Abstract
Background: Current SCT approaches consistently achieve rapid donor myeloid engraftment, but delayed immune recovery remains a significant obstacle and results in increased risk of infection and relapse. T cells are regenerated via 2 pathways, thymus-derived and peripheral expansion, processes for which IL-7 is critical. We postulated that non-myeloablative pre-transplant conditioning might preserve thymic function in pediatric SCT recipients thus enhancing thymus-derived naïve T cell regeneration.
Methods: We analyzed T cell subsets, T cell receptor excision circles (TREC), and IL-7 levels in peripheral blood after SCT in 21 pediatric pts with high-risk malignancies (median age 14, range 4–21). Fludarabine-based induction chemotherapy was administered for disease control and targeted CD4 count reduction. Pre-transplant conditioning consisted of cyclophosphamide (1,200 mg/m2/day) and fludarabine (30 mg/m2/day) × 4 days plus melphalan (100 mg/m2 × 1 dose in sarcoma pts). Grafts consisted of G-CSF mobilized unmodified peripheral blood stem cells from 5–6/6 HLA-matched first-degree relatives (median CD34 dose 11.7 × 10E6/kg, range 4.4–19.1; median CD3 dose 416 × 10E6/kg, range 228–815). Cyclosporine was used for GVHD prophylaxis.
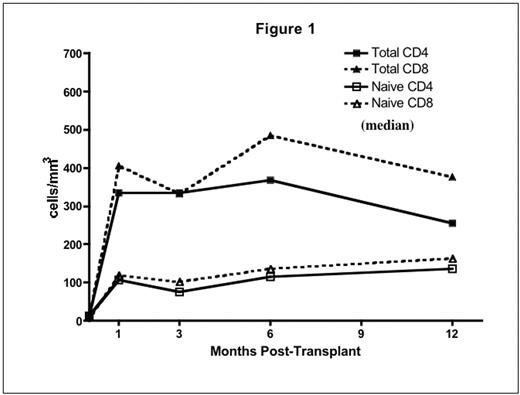
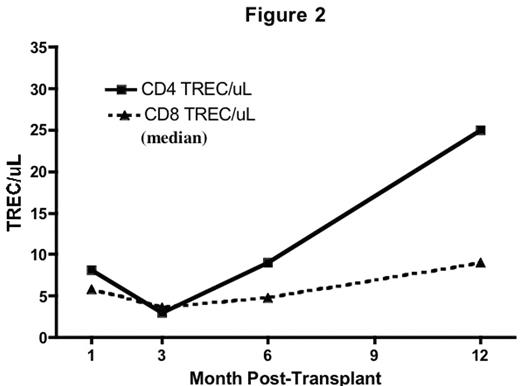
Results: Donor-derived engraftment was rapid (absolute neutrophil count > 500/uL median day 9, range 8–11). Complete donor lymphoid chimerism (>95% by VNTR-PCR on CD3 sorted peripheral blood) was achieved in all by day 28. Immune recovery was brisk and sustained. Substantial numbers of naïve (CD45RA+/CD62L+) CD4+ and CD8+ T-cells were detected at day 28 (Fig 1). There was a steady increase in TREC from 3 to 12 months consistent with early, robust thymic-dependant T cell generation (Fig 2). This was not seen in adult pts treated on a parallel trial (data not shown). IL-7 levels were elevated and inversely correlated with T cell counts (r=−0.56, p<0.0001).
Conclusions: Targeted immune depletion and NMSCT results in rapid, sustained immune reconstitution in pediatric pts with malignancy. Preserved thymic function appears to contribute to naïve T cell recovery in this setting. We postulate that non-myeloablative conditioning is thymus sparing and that this, in combination with immune depletion-induced IL-7 elevation, promotes early thymic-derived lymphoid recovery. This approach may serve as a strategy to overcome the prolonged immunodeficiency commonly encountered after allogeneic SCT in pediatrics and might be used as a platform to direct allogeneic anti-tumor immune responses in high-risk childhood cancers.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author