Abstract
RI-HSCT is now accepted practice in a number of clinical scenarios. However, two circumstances in which it is still often not covered by insurance carriers is a diagnosis of acute leukemia (AL) and relapse following an autograft. The aim of this analysis was to specifically study the influence of these 2 factors in the context of known prognostic variables in a series of 91 uniformly-treated patients with hematologic malignancies (19–71 years; median 53; 22% >60 years). 41 had relapsed after a prior autograft (6/29 AL, 20/38 lymphoma, 15/24 myeloma; P=0.005). 62% had refractory disease. Performance status (PS) was 0 in 31%, 1 in 47%, and 2/3 in 22%. Donors (55% >45 years) were HLA-matched siblings (n=51), 10/10 allele-matched unrelated (n=29), or 9/10 allele-matched (n=11). The conditioning comprised 100 mg/m2 melphalan with (no prior auto) or without (prior auto) 50 mg/kg cyclophosphamide. GVHD prophylaxis comprised cyclosporine-mycophenolate (HLA-matched sibs) or tacrolimus-mycophenolate (others). G/GM-CSF were not given routinely. Supportive care was uniform. As the table below shows, the 2-year cumulative incidence of relapse and non-relapse mortality (NRM) and 2-year disease-free (DFS) and overall (OS) survival rates were not significantly influenced by the diagnosis or prior autograft.
| . | Relapse . | NRM . | DFS . | OS . |
|---|---|---|---|---|
| Acute leukemia | 55% | 22% | 23% | 37% |
| NHL/Hodgkin | 42% | 29% | 28% | 47% |
| Myeloma | 66% | 18% | 16% | 36% |
| P | 0.35 | 0.49 | 0.89 | 0.51 |
| No prior autograft | 48% | 26% | 25% | 39% |
| Prior autograft | 60% | 21% | 19% | 42% |
| P | 0.82 | 0.55 | 0.88 | 0.52 |
| . | Relapse . | NRM . | DFS . | OS . |
|---|---|---|---|---|
| Acute leukemia | 55% | 22% | 23% | 37% |
| NHL/Hodgkin | 42% | 29% | 28% | 47% |
| Myeloma | 66% | 18% | 16% | 36% |
| P | 0.35 | 0.49 | 0.89 | 0.51 |
| No prior autograft | 48% | 26% | 25% | 39% |
| Prior autograft | 60% | 21% | 19% | 42% |
| P | 0.82 | 0.55 | 0.88 | 0.52 |
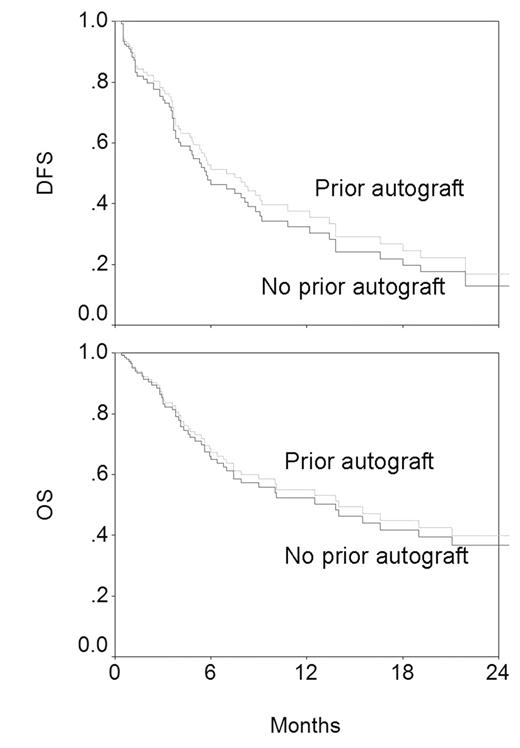
Next, diagnosis and prior autograft were entered into a Cox model with chemosensitivity, donor age, LDH and PS; variables known to influence outcome significantly in this group of patients. The 4 known variables retained their significance in influencing OS (LDH P=0.001, chemosensitivity P=0.003, PS P=0.003, donor age P=0.01) and DFS (chemosensitivity P=0.002, PS P=0.02, donor age P=0.02, LDH P=0.03). Prior autograft did not influence OS (P=0.77) or DFS (P=0.57) as shown in the figure below (derived from the Cox model; adjusted for the 4 significant variables).
Diagnosis did not influence DFS (P=0.49). However, it did affect OS (overall P=0.04; AL vs myeloma P=0.23; AL vs lymphoma P=0.01; myeloma vs lymphoma P=0.25) as shown in the figure below (derived from the Cox model; adjusted for the 4 significant variables.
The difference in OS likely reflects the fact that unlike for lymphoma and myeloma, salvage therapy is of limited benefit for AL relapsing after HSCT. However, comparable DFS suggests that the extent of benefit provided by the RI-HSCT procedure itself is independent of diagnosis. We conclude that prior autograft or the underlying diagnosis should not be used to determine eligibility for RI-HSCT.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author