Abstract
Regulatory T (Treg) cells are a subset of CD25+CD4+ T cells that constitutively express high levels of cytotoxic T lymphocyte antigen-4 (CTLA-4) and suppress T-cell activation and effector functions. Treg cells are increased in tissues of individuals infected with HIV-1 and macaques infected with simian immunodeficiency virus (SIVmac251). In HIV-1 infection, Treg cells could exert contrasting effects: they may limit viral replication by decreasing immune activation, or they may increase viral replication by suppressing virusspecific immune response. Thus, the outcome of blocking Treg function in HIV/SIV should be empirically tested. Here, we demonstrate that CD25+ T cells inhibit virus-specific T-cell responses in cultured T cells from blood and lymph nodes of SIV-infected macaques. We investigated the impact of CTLA-4 blockade using the anti–CTLA-4 human antibody MDX-010 in SIV-infected macaques treated with antiretroviral therapy (ART). CTLA-4 blockade decreased expression of the tryptophan-depleting enzyme IDO and the level of the suppressive cytokine transforming growth factor-β (TGF-β) in tissues. CTLA-4 blockade was associated with decreased viral RNA levels in lymph nodes and an increase in the effector function of both SIV-specific CD4+ and CD8+ T cells. Therefore, blunting Treg function in macaques infected with SIV did not have detrimental virologic effects and may provide a valuable approach to complement ART and therapeutic vaccination in the treatment of HIV-1 infection.
Introduction
The induction and maintenance of immune responses to antigens is tightly regulated. Activation of T cells requires the interaction between the T-cell receptor and the antigen presented on the surface of an antigen-presenting cell (APC; first signal) and engagement of CD28 (second signal) by the costimulatory molecules B7-1 (CD80) and B7-2 (CD86).1,2 Costimulation is particularly important for the initial T-cell response, promoting proliferation and survival. Following antigen stimulation, both CD28 and its negative regulatory, cytotoxic T lymphocyte antigen-4 (CTLA-4), are up-regulated on the cell surface and compete for their ligands, B7-1 and B7-2. CTLA-4 binds to both B7-1 and B7-2 with higher (10to 20-fold) affinity than CD28,3,4 and, in contrast to CD28, CTLA-4 suppresses T-cell activation. However, competition with CD28 for the costimulatory molecules is not likely to be the main mechanism responsible for CTLA-4 immunoregulatory activity.5-7
Several lines of evidence suggest that expression of CTLA-4 by CD25+CD4+ regulatory T (Treg) cells plays a role in controlling peripheral T-cell tolerance and differentiation.8,9 Treg cells are CD4+ T lymphocytes that express high levels of the interleukin-2 (IL-2) receptor α-chain (CD25), and constitutively express CTLA-4. Treg cells inhibit the proliferation of T cells through contactdependent or cytokine-mediated (IL-10, transforming growth factor-β [TGF-β]) inhibition of T-cell responses.10 Treg cells can induce activation of the enzyme IDO in APCs via CTLA-4–mediated ligation of CD80/CD86.11,12 IDO confers immunosuppressive activity to APCs.11,12
Two mechanisms have been suggested as mediators of the T-cell–suppressive action of IDO13 : degradation and consequent reduction of tryptophan, an essential amino acid required for T-cell proliferation; and generation of inhibitory tryptophan metabolites. Blocking CTLA-4 signals with monoclonal antibodies may provide an important tool to influence the host immune response in clinical settings. For example, synergy between anti-CD25 and anti–CTLA-4 monoclonal antibodies has been shown to be effective in antitumor therapy.14,15
Treg cells may suppress a potentially successful adaptive host immune response to a pathogen.16-21 In the case of HIV infection, CTLA-4 expression is higher in patients with advanced clinical symptoms compared with asymptomatic individuals,22,23 and the frequency of CTLA-4–expressing CD25+CD4+ Treg cells is increased in lymphoid tissues in untreated individuals infected with HIV-1.24 Primary infection of macaques with simian immunodeficiency virus (SIV) is associated with an increase in Treg cells, IDO, TGF-β, and IL-10.25 Similarly, chronic SIV infection is associated with an accumulation of Treg cells in lymphoid tissues, including the gut, and an increased level of immunosuppressive cytokines (A. B., M. V., A. H., D. F., J. N., Valentina Cecchinato, G. F., G. M. S., and C. C., our unpublished results, October 2006).
Here, we examined the virologic and immunologic consequences of CTLA-4 blockade in macaques chronically infected with SIVmac251. We demonstrated that CTLA-4 blockade was associated with a decrease in immunosuppressive molecules such as IDO and TGF-β in tissues. Plasma levels of the IDO catabolite were also decreased. Importantly, CTLA-4 blockade did not affect the responsiveness to antiretroviral therapy (ART) or increase plasma virus level when ART was suspended. On the contrary, viral levels in lymph nodes were decreased by treatment with anti–CTLA-4 antibody, concomitant with an increase in CD4+ and CD8+ T-cell effector function.
Materials and methods
Animals and protocol
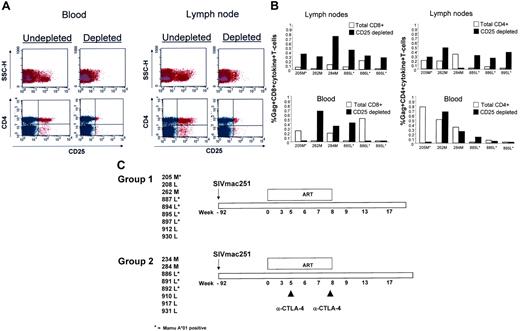
All animals were colony-bred rhesus macaques (Macaca mulatta) obtained from Covance Research Products (Alice, TX). The animals were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International, and the study was reviewed and approved by the animal care and use committees at Advanced BioScience Laboratories (Kensington, MD) and Southern Research Institute (Frederick, MD). The care and use of the animals were in compliance with all relevant institutional (National Institutes of Health) guidelines. At the time of purchase, all animals were in good health, were 2 to 4 years of age, and weighed 4 to 9 kg. Before the first study, all animals were seronegative for SIV, simian T-cell lymphotropic virus type 1 (STLV-1), and herpes virus B. ART consisted of intravenous administration of didanosine (DDI; 10 mg/kg/day), oral administration of stavudine (d4T) twice a day (1.2 mg/kg/dose), and subcutaneous administration of PMPA (20 mg/kg/day). In the first study, treatment began at 4 months after infection with SIVmac251 by the intravenous route,26 and the macaques were immunized as indicated in Table 1. ART was stopped at week 41. At week 92, the animals were enrolled in the present study, ART was started, and the treatment was continued daily for 8 consecutive weeks (Figure 1C). Group 1 included 6 Mamu-A*01– and 3 Mamu-A*01+ animals that received no additional treatment. The 8 macaques in group 2, which included 5 Mamu-A*01– and 3 Mamu-A*01+ macaques, were inoculated intravenously at weeks 5 and 8 with the blocking anti–CTLA-4 monoclonal antibody (MDX-010, 10 mg/kg/injection; Medarex, Bloomsbury, NJ).
RNA extraction, reverse transcription, and real-time PCR.
Total RNA was extracted from macaques' lymph nodes using the RNAeasy extraction kit (Qiagen, Valencia, CA), according to the manufacturer instructions. RNA (1 μg) was reverse transcribed into first-strand cDNA in a 20-μL reaction containing 1 μM random hexanucleotide primers, 1 μM oligo dT, and 200 U Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI).
cDNA quantification for IDO, IL-2, TGF-β, GAPDH, FoxP3, and CTLA-4 was performed by real-time polymerase chain reaction (PCR) conducted with the ABI Prism 7900HT (Applied Biosystems, Foster City, CA). All reactions were performed using a SYBR green PCR mix (Qiagen) according to the following thermal profile: denaturation at 95°C for 15 seconds, annealing at 60°C for 15 seconds, and extension at 72°C for 15 seconds (data collection was performed during the extension step). Data analysis was performed with the SDS 2.1 software, provided with the ABI Prism 7900HT. Primer sequences were designed using the Primer3 software, available online (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), and are presented in Table 2. Because the mRNA sequence for TGF-β was not directly available, the corresponding human mRNA sequences were compared with the macaque genome (http://www.hgsc.bcm.tmc.edu/projects/rmacaque) using the blast function available on the same site. The macaque genome contigs recognized were then aligned to the human genomic sequence for the same gene, to compare intron and exon sequences. Primers were designed on coding regions showing perfect identity between the macaque and the human sequence.
Viral load measurement
We quantified SIVmac251 RNA in plasma by nucleic acid sequence–based amplification (NASBA).27 Briefly, RNA was extracted from plasma and isothermally amplified using SIVmac251-specific primers. Quantification was made by electrochemiluminescence chemistry–based probe hybridization system using a coextracted internal standard. The copy number was expressed per 100 μL plasma, and the detection limit of the assay was 2 × 103 RNA copies.
CD4+ and CD8+ T-cell counts
CD4+ and CD8+ counts were periodically determined on 100 μL of whole blood and by fluorescence-activated cell sorter (FACS) analysis, according to the FACS/Lyse kit (BD Immunocytometry Systems, San Jose, CA) with minor modifications. Briefly, after incubating 10 μL of a mixture containing PerCP-CD4, allophycocyanin-CD8, PE-CD45, and FITC-CD3 monoclonal antibodies (BD Biosciences, San Jose, CA) for 30 minutes at room temperature, red cells were lysed by adding 2 mL of FACS lysing solution (BD Biosciences) for 15 minutes. Samples were then centrifuged for 5 minutes at 250 g at room temperature, washed (1% fetal calf serum [FCS] and 0.05% NaN3 in phosphate-buffered saline [PBS]), resuspended in 500 μL wash buffer, and stored at 4°C until analysis using a FACSCalibur flow cytometer (BD Biosciences).
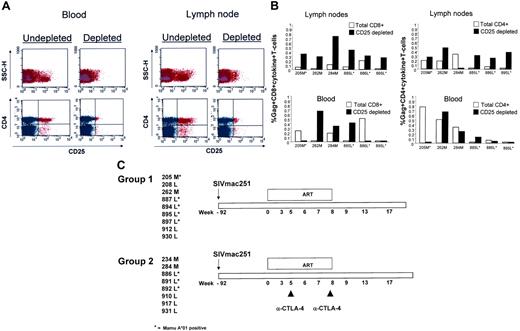
CD25 depletion reduces virus-specific immune responses. (A) FACS analysis of CD4+ T cells following depletion of CD25+ T cells. (B) Production of cytokines by total or CD25-depleted CD4+ and CD8+ T cells following Gag-specific stimulation in vitro. Cells were obtained from lymph nodes or blood before the initiation of the study. (C) Schematic representation of the study design and numerical designation of the animals enrolled. *Mamu-A*01+ macaques.
CD25 depletion reduces virus-specific immune responses. (A) FACS analysis of CD4+ T cells following depletion of CD25+ T cells. (B) Production of cytokines by total or CD25-depleted CD4+ and CD8+ T cells following Gag-specific stimulation in vitro. Cells were obtained from lymph nodes or blood before the initiation of the study. (C) Schematic representation of the study design and numerical designation of the animals enrolled. *Mamu-A*01+ macaques.
Tetramer staining
We screened rhesus macaques for the presence of the Mamu-A*01 allele using a PCR-based technique. Freshly prepared peripheral blood mononuclear cells (PBMCs; 5 × 105 per animal) were stained with anti–human CD3 antibodies (FITC; BD Immunocytometry Systems), anti–human CD8 (PerCP; BD Biosciences), and Mamu-A*01 tetrameric complexes refolded in the presence of a specific peptide and conjugated to allophycocyaninlabeled streptavidin (Beckman Coulter, Fullerton, CA). Gag181-189 CM9 (CTPYDINQM) (Gag_CM9)–specific tetramer was used.
After 30 minutes of incubation in the dark at room temperature, cells were washed with 1% FCS in PBS and fixed with 1% paraformaldehyde in PBS (pH 7.4). Samples were analyzed on a FACSCalibur (BD Biosciences), and the data are presented as percentage of tetramer+ cells in all CD3+CD8+ lymphocytes.
Identification of effector CD4+ and CD8+ T cells
Fresh PBMCs were isolated using lymphocyte separation medium (Cappel, Aurora, OH) density centrifugation. In some instances PBMCs were frozen (90% FCS/10% DMSO) until use. PBMCs (106) were incubated with 1 μg/mL each of the antibodies anti-CD28 and anti-CD49d and 1 μg/mL of Gag pool peptide in a 1 mL volume. Conjugated antibodies to the granular membrane protein CD107a were kindly supplied by M. Roederer (Laboratory of ImmunoTechnology, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) and were added to the cells prior to stimulation. A negative control (anti-CD28, anti-CD49d), and positive control (SEB, 1 μg/mL) were included to control for spontaneous activated production of cytokines and/or expression of CD107a, respectively. The cultures were incubated for 1 hour at 37°C in 5% CO2, followed by an additional 4 hours in the presence of the secretion inhibitor monensin (0.5 μL/mL; BD Pharmingen, San Diego, CA) and Brefeldin A (10 μg/mL; Sigma, St Louis, MO). After stimulation, PBMCs were washed, surface stained (anti-CD4 [BD Pharmingen] or anti-CD8β [Immunotech, Fullerton, CA]), washed again, and permeabilized using FACS Perm/Wash buffer (BD Biosciences). After permeabilization, the cells were washed and stained with antibodies specific for cytokines (interferon-γ [IFN-γ] and tumor necrosis factor-α [TNF-α] monoclonal antibodies; BD Pharmingen). The cells were washed a final time and resuspended in 1% paraformaldehyde (Electron Microscopy Systems, Fort Washington, PA) in PBS. Four-parameter flow cytometry analysis was performed using a FACSCalibur flow cytometer. List mode data files were analyzed using FlowJo software (Tree Star, San Carlos, CA). In all cases at least 100 000 live events were collected for analysis.
Tryptophan and kynurenine concentration measurement by high-performance liquid chromatography
Detection of tryptophan and kynurenine was performed on plasma collected from animals before initiation of ART and 1 week after anti–CTLA-4 administration by high-performance liquid chromatography as previously described.28
CD25+ T-cell depletion
To deplete CD25+ T cells, PBMCs and T cells purified from lymph nodes were passed through a prewetted 30-μm nylon mesh (Miltenyi Biotec, Auburn, CA) to remove clumps. Cells were washed, pelleted, resuspended in 100 μL selection buffer (PBS supplemented with 0.5% FCS and 2 mM EDTA), and stained with CD25 conjugated with r-PE antibody (clone 2A3; Becton Dickinson) for 10 minutes at 4°C to 8°C in the dark. Following incubation, the cells were washed twice and resuspended in 80 μL selection buffer per 107 cells, and 20 μL per 107 cells of anti-PE microbeads (Miltenyi Biotec) were added and incubated at 4°C to 8°C for 15 minutes. The cells were washed, resuspended in 500 μL selection buffer, and passed through magnetic beads for depletion of CD25+ T cells. Unlabeled effluent was collected and analyzed. To assess the degree of CD25 depletion, macaque PBMCs or T cells purified from lymph nodes, as previously described,29 were stained with CD3ϵ-FITC, CD4-PerCP, CD8-allophycocyanin, and CD25-PE (BD Biosciences) for 20 minutes in the dark at 4°C to 8°C and washed with wash buffer (PBS supplemented with 1% FCS). Following incubation, the cells were washed and resuspended in 200 μL 1% paraformaldehyde (Sigma). Analysis was performed using the FACSCalibur.
Intracellular cytokine staining was performed using both the anti–TNF-α and anti–IFN-γ antibodies. Fresh PBMCs (106) in 0.2 mL complete medium were incubated for 1 hour at 37°C in the absence or presence of a pool of Gag peptides (2 μg/mL) and in the presence of CD28 and CD49d (1 μg/mL each) on a V-bottom–well plate. After addition of 10 μg/mL Brefeldin A (Sigma), cells were incubated for 5 hours at 37°C and processed for surface and intracellular cytokine staining. Briefly, cells were washed with 1% FCS in PBS, surface-stained for 20 minutes with CD3ϵ-FITC and CD8-PerCP (4 μL each; Becton Dickinson), washed again, and permeabilized with FACSPerm (BD Pharmingen) for 10 minutes at room temperature in the dark. Following 2 further washes, cells were intracellularly stained with PE-conjugated anti–TNF-α with or without PE-conjugated anti–IFN-γ (4 μL/well each; BD Pharmingen), with or without allophycocyaninlabeled CD69 (1.5 μL/well each; BD Pharmingen), incubated for 20 minutes at 37°C, fixed with 180 μL 1% paraformaldehyde in PBS, and analyzed on a FACSCalibur.
FoxP3 staining
Frozen PBMCs (1 × 106) were surface stained with anti–human CD4 PerCP (BD Pharmingen), anti–human CD8β (Immunotech), allophycocyanin, and anti–human CD25 PE (BD Pharmingen). After 30 minutes of incubation in the dark at room temperature, cells were washed with cold 1% FCS in PBS. Cell pellets were resupended with pulse vortex and 1 mL freshly prepared Fix/Perm Buffer (catalog no. 00-5123; eBioscience, San Diego, CA) was added. Samples were incubated at 4°C for 30 minutes in the dark. After incubation, cells were washed once by adding 1% FCS in PBS followed by centrifugation and decanting of supernatant. Cells were washed with 2 mL 1 × Permeabilization Buffer (catalog no. 00-8333; eBioscience) and centrifuged. Supernatants were then decanted and washed again, and cells were blocked with 2% normal rat serum (catalog no. 24-5555; eBioscience) in 1 × Permeabilization Buffer, in approximately 100 μL volume, at 4°C for 15 minutes. Without washing after the blocking step, cells were stained with anti–human FoxP3 antibody FITC (catalog no. 77-5776; eBioscience) and incubated at 4°C for 30 minutes in the dark. After incubation, cells were washed twice with 2 mL 1 × Permeabilization Buffer. After centrifugation and decanting of the supernatant, cells were resuspended in 1% paraformaldehyde (Electron Microscopy Systems) in PBS. Four-parameter flow cytometry analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems). List mode data files were analyzed using FlowJo software (Tree Star). In all cases at least 100 000 live events were collected for analysis.
Statistical analysis
Statistical analyses for mRNA expression, tryptophan and kynurenine ratio, and lymph node viral load were performed using the SPSS 13.0 software (SPSS, Chicago, IL). Differences between groups were assessed by nonparametric Mann-Whitney U test. Differences before and after treatment within the same group were assessed using the Wilcoxon test. All P values shown in the text and figures are 2-tailed.
Results
CD25+ T cells inhibit virus-specific T-cell response in tissues of SIV-infected macaques
Removal of Treg cells from PBMCs of individuals infected with HIV-1 results in the restoration of virus-specific T-cell responses.30 Before assessing the effect of CTLA-4 blockade, we investigated whether removal of CD25+ T cells affected the ability of macaque T cells to respond to SIV antigens in vitro. Lymph nodes were excised from 6 animals infected with SIV before ART treatment. In parallel, PBMCs were also isolated from the blood of the same animals. Undepleted or CD25-depleted T cells from both compartments were stimulated in vitro with a peptide pool encompassing the entire SIV Gag protein, and their ability to produce both TNF-α and IFN-γ was assessed by FACS analysis. Representative data of CD25+ T-cell depletion from 1 animal are presented in Figure 1A. Interestingly, CD8+ T-cell responses were increased upon removal of CD25+ T cells from the lymph nodes of all animals studied, whereas depletion of CD25+ T cells from PBMCs led to increased CD8+ T-cell response only in 50% of the animals (Figure 1B). Similarly, CD4+ T-cell responses were increased upon CD25+ T-cell depletion in the lymph nodes of 5 (83%) of 6 animals analyzed following removal of CD25+ T cells from circulating PBMCs. The increase of virus-specific immune response following CD25+ T-cell depletion was not observed consistently in blood, and in some cases a decrease rather than an increase was observed, likely reflecting the depletion of activated T cells that may have been antigen specific. All together, these data are consistent with the idea that Treg cells may be more frequent in tissues,24,25 and are in agreement with the finding in humans that Treg cells downmodulate specific anti-HIV responses.30-32
Biological effects of CTLA-4 blockade in vivo
Because we found that removal of CD25+ cells resulted in enhanced SIV-specific T-cell responses in vitro, we addressed whether blocking of CTLA-4 (a marker expressed on activated T cells as well as on Treg cells) in vivo affected the expression of immunosuppressive modulators, viral replication, and SIV-specific immune responses. Seventeen macaques chronically infected with SIVmac251 were divided into 2 groups of 9 and 8 macaques each. The macaques were treated daily with a triple-drug regimen of HIV-1 reverse-transcriptase inhibitors (nucleoside analogs): PMPA, DDI, and d4T for 8 weeks. Animals in group 2 received 2 inoculations of the CTLA-4–blocking MDX-010 antibody at 5 and 8 weeks after initiation of ART (Figure 1C). At 8 weeks, ART was suspended to assess the effect of CTLA-4 blockade on virus rebound.
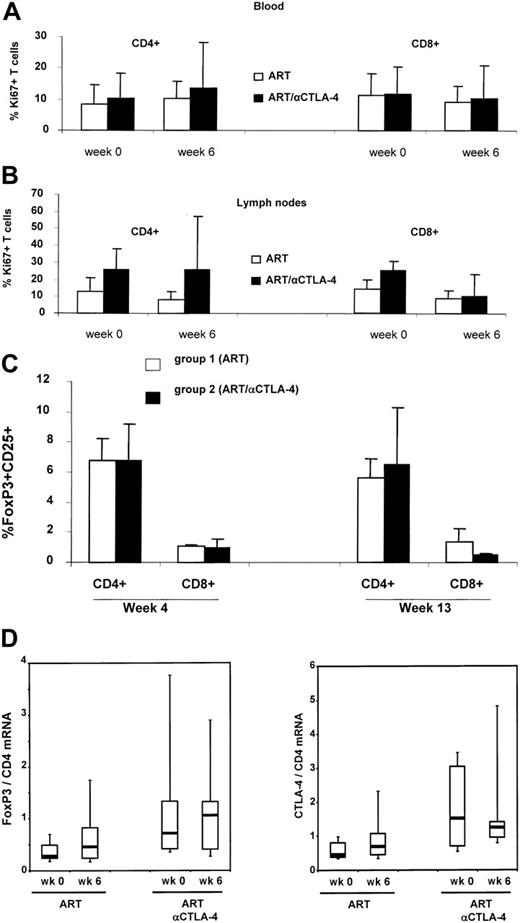
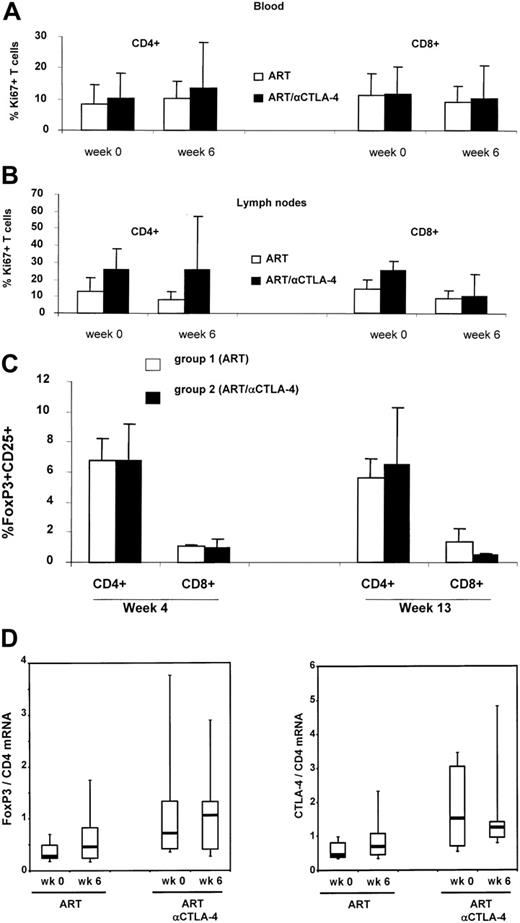
Because CTLA blockade may result in T-cell activation or removal of suppression of activation, we compared the basal expression of the cell-cycle progression marker Ki-67 by flow cytometry on lymph nodes and peripheral blood T cells at the time of ART initiation, and at 1 week after the first inoculation of MDX-010 in all treated animals. The frequency of Ki-67+ T cells in blood or lymph nodes of infected macaques treated with MDX-010 was not significantly higher than that in macaques treated with ART alone. However, the lack of significant differences may be due to the degree of variation of the basal level of T-cell activation observed in SIV infection and the small number of animals in the study (Figure 2A-B). MDX-010 is a blocking antibody, not a depleting antibody; therefore, one would not expect changes in the number of FoxP3+ or CTLA-4+ CD4+ T cells following MDX-010 administration. The percentage of FoxP3+ CD4+ and CD8+ T cells in blood was not significantly altered by the 2 inoculations of MDX-010 (Figure 2C). Similarly, no significant changes were observed in the level of FoxP3 and CTLA-4 mRNA expression in lymph nodes before and after ART treatment and before and after ART and MDX-010 treatment (Figure 2D).
Ki-67, FoxP3, and CTLA-4 expression in treated macaques. Flow cytometry analysis of the Ki-67 marker on CD4+ or CD8+ T cells at the time of ART initiation (week 0) and 1 week after MDX-010 inoculation (week 6) in blood (A) or lymph nodes (B). FoxP3 expression measured by FACS in CD25+CD4+ T cells and CD25+CD8+ T cells in blood at weeks 4 and 13 (C). (D) Level of FoxP3 and CTLA-4 expression in lymph nodes of macaques before treatment (time 0) and 1 week after CTLA-4 treatment (week 6). For panels A-C, mean values plus standard error are shown. For panel D, horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; and vertical lines extend to the 10th and 90th percentiles.
Ki-67, FoxP3, and CTLA-4 expression in treated macaques. Flow cytometry analysis of the Ki-67 marker on CD4+ or CD8+ T cells at the time of ART initiation (week 0) and 1 week after MDX-010 inoculation (week 6) in blood (A) or lymph nodes (B). FoxP3 expression measured by FACS in CD25+CD4+ T cells and CD25+CD8+ T cells in blood at weeks 4 and 13 (C). (D) Level of FoxP3 and CTLA-4 expression in lymph nodes of macaques before treatment (time 0) and 1 week after CTLA-4 treatment (week 6). For panels A-C, mean values plus standard error are shown. For panel D, horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; and vertical lines extend to the 10th and 90th percentiles.
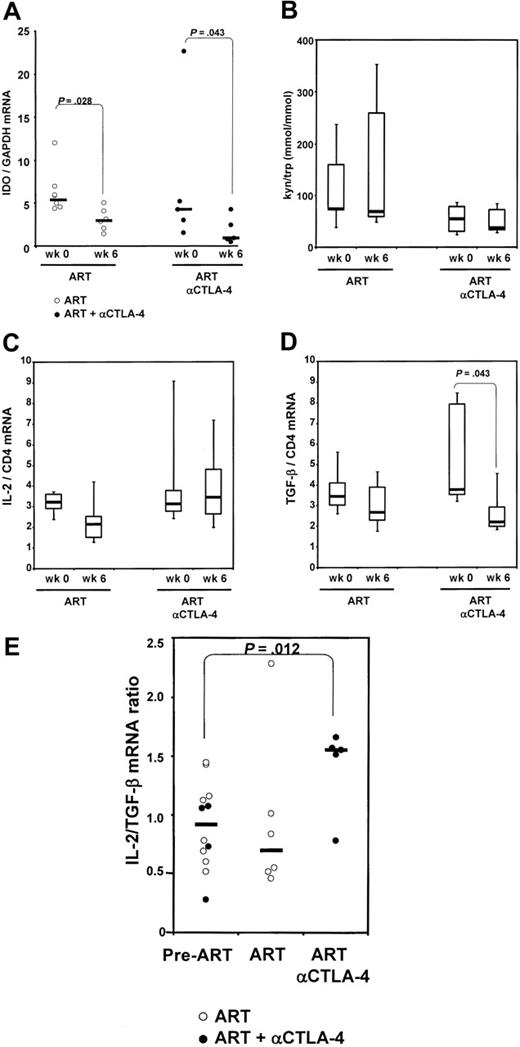
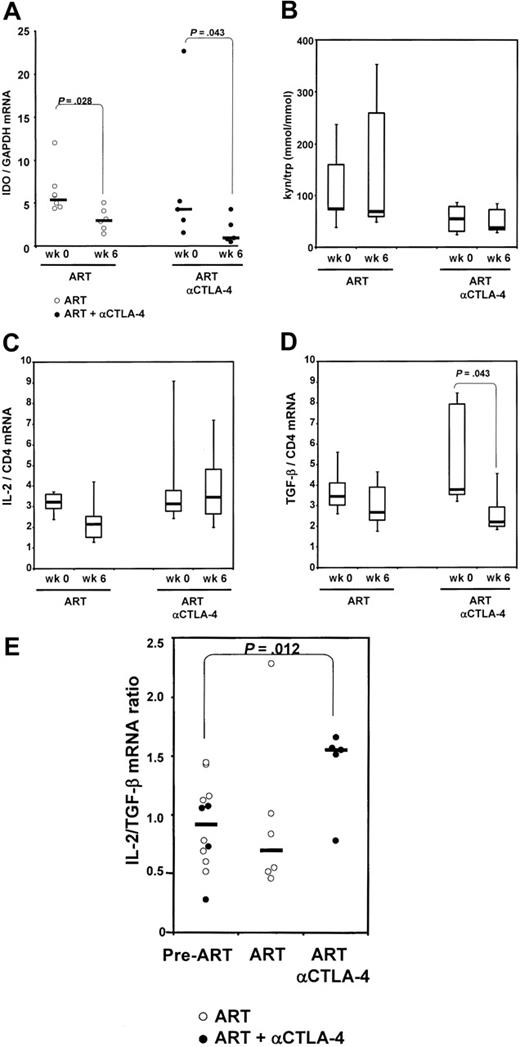
The binding of CTLA-4 to the B7 costimulatory molecules induces expression of IDO in antigen-presenting cells. We quantified mRNA expression for IDO in axillary lymph nodes excised from the animals included in the study before the initiation of ART (n = 11) and after 6 weeks of ART (n = 6) or of ART plus anti–CTLA-4 antibody (n = 4). No significant difference in IDO levels was found between the groups before initiation of ART (P = .284). A paired analysis of the changes within each of the animals was performed separately for each group. The basal level of IDO expression was decreased significantly by treatment with ART alone and, interestingly, the combination of ART and MDX010 treatment resulted in a further decrease of IDO mRNA than ART alone (Figure 3A).
To determine whether the differences we observed for IDO mRNA expression resulted in comparable differences in IDO enzymatic activity, we measured the plasma concentration of kynurenine and tryptophan, the product and the substrate of IDO enzymatic activity, respectively, by high-performance liquid chromatography. Figure 3B shows that animals within each group treated with ART alone (n = 6) had highly variable levels of the tryptophan and kynurenine ratio at week 6 in blood, and these levels did not differ from those measured before treatment. Animals treated with anti–CTLA-4 (n = 7) showed a trend to lower levels of the tryptophan and kynurenine ratio (Figure 3B). Interestingly, 2 of 6 animals undergoing ART alone showed extremely high levels of kynurenine/tryptophan (> 300 μmol/mmol). These data suggest that administration of anti–CTLA-4 in combination with ART may reduce the immunosuppressive effect of tryptophan catabolism.
To investigate the effect of CTLA-4 blockade on Treg cytokine–mediated immunosuppression, we measured the level of TGF-β, 1 of the mediators of the suppressive effect of Treg cells.33 We also measured the level of IL-2, a positive regulator of immune response. When intergroup variations before and after ART were measured from all animals in each group, we observed that treatment with ART or MDX-010 did not affect IL-2 mRNA expression but decreased significantly TGF-β expression in lymph nodes (Figure 3C-D). Indeed, the ratio between IL-2 and TGF-β as a measure of T-cell activation versus T-cell suppression was unchanged when ART alone was administered, but was significantly increased in animals treated with ART plus MDX-010 (P = .012) (Figure 3E). Taken together, these data suggest that CTLA-4 blockade decreases the level of the immunosuppressive cytokine TGF-β as well as the level of IDO expression, which in turn results in an alteration of the tryptophan and kynurenine ratio. Such changes would presumably favor T-cell activation over immune suppression.
Virologic and clinical outcome
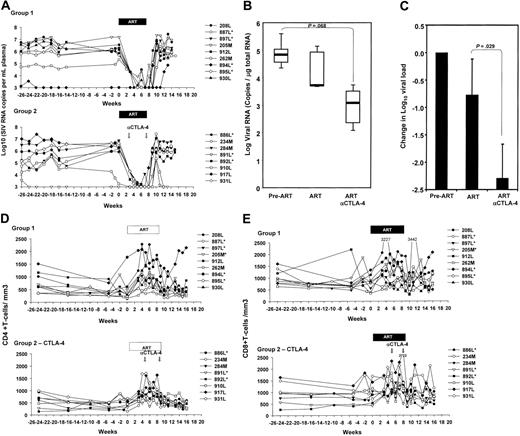
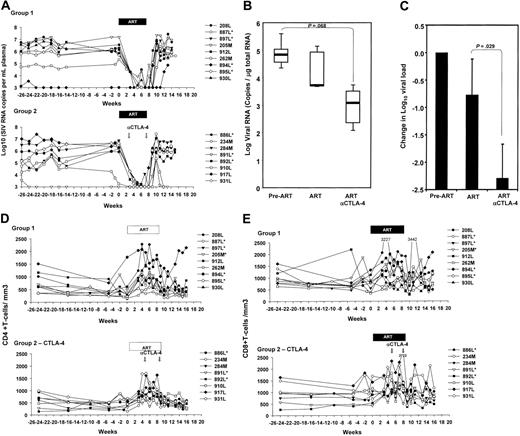
The plasma virus levels before treatment with ART were comparable in the 2 groups, and, as expected,34 treatment with this regimen of ART suppressed viral replication in most macaques (Figure 4A). No difference in the responsiveness to ART was observed in the macaques treated with the anti–CTLA-4 blockade, indicating that, even though CTLA-4 blockade blunted the expression of immunosuppressive effector molecules, virus replication was not increased (Figure 4A). Similarly, following ART suspension, the extent of viral rebound and set-point plasma virus levels did not differ significantly in the 2 groups of animals.
IDO mRNA expression and enzymatic activity. (A) IDO mRNA expression, analyzed by real-time PCR, on total RNA extracted from macaques' lymph nodes before initiation of ART (week 0), and after 6 weeks of treatment (ART) and MDX-010 treatment (ART and anti–CTLA-4) for each group. (B) Plasma kynureninetryptophan ratio measured by high-performance liquid chromatography in each group before initiation of ART (week 0), and after 6 weeks of treatment (ART) and MDX-010 (ART and anti–CTLA-4). Changes in IL-2 mRNA (C) and TGF-β (D) induced by ART and ART and anti–CTLA-4 after 6 weeks of treatment in each group. (E) Ratio between the level of IL-2 and TGF-β mRNA. Pre-ART indicates before initiation of ART. For panels A and E, dots represent individual values for each animal; horizontal bars correspond to the median. For panels B-D, horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; and vertical lines extend to the 10th and 90th percentiles.
IDO mRNA expression and enzymatic activity. (A) IDO mRNA expression, analyzed by real-time PCR, on total RNA extracted from macaques' lymph nodes before initiation of ART (week 0), and after 6 weeks of treatment (ART) and MDX-010 treatment (ART and anti–CTLA-4) for each group. (B) Plasma kynureninetryptophan ratio measured by high-performance liquid chromatography in each group before initiation of ART (week 0), and after 6 weeks of treatment (ART) and MDX-010 (ART and anti–CTLA-4). Changes in IL-2 mRNA (C) and TGF-β (D) induced by ART and ART and anti–CTLA-4 after 6 weeks of treatment in each group. (E) Ratio between the level of IL-2 and TGF-β mRNA. Pre-ART indicates before initiation of ART. For panels A and E, dots represent individual values for each animal; horizontal bars correspond to the median. For panels B-D, horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; and vertical lines extend to the 10th and 90th percentiles.
Because it is believed that low levels of viral replication still occur in tissues during ART treatment, we analyzed viral replication in lymph nodes that were excised from the animals before initiation of ART (n = 11) and 6 weeks after treatment with ART (n = 4) or ART plus MDX-010 (n = 4). Viral RNA was quantified in the total RNA from lymph nodes by NASBA. Although no difference was found between the groups before ART was initiated (P = .876), animals treated with ART plus MDX-010 showed a trend to a lower number of viral RNA copies in lymph nodes than did animals treated with ART alone, and the reduction induced by ART plus anti–CTLA-4 approached statistical significance (P = .068) (Figure 4B). Importantly, animals treated with ART plus MDX-010 showed a change in log viral load significantly larger than did animals undergoing ART alone (P = .029) (Figure 4C), suggesting that CTLA-4 blockade with MDX-010 may facilitate the control of viral replication in lymphoid tissues.
The mean absolute CD4+ and CD8+ T-cell counts per cubic milliliter of blood were similar at the time of ART initiation between the 2 groups, and, after administration of anti–CTLA-4 antibody, no significant changes in CD4+ or CD8+ T-cell counts was observed. Similarly, no difference in T-cell counts was observed after cessation of ART in either group (Figure 4D-E).
Treatment with anti–CTLA-4 antibodies enhances virus-specific effector T cells
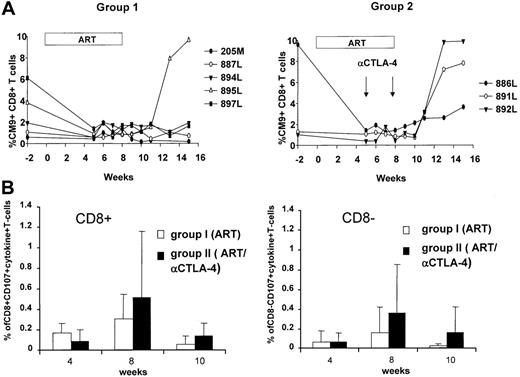
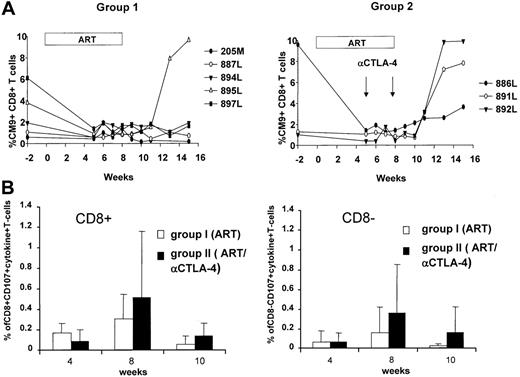
Because CTLA-4 blockade may result in an augmentation of effector T-cell responses, we studied the SIV-specific immune response using different approaches. Because we had included Mamu-A*01+ macaques in both groups, we quantified the dominant response to Gag181-189 CM9 by tetramer staining. The frequency of this response in blood decreased following ART treatment, as expected.35 However, following ART cessation, viral rebound increased the frequency of the CD8+ T-cell response, and this increase was more evident in the group of macaques treated with anti–CTLA-4 antibodies (Figure 5A). The increased frequency in PBMCs of the Gag CM9 Mamu-A*01 tetramer response 3 weeks after cessation of ART was observed in all 3 Mamu-A*01+ animals treated with anti–CTLA-4 antibodies, whereas it occurred only in 1 (20%) of 5 animals in the control ART-only group (Figure 5A). The difference observed is likely not due to a difference in the extent of viral rebound because within the group treated with the anti–CTLA-4 antibody, animals 886, 891, and 892 experienced viral rebound at a level equivalent to or lower than animals 884, 895, and 897, which were not treated with the antibody (Figures 3A, 5A).
The function of Gag-specific effector T cells was also assessed for their ability to degranulate (CD107) and produce cytokines (IFN-γ and TNF-α), as this combination is thought to better characterize effector T cells.36 At week 4, no differences in the responses to the peptide pool encompassing the entire Gag protein were found in the 2 groups. However, a trend for an increased frequency in both the CD4+ and CD8+ T cells was found both during ART treatment and after ART suspension in the animals treated with MDX-010 (Figure 5B).
Discussion
Chronic HIV-1 infection is characterized by progressive loss of immune function and failure of the immune system to eradicate the infection.37-39 Lack of appropriate T-cell help, defective CD8+ T-cell proliferation and effector function, apoptosis of virusspecific T cells, and the immunosuppressive effect of cytokines have been proposed as underlying mechanisms of the failure of the host to clear viral infection.40-44 The observations that CTLA-4 is overexpressed in HIV-1 infection,23 that the frequency of Treg cells is increased in tissues of individuals infected with HIV-1,24 and that HIV-1–specific immune responses are restored in vitro by removal of Treg cells30 suggest that Treg cells may contribute to the inability of the host to clear viral infection by dampening the adaptive immune response. Thus, blocking Treg function or decreasing negative costimulation could be a rational approach to ameliorate the adaptive immune response to HIV.
Viral RNA levels in plasma and lymph nodes. (A) Virus levels in plasma of the macaques treated with ART alone (top panel) or ART plus anti–CTLA-4 (bottom panel). (B) SIVmac251 RNA quantified on 1 μg total RNA extracted from macaque lymph nodes before initiation of ART (pre-ART), and after 6 weeks of treatment (ART) and MDX-010 (ART and anti–CTLA-4). Horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; and vertical lines extend to the 10th and 90th percentiles. (C) Changes in SIVmac251 RNA induced by ART and ART plus MDX-010 (ART and anti–CTLA-4) after 6 weeks of treatment. Mean values plus standard error are shown. (D-E) CD4+ (D) and CD8+ (E) absolute counts per cubic millimeter in blood of the macaques over time.
Viral RNA levels in plasma and lymph nodes. (A) Virus levels in plasma of the macaques treated with ART alone (top panel) or ART plus anti–CTLA-4 (bottom panel). (B) SIVmac251 RNA quantified on 1 μg total RNA extracted from macaque lymph nodes before initiation of ART (pre-ART), and after 6 weeks of treatment (ART) and MDX-010 (ART and anti–CTLA-4). Horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; and vertical lines extend to the 10th and 90th percentiles. (C) Changes in SIVmac251 RNA induced by ART and ART plus MDX-010 (ART and anti–CTLA-4) after 6 weeks of treatment. Mean values plus standard error are shown. (D-E) CD4+ (D) and CD8+ (E) absolute counts per cubic millimeter in blood of the macaques over time.
Virus-specific T cell response. (A) Gag CM9 tetramer staining of blood lymphocytes over time in the Mamu-A*01+ animals of groups 1 and 2. (B) CD8+ and CD8– T cells expressing CD107, TNF-α, and IFN-γ following stimulation with peptide pools encompassing the entire SIV Gag protein. The mean values and SD of the percentage of T cells positive for all markers are shown.
Virus-specific T cell response. (A) Gag CM9 tetramer staining of blood lymphocytes over time in the Mamu-A*01+ animals of groups 1 and 2. (B) CD8+ and CD8– T cells expressing CD107, TNF-α, and IFN-γ following stimulation with peptide pools encompassing the entire SIV Gag protein. The mean values and SD of the percentage of T cells positive for all markers are shown.
In the present study, we demonstrated that SIV-specific immune responses can be restored in vitro by removing CD25+ T cells, as observed in individuals infected with HIV-1. Because Treg cells are CD25+ T cells that are augmented early during SIV infection, and Treg cells constitutively express CTLA-4, we investigated the virologic and immunologic consequences of inhibiting Treg activity using the MDX-010 antibody that blocks CTLA-4 in macaques infected with SIV and treated with ART. The anti–CTLA-4–blocking antibody used here has been shown to be cross-reactive and effective in macaques,45 and is currently being tested in clinical trials on human melanoma.14,15 Patients with melanoma who experienced tumor regression also developed signs of autoimmunity, suggesting that blockade of CTLA-4 results in both desirable and deleterious immune responses.14,15 Indeed, a correlation between autoimmunity and durable objective tumor regression has been evoked.46
In the HIV/SIV setting, there is also the risk of favoring viral replication, as activated CD4+ T cells are the primary targets of HIV/SIV.47 In our study, administration of anti–CTLA-4 antibody was not associated with an evident increase in viral replication. In contrast, CTLA-4 blockade appeared to augment further the ability of ART to reduce virus levels in tissues. This finding goes hand in hand with the observation provided here that CTLA-4 blockade was associated with a trend for an increase in virus-specific effector immune responses.
We speculate in here that the effect observed on viral replication is related to CTLA-4 blockade, as this treatment may have interfered with either Treg function or a more general blockade of negative costimulation. However, the possibility that this antibody may decrease the differentiation of recently activated T cells into the FoxP3 pathway needs to be entertained as well. The immunoregulatory activity of Treg cells is mediated by activation of IDO.13,48,49 We observed that ART plus CTLA-4 blockade produced a larger reduction of IDO mRNA expression and enzymatic activity than ART alone. Accordingly, the expression of TGF-β is also significantly reduced in the animals treated with MDX-010. Although it has been suggested that production of TGF-β is not directly associated with CTLA-4 engagement,50 our findings suggest that CTLA-4 blockade affects the production of this cytokine.
The ultimate goal of CTLA-4 blockade in HIV-1 infection would be to enhance antiviral immune responses. Here, we observed a trend of an increase in virus-specific peripheral CD4+ and CD8+ T-cell effector function in anti–CTLA-4–treated animals. Furthermore, the response against the CM9 Gag peptide was expanded by viral rebound in all 3 Mamu A*01+ animals treated with anti–CTLA-4 plus ART, compared with only 1 of 5 of the animals treated with ART alone. We believe that it is unlikely that the lesser expansion of tetramer+ cells in the untreated animals of group 1 was due to potential mutation in the CM9 epitope that would lead to viral immune escape, as the frequency of this response was high in most animals of this group before the initiation of ART. The apparent increase in anti-SIV responses that we observed may be either the result of a non–antigen-specific effect of anti–CTLA-4 or related to the potentiation of the immunogenicity of residual SIV antigens, as ART does not completely block viral replication. Regardless, CTLA-4 blockade may synergize with an SIV/HIV vaccine and augment further virus-specific immune response.
In our study, we observed a decrease of the virus level in the lymph nodes during ART and CTLA-4 treatment. However, the extent of viral rebound after ART cessation did not differ significantly among the animals treated with MDX-010. CTLA-4 blockade may favor elimination of infected cells by allowing an adequate proliferation and differentiation of virus-specific CD8+ T cells because of the increased tryptophan availability. However, the frequency of CD8+ effector T-cell responses at the time of ART suspension may be insufficient to fully curtail viral replication once ART is discontinued. We anticipate that pairing anti–CTLA-4 to a therapeutic vaccination under an ART regimen34,35 may constitute a strategy to further strengthen the immune response against HIV/SIV. Indeed, vaccination of macaques with hepatitis surface antigen or a human melanoma antigen in conjunction with MDX010–induced CTLA-4 blockade resulted in amplification of the immune response to the vaccines.51 Last, repeated anti–CTLA-4 treatment during ART may also represent an intriguing approach, particularly during primary infection, to decrease the number of infected cells in tissues.
In summary, our results provide evidence on the safety of this approach and provide the rationale for other interventions that target negative immune regulatory pathways. The recent demonstration in murine models of another negative regulatory pathway mediated by PD-1, which at least in mice outweighs the impact of CTLA-4 blockade,52 provides additional targets for the development of immune strategies that hopefully could ameliorate the host response to HIV.
Authorship
Two of the authors (D.B., I.L.) are employed by Medarex, whose product was studied in the present work.
Prepublished online as Blood First Edition Paper, August 8, 2006; DOI 10.1182/blood-2006-04-010637.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Steven Snodgrass for editorial assistance. We acknowledge the assistance of Jim Treece, Stephen Whitney, and Deborah Weiss of Advanced BioScience Laboratories, Inc.
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), Center for Cancer Research. This study was partially supported by NIH contract N01-AI-15451 to Southern Research Institute.