained in 2 clinical responders to lestaurtinib. (A) Patient 15, a 74-year-old woman with an ITD mutation, presented with a WCC of 140.7 × 109/L, and was initially cytoreduced with hydroxyurea (HU). HR was achieved on 60 mg of lestaurtinib, with complete clearance of peripheral-blood blasts. There was rapid disease progression after lestaurtinib was withdrawn at day 17 due to the onset of grade 4 fatigue. (B) Patient 19, a 74-year-old man with a D835Y point mutation, presented with a WCC of 42.4 × 109/L and was initially cytoreduced with hydroxyurea. HR was achieved on 60 mg of lestaurtinib, with peripheral-blood blast clearance, transient normalization of neutrophil and platelet counts, and transfusion independence lasting 2 months before disease progression.
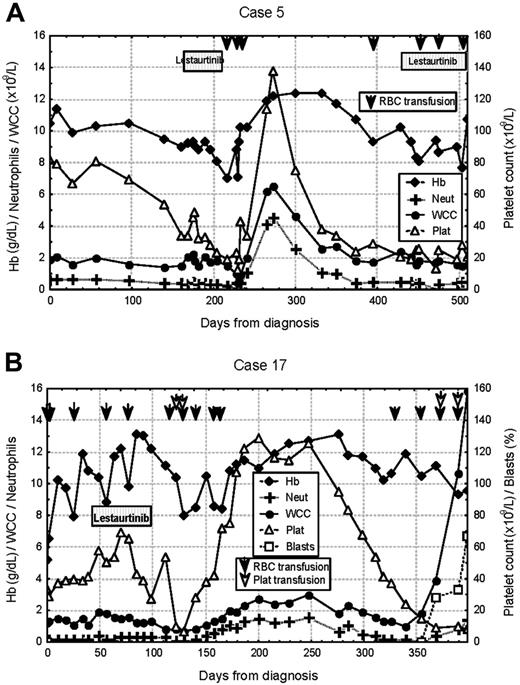
Serial blood counts obtained in 2 delayed responders to lestaurtinib. (A) Patient 5, a 75-year-old man with an I836 deletion point mutation, presented with pancytopenia. No peripheral-blood or bone marrow response was seen in 56 days of lestaurtinib therapy. Transfusion independence was subsequently achieved for a period of 4 months from trial day 104, which was associated with transient normalization of WCC and neutrophil and platelet counts. There was no second response following hematologic deterioration and reinstitution of lestaurtinib therapy. (B) Patient 17, a 72-year-old woman with WT FLT3, also presented with pancytopenia. Bone marrow blast numbers fell from 80% to 30% over 56 days of lestaurtinib treatment, after which the drug was withdrawn following an episode of febrile neutropenia. The patient made a marked delayed hematologic response, with improvements in platelet and neutrophil counts and sustained transfusion independence between days 123 and 284. At the time of subsequent disease progression, the patient declined the option of a second course of lestaurtinib treatment and died on trial day 364.
Serial blood counts obtained in 2 delayed responders to lestaurtinib. (A) Patient 5, a 75-year-old man with an I836 deletion point mutation, presented with pancytopenia. No peripheral-blood or bone marrow response was seen in 56 days of lestaurtinib therapy. Transfusion independence was subsequently achieved for a period of 4 months from trial day 104, which was associated with transient normalization of WCC and neutrophil and platelet counts. There was no second response following hematologic deterioration and reinstitution of lestaurtinib therapy. (B) Patient 17, a 72-year-old woman with WT FLT3, also presented with pancytopenia. Bone marrow blast numbers fell from 80% to 30% over 56 days of lestaurtinib treatment, after which the drug was withdrawn following an episode of febrile neutropenia. The patient made a marked delayed hematologic response, with improvements in platelet and neutrophil counts and sustained transfusion independence between days 123 and 284. At the time of subsequent disease progression, the patient declined the option of a second course of lestaurtinib treatment and died on trial day 364.
Figure 2 illustrates the clinical responses seen in 2 patients (patients 5 and 17) who made unanticipated delayed hematologic responses that occurred after completion of 56 days of lestaurtinib therapy. Both of these patients were profoundly neutropenic and thrombocytopenic prior to commencing lestaurtinib, showed little change in counts during the period of study drug treatment, but subsequently achieved blood-count normalization, with prolonged transfusion independence lasting 130 and 161 days, respectively.
Correlative laboratory studies
A number of correlative laboratory assays were performed using patient bone marrow, peripheral blood, and plasma samples obtained at trial enrollment and at day-14, -28, and -56 assessments to help understand the basis of the clinical activity of lestaurtinib in individual patients.
FLT3-expression assays
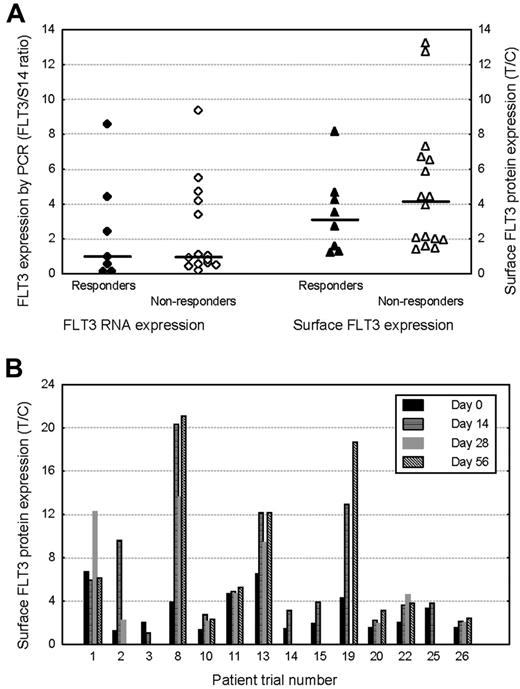
Sufficient material was available for flow cytometric quantification of pretreatment blast surface FLT3 expression in 26 of 29 patients. In 23 patients, pretreatment FLT3 RNA expression was also quantified by real-time PCR, relative to expression of the housekeeping gene S14. No relationship was evident between pretreatment FLT3 expression, quantified in terms of both surface protein or RNA expression, and the degree of subsequent clinical response to lestaurtinib (Figure 3A). In 14 patients, sufficient material was available to allow quantification of blast FLT3 surface-protein expression at day 0 and at least 1 later timepoint during lestaurtinib therapy. Nearly all (13) of the 14 patients demonstrated increases in blast surface FLT3 expression during in vivo FLT3 inhibitor treatment (Figure 3B).
Inhibition of FLT3 signaling
It is highly desirable that any clinical benefit attributed to lestaurtinib is assessed in the context of the degree of target inhibition achieved. Simple pharmacokinetic measurement of plasma drug concentration is unreliable as a surrogate of FLT3 inhibitory activity because lestaurtinib is highly protein bound with potentially wide variability in levels of free, active drug. Preclinical in vitro studies suggest that continuous inhibition of FLT3 autophosphorylation to below 15% of baseline activity is necessary to achieve a cytotoxic effect on leukemic blasts.28,33 In the clinical setting, however, direct measurement of changes in in vivo FLT3 phosphorylation over time by immunoblotting is made difficult by variation in blast numbers during lestaurtinib treatment, with some patients losing the FLT3 signal entirely after clinical drug response.
FLT3 expression and clinical response. (A) Comparison of pretreatment FLT3 expression in clinically lestaurtinib-responsive (hematologic response or greater) and nonresponsive patients. FLT3 expression was quantified at RNA level in BM MNCs by real-time PCR relative to the stably expressed housekeeping gene S14. Surface FLT3 protein expression was measured flow cytometrically using PE-conjugated anti-CD135. Blasts were selected using a CD45/side-scatter gating strategy. The horizontal bars represent the median expression levels in each group. (B) Changes in surface FLT3 protein expression in 14 patients assayed serially, prior to, and during lestaurtinib treatment.
FLT3 expression and clinical response. (A) Comparison of pretreatment FLT3 expression in clinically lestaurtinib-responsive (hematologic response or greater) and nonresponsive patients. FLT3 expression was quantified at RNA level in BM MNCs by real-time PCR relative to the stably expressed housekeeping gene S14. Surface FLT3 protein expression was measured flow cytometrically using PE-conjugated anti-CD135. Blasts were selected using a CD45/side-scatter gating strategy. The horizontal bars represent the median expression levels in each group. (B) Changes in surface FLT3 protein expression in 14 patients assayed serially, prior to, and during lestaurtinib treatment.
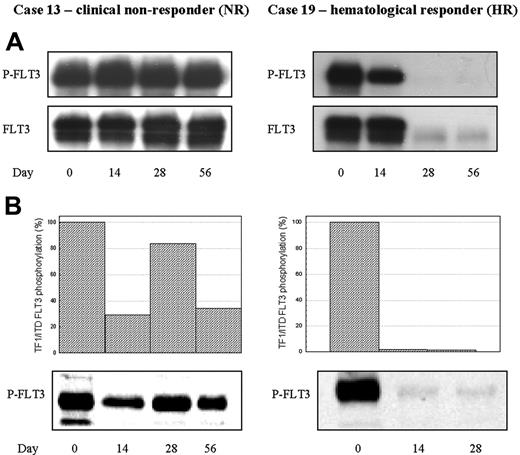
In vivo and ex vivo bioassay of FLT3 inhibition by lestaurtinib in 2 representative patients. (A) Direct in vivo bioassay. FLT3 was immunoprecipitated from BM MNCs, and sequential Western blot was performed using 4G10 antiphosphotyrosine antibodies and anti-FLT3 antibodies. (B) “Ex vivo” FLT3 PIA assay. TF/ITD cells were exposed to patient plasma obtained 12 hours after lestaurtinib administration on trial days 14, 28, and 56, then lysed and analyzed by sequential immunoblotting with 4G10 and anti-FLT3 antibodies. Densitometric analysis of phosphorylated FLT3, normalized for the amount of total FLT3, is displayed graphically. Patient 13, a FLT3 WT patient, made no clinical response to lestaurtinib, with direct assay revealing no evidence of in vivo FLT3 inhibition, and ex vivo assay confirming incomplete inhibition of TF/ITD FLT3 by patient plasma at all timepoints. In contrast, Patient 19, a clinically responsive patient with a FLT3 point mutation, showed significant in vivo reduction in FLT3 phosphorylation by day 14. Reductions in blast numbers limited the validity of the direct assay on days 28 and 56, by which time the FLT3 signal had become almost undetectable. Ex vivo assay, however, confirmed the presence of sufficient continuing FLT3 PIA on days 14 and 28 to almost fully inhibit TF1/ITD FLT3 phosphorylation.
In vivo and ex vivo bioassay of FLT3 inhibition by lestaurtinib in 2 representative patients. (A) Direct in vivo bioassay. FLT3 was immunoprecipitated from BM MNCs, and sequential Western blot was performed using 4G10 antiphosphotyrosine antibodies and anti-FLT3 antibodies. (B) “Ex vivo” FLT3 PIA assay. TF/ITD cells were exposed to patient plasma obtained 12 hours after lestaurtinib administration on trial days 14, 28, and 56, then lysed and analyzed by sequential immunoblotting with 4G10 and anti-FLT3 antibodies. Densitometric analysis of phosphorylated FLT3, normalized for the amount of total FLT3, is displayed graphically. Patient 13, a FLT3 WT patient, made no clinical response to lestaurtinib, with direct assay revealing no evidence of in vivo FLT3 inhibition, and ex vivo assay confirming incomplete inhibition of TF/ITD FLT3 by patient plasma at all timepoints. In contrast, Patient 19, a clinically responsive patient with a FLT3 point mutation, showed significant in vivo reduction in FLT3 phosphorylation by day 14. Reductions in blast numbers limited the validity of the direct assay on days 28 and 56, by which time the FLT3 signal had become almost undetectable. Ex vivo assay, however, confirmed the presence of sufficient continuing FLT3 PIA on days 14 and 28 to almost fully inhibit TF1/ITD FLT3 phosphorylation.
A more standardized comparison of continuous FLT3 inhibitory activity, attributable to free-plasma lestaurtinib, and not reliant on the presence of large numbers of leukemic blasts, was made by measuring “FLT3 plasma inhibitory activity” (PIA) as a surrogate assay. In this technique, FLT3 phosphorylation levels were compared in FLT3/ITD-expressing TF/ITD cells treated with patient plasma obtained at trial entry and immediately prior to lestaurtinib dosing on days 14, 28, and 56 (trough drug levels). This “ex vivo” measurement of PIA against FLT3 allowed the assessment of sustained target inhibition by the study drug. To validate the ex vivo assay as a reliable indicator of the in vivo FLT3 inhibitory activity of lestaurtinib, a comparison was made, where sufficient cells were available, with direct assay of FLT3 phosphorylation in the patient's blasts. This comparison is illustrated in 2 representative cases, 1 clinically responsive to lestaurtinib and 1 resistant, in Figure 4.
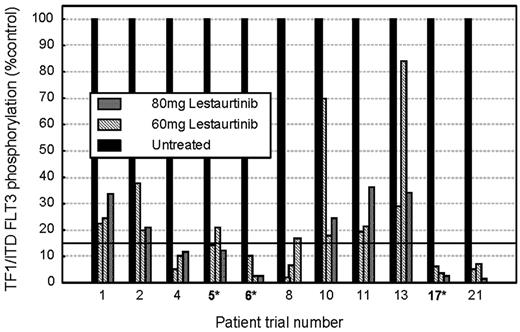
Variation in FLT3 PIA over time. FLT3 PIA assay was performed as described in “Patients, materials, and methods.” For each patient the 4 histogram columns show the FLT3 PIA, which was measured prior to commencement of lestaurtinib treatment and predosing on trial days 14, 28, and 56. *Clinically responsive patients (hematologic response or greater). No clinical responses were seen in patients who failed to demonstrate more than 85% sustained FLT3 PIA.
Variation in FLT3 PIA over time. FLT3 PIA assay was performed as described in “Patients, materials, and methods.” For each patient the 4 histogram columns show the FLT3 PIA, which was measured prior to commencement of lestaurtinib treatment and predosing on trial days 14, 28, and 56. *Clinically responsive patients (hematologic response or greater). No clinical responses were seen in patients who failed to demonstrate more than 85% sustained FLT3 PIA.
An Assay of FLT3 PIA was performed using plasma samples obtained from 24 of 27 evaluable trial patients. Overall, the target level of sustained FLT3 inhibition to below 15% of baseline activity was achieved in 16 of 24 patients at the dose of 60 mg twice daily; all 8 clinical responders to the drug were among these 16 patients. No clinical responses were seen in the 8 patients in which 85% sustained target inhibition was not achieved. Figure 5 illustrates changes in FLT3 PIA over time in 11 trial patients in whom plasma samples were available at all 4 timepoints: 3 clinically responsive patients are marked with an asterisk. Increases in lestaurtinib dose to 80 mg twice daily disappointingly failed to increase FLT3 PIA levels to more than 85% in any of the 5 patients who were reassessed after they had failed to achieve satisfactory inhibitory levels at the 60-mg dose.
In vitro cytotoxicity assays
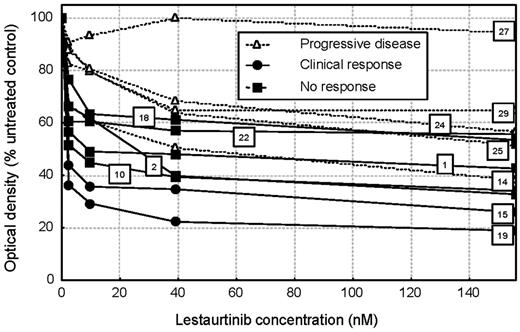
In vitro cytotoxic response of pretreatment BM MNCs to lestaurtinib was assessed by MTS assay in 12 patients for whom adequate cell numbers were available (Figure 6). Cytotoxic dose responses were highly heterogeneous: only 1 patient (patient 27) demonstrated complete in vitro cytotoxic resistance to lestaurtinib, although 5 other patients, particularly those with clinically unresponsive disease, displayed relative lestaurtinib resistance, with more than 50% of blasts surviving at maximal drug concentrations.
Direct correlation of in vitro cytotoxic response data, in vivo FLT3 inhibitory activity (quantified by ex vivo bioassay), and clinical response data were possible in 10 patients, summarized in Table 5. Sustained FLT3 inhibition to below 15% baseline activity was achieved in 7 of these 10 patients. Blasts from 2 of these 7 patients (patients 15 and 19) showed a significant in vitro cytotoxic response to lestaurtinib: these 2 patients also responded clinically. The other 5 patients displayed relative in vitro cytotoxic resistance to lestaurtinib and failed to respond clinically, suggesting that despite achieving adequate FLT3-inhibitory free-plasma lestaurtinib levels, blasts from these patients were inherently resistant to the study drug. In contrast, although the 3 patients who failed to achieve sustained 85% FLT3 PIA (patients 1, 2, and 10) also failed to respond clinically, the in vitro cytotoxic responses of blasts from these patients suggested a potential for clinical response if sustained plasma inhibitory concentration of lestaurtinib was to be achieved (for example, by future dose manipulation).
In vitro cytotoxicity assay. In vitro cytotoxic response of BM MNCs from 12 patients quantified by MTS assay after 72 hours of incubation with a range of concentrations of lestaurtinib. The trial number and clinical response status of each patient is indicated to facilitate comparison with Tables 2 and 5 and Figure 5.
In vitro cytotoxicity assay. In vitro cytotoxic response of BM MNCs from 12 patients quantified by MTS assay after 72 hours of incubation with a range of concentrations of lestaurtinib. The trial number and clinical response status of each patient is indicated to facilitate comparison with Tables 2 and 5 and Figure 5.
Discussion
The preclinical rationale to support the clinical use of FLT3 inhibitors in AML is strong. This study is the first clinical trial to examine the effects of FLT3 inhibitor monotherapy in patients with AML whose disease had not failed prior chemotherapy. It is also the first study of its kind to recruit patients irrespective of their FLT3 mutation status.
Oral lestaurtinib was generally well tolerated in this group of older patients, with reported toxicities being mild in comparison with those expected with traditional cytotoxic chemotherapy. The most commonly reported events were mild gastrointestinal side effects that were somewhat more marked following dose escalation to 80 mg.
For the older AML age group under study, a typically representative population with a FLT3 mutation rate of 17% was recruited. The limited clinical responses to lestaurtinib were of a similar frequency, depth, and duration to those reported in previous studies of FLT3 inhibitor monotherapy in relapsed/refractory patients with FLT3-activating mutations.26-30 No complete or partial remissions were seen by standard criteria and the median time until evidence of disease progression was 25 days, although some more prolonged responses with extended transfusion independence were seen. It seems likely that any potential increase in drug sensitivity to be expected from recruiting a previously untreated population was offset by the selection of older patients with fewer FLT3 mutations. Similar clinical response rates were seen irrespective of the need for pre-lestaurtinib cytoreductive treatment with hydroxyurea, although in patients with high presenting WCC it can be very difficult to completely exclude the possibility of hydroxyurea influencing the initial clinical response.
Preclinical studies suggest that AML blasts that express only WT FLT3 have variable, but often significant dependence on FLT3 signaling for survival, with cells from many WT patients showing significant in vitro susceptibility to the cytotoxic effects of lestaurtinib.32,33 In the current trial, clinical responses in WT patients were less frequent (response rate, 23% vs 60%) but generally of a similar magnitude and duration to those described in FLT3 mutant patients.
Two patients in the current study, an FLT3 point mutant patient and a WT patient, made prolonged delayed clinical responses to lestaurtinib that peaked several weeks after the study drug was withdrawn, achieving blood-count improvements and transfusion independence for periods of 4 and 6 months, respectively. Recent data have demonstrated that FLT3-activating mutations are present in leukemia stem cells and that these cells may be dependent on FLT3 signaling for survival.39 Lestaurtinib inhibits engraftment of FLT3/ITD stem cells in mice, suggesting a direct cytotoxic effect on stem cells,39 and it has been hypothesized that stem-cell–directed therapeutic effects could underlie such delayed responses.40 These interesting responses, if repeated, could suggest a future “adjuvant” role for FLT3 inhibitors in the elimination of residual leukemic stem cells after the induction of conventional disease remission by traditional means.
Correlative laboratory studies were performed to help understand the basis of the variation in clinical activity of lestaurtinib between different patients. No link was established between baseline levels of FLT3 expression and the likelihood of clinical response to lestaurtinib, providing no clear rationale for the future selection of patients for FLT3 inhibitor therapy based on this parameter. Nearly all (13 of 14) patients who were assayed serially demonstrated increases in blast-surface FLT3 expression during FLT3 inhibitor therapy, and it is uncertain whether this represents a potential drug resistance mechanism or merely reflects that, following lestaurtinib exposure, a larger proportion of total cellular FLT3 is deactivated and returns to the cell membrane. This up-regulated FLT3 expression may have been 1 factor that limited the benefits of midtreatment dose escalation from 60 mg to 80 mg in the current study.
Given the contrast between generally favorable preclinical data and the relatively modest in vivo responses seen in single-agent clinical trials to date, it is important to consider why some patients who are responsive to FLT3 inhibitors in vitro show little or no in vivo response. In the current study, a clear relationship was seen between the results of assays of plasma FLT3 inhibitory activity, in vitro cytotoxicity, and clinical response. All 8 patients in whom clinical activity was evident achieved sufficient sustained plasma lestaurtinib levels, as quantified by the ex vivo bioassay, to dephosphorylate FLT3 to a level less than 15% of baseline. One third of the patients failed to achieve these 85% FLT3-inhibitory plasma levels at the 60-mg dose of lestaurtinib and, disappointingly, inhibitory levels failed to improve consistently following dose escalation to 80 mg twice daily. Lestaurtinib is highly protein bound, and this effect may vary between patients. This is an important area of further research: by judicious monitoring of plasma drug levels in future studies it may be possible to tailor dosing in individual patients to ensure sustained FLT3-inhibitory levels in all cases.
In patients where the ex vivo assay indicated that adequate plasma FLT3-inhibitory activity was present, 8 of 16 cases failed to achieve a clinical response. In vitro cytotoxicity assay showed blasts from these patients to display relative intrinsic resistance to lestaurtinib. Preclinical studies have previously demonstrated a lack of cytotoxic response to lestaurtinib in the face of successful inhibition of FLT3, both in leukemia cell lines and in a proportion of primary blast samples.21,33 This intrinsic resistance can be hypothesized to occur by several mechanisms. Some patients may have relatively little dependence on FLT3 signaling for blast proliferation, as evidenced by the relative lack of FLT3 activation seen in some cases at baseline. Other patients, particularly those with WT FLT3, may activate signaling pathways downstream of FLT3 by alternative FLT3-independent mechanisms, meaning that the inhibition of FLT3 alone is not sufficient to cause cytotoxicity. In this second group of patients, FLT3 inhibition could still be clinically beneficial if used in combination with other targeted or conventional cytotoxic agents.
In summary, in this group of older, previously untreated patients with AML who were considered unfit for conventional cytotoxic chemotherapy, lestaurtinib was well tolerated, but achieved only relatively modest clinical efficacy. Clinical responses occurred in patients in whom sustained plasma FLT3 inhibitory activity was combined with an inherent sensititivity of blasts to the cytotoxic effects of lestaurtinib. FLT3 inhibitors may only have a limited future role as single therapeutic agents: this is not surprising when considered in the context of the relatively limited effects achieved with an extensively clinically proven targeted agent such as imatinib mesylate when used in CML blast crisis.41 It is now well established that clinical antileukemic effects result from inhibition of FLT3, however, and there is also a growing body of preclinical evidence to support a synergistic relationship between lestaurtinib and conventional cytotoxic agents if the FLT3 inhibitor is employed simultaneously with or immediately following chemotherapy.42,43 A prospective trial of lestaurtinib in combination with standard chemotherapy is already under way,44 with further studies in preparation. The current study has also provided important proof of principle that inhibition of FLT3 can result in clinical response in some FLT3 WT patients, and there is thus a continued rationale for including such patients in future combination trials.
Prepublished online as Blood First Edition Paper, July 20, 2006; DOI 10.1182/blood-2006-04-015560.
S.K. coordinated the clinical trial, performed the in vitro cytotoxicity assays, flow cytometric FLT3-expression assays, and immunoblotting experiments, analyzed the clinical and laboratory data, and wrote the manuscript. A.K.B. designed and wrote the protocol, was the Chief Investigator, contributed to the “Introduction” and “Discussion,” and reviewed the manuscript. W.J.K. coordinated the clinical trial and supervised computer data collection and processing. T.L., S.A., R. Chopra, and R. Clark contributed patients to the trial. M.J.L. and D.S. developed and performed the ex vivo FLT3 plasma inhibitory activity assay and reviewed the manuscript.
The authors declare no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We would like to thank Dr J. I. O. Craig, Prof N. Russell, Prof C. Craddock, Dr S. Killick, Dr M. F. McMullin, Dr D. Milligan, and Dr D. Culligan for contributing patients to the study; Dr K. I. Mills, Ms A. Gilkes, and Mrs V. Walsh for FLT3 molecular characterization of patients and technical advice; and Dr Peter Brown of Cephalon for providing lestaurtinib and advice for the study.