Abstract
CAM307 is a randomized Phase III trial comparing the efficacy and safety of alemtuzumab (CAM) with chlorambucil (CHLO). The trial enrolled 297 previously untreated patients (pts) requiring therapy according to NCI-WG criteria. Pts were randomized 1:1 to CAM (n=149) vs CHLO (n=148) using standard dosing regimens. Fluorescence in situ hybridization (FISH) on interphase nuclei of lymphocytes isolated from blood was analyzed for cytogenetic abnormalities prior to the start of therapy. FISH analysis was performed using 13 DNA probes to detect chromosomal aberrations in 17p13.1 (P53), 13q14 (RB1, D13S319 and D13S25), 11q22.3-11q23 (ATM and MLL), 6q27 (subtelomere), 6q21 (chromosome 6q21/alphasatellite 6 cocktail probe), trisomy 8q24 (c-myc), trisomy 12 (CEP12) and translocations involving the locus of immunoglobulin heavy chain gene (IGH, 14q32.33). Samples were analyzed in 282 pts (95%); chromosomal aberrations were detected in 231 pts (82%) while 51 pts (18%) exhibited a normal interphase FISH pattern. The most frequent abnormalities were deletions (del) at loci 13q (49%), sole del 13q (24%), 11q (19%), 17p (7 %), 6q (4 %), and trisomies 12 (14%) and 8q (5%). Translocations IGH, 14q32.33 were detected in 10 pts (4%). An exploratory analysis was performed to correlate time to event variables (assessed by an independent response review panel) with cytogenetics.
Overall 165 pts (59%) revealed combination abnormalities. The most frequently observed chromosomal associations were: del 13q + del 14q (N=20, 12%), del 11q + del 13q (N=17, 10%), del 11q + del 13q + del 14q (N=11, 7%), del 11q + del 14q (N=7, 4%), trisomy 12 + del 13q (N=5, 3%), del 13q + del 17p (N=4, 2%), del 11q + trisomy 12 (N=3, 2%) and del 17p + del 6q (N=3, 2%). Coexistence of del 17p and del 11q was not observed. Although del 13q was observed with all chromosome abnormalities, nearly half of the cases del 13q14.3 (D13S25 and D13S319) coincided with an ATM deletion (11q22.3). FISH analysis has allowed the detection of uncommon abnormalities: tetraploidy (n=1), hyperdiploidy (n=1), trisomy 18 (n=1) and c-myc oncogene amplification (>15 copies per nuclei) (n=2). The latter is a well known abnormality in solid tumors but rarely seen in leukemia. In addition, del of the IGH variable region was detected in 70 pts. The biological and clinical significance of this abnormality is to be investigated.
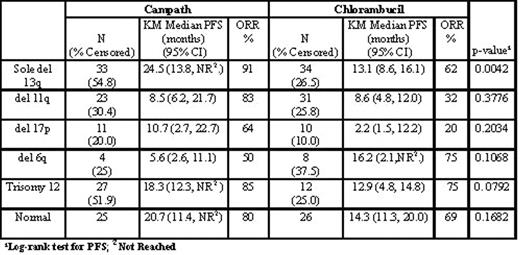
Conclusions: Overall, 82% of treatment naïve BCLL pts revealed cytogenetic aberrations and 59% were combination abnormalities. CAM307 demonstrates a significant improvement in PFS in pts treated with CAM vs CHLO who present with del 13q as the sole abnormality; no difference in pts with del 11q. However, a trend towards improved PFS was observed in pts with trisomy 12 and del 17p, which did not reach significance due to small sample size. Further investigation of CAM therapy in high risk cytogenetic subgroups is warranted.
Disclosures: SIRARD - Medical Director at Genzyme Corporation; GOLDBERG - Sr. VP, Clinical Research at Genzyme Corporation.; HILLMEN - Schering AG/Genzyme Corporation.; Sirard/Goldberg - stock options and employee stock purchase plan at Genzyme Corporation.; Robak/Skotnicki - with Genzyme Corporation; Hillmen - with Schering AG.; Jaksic - from Genzyme Corporation.; Hillmen - Speakers Bureau for Schering AG/Genzyme Corporation.
Author notes
Corresponding author