Abstract
The chemokine CXCL12 influences self-renewal and differentiation of hematopoietic stem cell precursors in bone marrow by directing them toward specific stromalcell components. CXCL12 up-regulates members of the SOCS family through JAK/STAT activation, a mechanism that attenuates chemokine responses. SOCS expression may thus modulate retention of hematopoietic precursors (Sca-1+ c-Kit+Lin– cells) in bone marrow. We show that in bovine growth hormone transgenic mice and in growth hormone–treated mice, SOCS up-regulation correlated with a large number of Sca-1+ c-Kit+Lin– cells in blood. Retroviral transduction of SOCSs blocked in vitro migration of Sca-1+c-Kit+Lin– cells, as well as their capacity to reconstitute lethally irradiated mice. Furthermore, in lethally irradiated mice reconstituted with bone marrow infected by a tetracycline-regulated, SOCS-expressing lentiviral vector, doxycycline treatment promoted rapid, extensive precursor mobilization to the periphery. The results indicate that by blocking CXCR4-mediated functions, SOCSs modulate hematopoietic precursor cell retention in bone marrow, and suggest the therapeutic interest of SOCS manipulation in several pathologic situations.
Introduction
Bone marrow (BM) is the major definitive adult hematopoietic organ, which replaces the hematopoietic function of the embryonic liver. Adult BM contains a small proportion of hematopoietic stem cells (HSCs) included in the Sca-1+c-Kit+Lin– population in mice1-3 and in the CD34+Lin– population in humans4-6 ; these populations represent less than 0.01% of total BM cells. During hematopoiesis, HSCs are committed as specific lineage progenitors and mature, and their progeny enter the bloodstream. Hematopoietic precursors are thought to remain mainly in BM to receive specific differentiation and proliferation signals. Hematopoiesis depends on the BM microenvironment and is modulated by stromal-cell–secreted factors, including IL-7, SCF, GM-CSF, G-CSF, IL-3, and CXCL12.7,8
The chemokine CXCL12 (SDF-1α), first isolated from stromal cell–culture supernatants,9 is the specific ligand for the G protein–coupled receptor CXCR4. It is expressed in HSCs, B-cell precursors, and peripheral-blood lymphocytes (PBLs).8,10,11 In contrast to proinflammatory chemokines, CXCL12 is produced constitutively in many organs, including BM. This suggests a role for CXCL12/CXCR4 interactions in steady-state homeostatic processes such as control of leukocyte trafficking and retention of nondifferentiating and maturing hematopoietic cells in BM. Both CXCR4- and CXCL12-deficient mice die perinatally,12-14 with abnormalities including lack of blood-vessel formation in the gut, severe ventricular septal defects, altered cerebellar neuron migration,13-15 and anomalous B-cell lymphopoiesis and BM myelopoiesis. B-cell precursors are reduced in fetal liver and BM; hematopoietic precursors and myeloid cells are found in blood but are barely detectable in liver.13-15 Mice reconstituted with CXCR4-deficient fetal liver cells have abnormally large numbers of pro-B and pre-B cells and granulocytes in circulation, with a parallel reduction in BM. These observations implicate CXCL12/CXCR4 in retention of B-lineage and granulocytic precursors in BM.16 CXCL12 directs hematopoietic precursors toward stromal cells to complete their differentiation,16 but also induces the integrin-mediated arrest of HSCs that retains these cells in BM.17
CXCL12 exerts its effects via receptor dimerization and activation of JAK,18 a kinase family originally implicated in cytokine signaling.19 JAK proteins phosphorylate chemokine receptors on tyrosines, activate STAT transcription factors, and facilitate receptor association of guanine nucleotide–binding proteins (G proteins).20 Via this signaling cascade, chemokines mediate changes in the cytoskeletal apparatus and transcription factors that regulate cell growth.21,22 Through JAK/STAT activation, CXCL12 binding of CXCR4 up-regulates SOCS proteins, which block chemokine-induced functions in a B-cell line, as well as in B220low and CD11+ cells. SOCS overexpression or cytokine-mediated up-regulation interfere with CXCL12-mediated signaling and response, in vivo and in vitro.23
Growth hormone (GH) is a pleiotropic cytokine with many biologic effects, including skeletal growth in childhood and regulation of various anabolic processes in adults. Specific leukocyte populations express GH receptors, and GH is implicated as a hematopoietic growth and differentiation factor.24 After ligand binding, the GH receptor dimerizes, signals through JAK2 kinase,25 and activates STATs. This leads to up-regulation of a variety of genes in vitro and in vivo, including SOCS.26 The SOCS family members CIS, SOCS1, SOCS2, and SOCS3 have an important role in regulating GH action27,28 ; SOCSs inhibit receptor signaling to STAT5b via phosphotyrosine-dependent binding to JAK2 (SOCS1) and/or the cytoplasmic tail of GHR (CIS, SOCS3).29 Recent findings indicate that SOCS proteins also act as adaptors that regulate turnover of certain substrates by interacting with and activating an E3 ubiquitin ligase.30,31
Here we show that bGH transgenic (bGHTg) mice have a larger number of Sca-1+c-Kit+Lin– precursor cells in peripheral blood than control mice; this phenotype is also observed following intramuscular GH injection into wild-type (wt) mice. Sca-1+cKit+Lin– cells isolated from bGHTg mice show SOCS1 and SOCS3 up-regulation and impaired CXCL12-induced function, but no difference in plasma membrane CXCR4 expression; SOCS1 and SOCS3 down-regulation due to GH depletion restored CXCL12-mediated functions. All together, these observations suggest a role for SOCSs in controlling CXCL12-mediated HSC retention in BM. We transduced BM Lin– cells with retroviral SOCS1 and SOCS3 vectors, or with lentiviral vectors in which SOCS protein expression is under the control of a tetracycline-regulated promoter. In both cases, SOCS1- or SOCS3-expressing BM Lin– cells did not migrate toward CXCL12 gradients or reconstitute lethally irradiated mice. Mice reconstituted with Sca-1+c-Kit+Lin– cells transduced with the lentiviral constructs were treated with doxycycline, which promoted SOCS up-regulation and precursor mobilization from BM to the periphery. The mechanism involves SOCS1 and SOCS3 association to JAK and CXCR4, respectively, and subsequent abrogation of CXCL12-mediated functions. As a consequence, SOCS-expressing HSCs have irregular interactions with the hematopoietic microenvironment, leading to cell egress from BM. This indicates that SOCSs are key molecules in controlling HSC retention in BM and reinforces the importance of cytokine/chemokine crosstalk in the physiologic microenvironment.
Materials and methods
Mice
bGHTg and control wt mice on the C57BL/6 background were from Drs J. P. García-Ruiz and E. Delgado (Centro de Biología Molecular, Universidad Autónoma de Madrid/Consejo Superior de Investigaciones Científicas [CBM UAM/CSIC], Madrid, Spain). Procedures were approved by the Centro Nacional de Biotecnología (CNB) Animal Care and Use Committee, in compliance with national and European legislation.
Biologic materials
We used HEK-293 cells (TIB202; American Type Culture Collection, Manassas, VA), MS-5 cells (ACC441; German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), and FBMD-1 (a gift of Dr J. A. Cancelas, Cincinnati Children's Research Foundation, Cincinnati, OH). Antibodies include anti-CXCR4 mAb,18 anti–CD2-PE, –CD4-PE, –CD4-BIOT, –CD8-BIOT, –CD11b-FITC, –CD11b-BIOT, –CD43-BIOT, –cKIT-APC, –cKIT-PE, –Gr1-PE, –Gr1-BIOT, –SCA1-FITC, –TER119-BIOT (Pharmingen, San Diego, CA), –CD3-FITC, –CD8-SPRD, –B220-FITC, –B220-BIOT, –IgM-PE (Southern Biotechnologies, Birmingham, AL), and –NGFR-BIOT (Chemikon, Temecula, CA); we also used Av-PeCy7 (Bioscience, San Diego, CA), Av-SPRD, and goat anti–mouse IgG1-SPRD (Southern Biotechnologies). For Western blot, we used anti–FLAG M2 mAb (Sigma, St Louis, MO), polyclonal anti–SOCS-1 (H-93; Santa Cruz Biotechnology, Santa Cruz, CA), –SOCS-3 (H-103; Santa Cruz Biotechnology), –P-JAK2 (Y1007, Y1008; UBI, Lake Placid, NY), and anti–β-actin antibody (AC-15; Sigma).
Drugs used were tetracycline, doxycycline, 5′-bromo-2′-deoxyuridine, and hexadimethrine bromide (Sigma); cytokines were human erythropoietin (Stem Cell Technologies, Vancouver, BC) and murine IL-6, IL-7, SCF, and FLT-3 ligand (PeproTech, Rocky Hill, NJ).
Flow cytometry analysis and cell sorting
Flow cytometry was as described,18 using FITC-, PE-, SPRD-, or biotinlabeled antibodies (1 μg/50 μL/well, 60 minutes, 4°C), and Av-PeCy7 or Av-SPRD. Cell-bound fluorescence was determined in a Cytomics FC 500 (Beckman-Coulter, Miami, FL).
Peripheral blood cells were stained (25 μL/well, 30 minutes, room temperature) as described in the previous paragraph and erythrocytes lysed with OptiLyse C (Beckman-Coulter).
BM cells were harvested from mouse femurs, erythrocytes lysed with NH4Cl (5 minutes, 37°C), and cells stained as for peripheral blood cells. BM cells were stained with Sca-1+c-Kit+Lin– and sorted in an Epics Altra Hypersort (Beckman-Coulter). We isolated Lin– cells by negative sorting of BM cells using biotin-labeled antibodies (anti-CD4, -CD8, -CD11b, -CD43, -Gr1, -TER119, -B220) and streptavidin M-280 Dynabeads (Dynal, Oslo, Norway).
BrdU-labeled cells were stained32 with FITC-conjugated anti-BrdU mAb (Beckton-Dickinson, San Jose, CA). To evaluate lentivirus-transduced cells, BrdU-labeled cells were stained with anti-BrdU mAb, followed by SPRD-conjugated goat antimouse antibody.
In vitro BrdU labeling of BM cultures was as described33 using MS-5 stromal cells. We added lentivirus-infected Lin– cells (105 cells/well) and cocultured (20 or 40 hours, 37°C) alone or with tetracycline (1 μg/mL).
Mouse treatment
Three-month-old C57BL/6 mice received daily intramuscular injections of rhGH (recombinant human GH; Pfizer, New York, NY) (10 μg/mL in 50 μL PBS) or PBS for 7 or 25 days, followed by flow cytometric analysis of BM and peripheral precursor cells. For induction experiments, doxycycline (Sigma) was administered ad libitum in drinking water (2 mg/mL) containing 5% sucrose from the start of the experiment (4 days before intrafemoral reconstitution or 20 days after reconstitution). For in vivo BrdU incorporation, mice were given BrdU (0.8 mg/mL) in drinking water, prepared freshly every 2 days, for a 9-day period.
Constructs
Flag-SOCS1 and -SOCS3 sequences were recovered by polymerase chain reaction (PCR) from pEF-FLAG-I/mSOCS1 or mSCOS3 constructs (from Dr T. Willson, Walter and Eliza Hall Institute, Victoria, Australia) using oligonucleotides 5′BamHI-SOCS1/3 (5′ATGGATCCATGGCGCGCCAGGACTA CAAG3′), 3′XhoI-SOCS1 (5′ATCTCGAGTCAGATCTGGAAGGGGAAGG3′), and 3′XhoI-SOCS3 (5′CGCTCGAGTTAAAGTGGAGCATCATACTG3′) and inserted in the pRV-IRES/ΔNGFR retroviral vector (Genetrix, Madrid, Spain) to obtain pRV-IRES/ΔNGFR-Flag-SOCS-1 and pRV-IRES/ΔNGFR-Flag-SOCS-3 constructs.
For the lentiviral “Tet-on” system, we recovered SOCS1 and SOCS3 sequences from pEF-FLAG-I/mSOCS1 or mSOCS3 constructs using oligonucleotides 5′BamH1-SOCS1/3 (5′ATGGATCCATGGCGCGCCAGGACTACAAG3′), 3′XhoI-SOCS1 (5′ATCTCGAGTCA GATCTGGAAGGGGAAGG3′), and 3′XhoI-SOCS3-mut (5′ATCTCGAGTTAAAGTGGAG CATCATACTGATCCAGGAACTC3′) and cloned into pCDNA3.1. SOCS sequences were extracted from PCDNA3.1 plus cytomegalovirus (CMV) promoter and BGH polyadenylation sequences using oligonucleotides 5′EcoRI-pCMV (5′TAGAATTCCGCGTTGACATTGATTATTGAC3′) and 3′MluI-BGH polyA (5′ATACGCGTGCCATAGAGCCCACCGCATC3′) and cloned in pLVTHM-GFP to obtain pLVTHM-SOCS1-GFP and pLVTHM-SOCS3-GFP constructs (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
Cell transfection and infection
HEK293 cells were transiently transfected with pRV-IRES/ΔNGFR, pRV-IRES/ΔNGFR-Flag-SOCS1, or pRV-IRES/ΔNGFR-Flag-SOCS3 plus the pCLEco envelope to obtain retroviral particles, or with pLV-tTRKRAB-dsRed (Tronolab, Lausanne, Switzerland), pLVTHM-GFP, pLVTHM-SOCS1-GFP, or pLVTHM-SOCS3-GFP plus RRE, REV, and VSVg envelopes to obtain lentiviral particles using JetPEI reagent (PolyPlus Transfection, Illkirch, France). We used culture supernatants (48-72 hours after transfection) to infect isolated Lin– cells with retro- or lentiviral constructs. Retrovirus-infected Lyn– cells were monitored by ΔNGFR analysis. Lentivirus-infected Lin– cells were monitored for coexpression of dsRed (a red fluorescent protein mutant) and GFP.
Intravenous and intrafemoral reconstitution
Lethally irradiated C57BL/6 mice (2 doses, 5.25 Gy, 4 hours apart) were reconstituted by intravenous injection with whole blood (300 μL/mouse) or BM cells (106/mouse) from wt or bGHTg mice. Alternatively, irradiated C57BL/6 mice were reconstituted by intrafemoral injection with whole blood (50 μL/mouse) from bGHTg or GH-injected wt mice. Irradiated C57BL/6 mice were intrafemorally reconstituted using retrovirus- or lentivirus-infected Lin– cells (5 × 104 cells/50 μL/mouse). Two unreconstituted, lethally irradiated control mice were included in each experiment; only experiments in which these controls died were included.
Chromosome painting
Total BM cells from transplanted femurs were incubated with 0.03 M sodium citrate (37°C, 25 minutes), fixed twice in methanol–acetic acid (3:1), and dropped onto glass slides. Chromosome painting was performed in interphase nuclei with a Cy-3–labeled mouse chromosome Y painting probe (Cambio, Cambridge, United Kingdom), with DAPI counterstaining, followed by imaging on a Leica DMRB fluorescence microscope (Leica, Heidelberg, Germany) and an Olympus DP70 digital camera (100 × magnification; Olympus, Melville, NY).
Hemogram analysis
We performed hemogram analysis of mouse blood using a Procount veterinary hematology analyzer (Diatron, Vienna, Austria).
Analysis of in vitro colony formation
We assayed cells (5 × 105) from blood or BM for CFU-GM and BFU-E colony formation by culture in Methocult GF M3534 or M3630 (Stem Cell Technologies) selective media, supplemented with 6 U/mL recombinant human erythropoietin, 15% IL-3–producing WEHI-conditioned medium, and 50 ng/mL recombinant murine SCF. Colonies were analyzed after 10 days in culture.
Western blot
RT-PCR analysis
Total cell RNA was extracted from BM-derived Sca-1+c-Kit+Lin– precursors using TriReagent (Sigma). Total RNA (5 μg) was reverse-transcribed using random hexamers and 100 U Superscript II RT (Invitrogen, Carlsbad, CA). Real-time PCR was performed on an ABI Prism 7700 with SYBR Green PCR Reagents (Applied Biosystems, Foster City, CA) (2 minutes at 50°C; 10 minutes at 95°C; 40 cycles, 15 seconds at 95°C; 90 seconds at 67°C). We used specific primers to amplify sequences spanning different exons, except for the β-actin pair, which was used to correct for cDNA loading differences. We detected the cycle (Ct) in which reactions achieve a determined fluorescence level; higher Ct values represent low mRNA levels, and vice versa. mRNA expression was estimated as ΔCt values, which express the cycle threshold difference between the indicated primer and the β-actin pair. Relative expression between bGHTg and wt mice was calculated using the 2ΔΔCt formula: fold expression = 2ΔΔCt, where ΔΔCt =ΔCtwt –ΔCtbGHTg.34
Primers used were CXCR4 (sense) CCATGGAACCGATCAGTGTG, (antisense) TTTTCATCCCGGAAGCAGG; SOCS1 (sense) CGCGTCCTGCCGCCA, (antisense) AGTTCCGTTGGCGACTGTC; SOCS3 (sense) CCCTTTGTAGACTTCACGGC, (antisense) GAAACTTGCTGTGGGTGACC; mCXCL12 (sense) TCGGGTCAATGCACACTTGT, (antisense) AGCCAACGTCAAGCATCTGA.
Cell migration
We used 5 × 105 cells in 100 μL with several CXCL12 concentrations (PeproTech); the cell-migration index was calculated23 and expressed as the percentage of input cells. Migrated cells were stained with anti-Sca, –c-Kit, and -Lin antibodies and analyzed by flow cytometry. Retro- and lentivirus-infected cells were sorted before evaluation in migration assays.
Cell adhesion
Untreated or GH-depleted (37°C, 2 hours) Lin– cells from bGHTg mice or control littermates were labeled with BCECF (Molecular Probes, Eugene, OR). Cells (105 cells/well) were seeded on a 96-well plate precoated with fibronectin (20 μg/mL), alone or with 50 nM CXCL12, then incubated (37°C, 5 minutes) and washed, and luminescence was quantified on a Cytofluor 2300 (Millipore, Bedford, MA). Luminescence was directly proportional to cell number/well in the 2.5 to 25 × 103 cell range.
To evaluate cell adhesion to stromal cells, MS-5 cells were cultured to confluence in α-MEM (BioWhittaker, Walkersville, MD) with 10% FCS and antibiotics. Untreated or tetracycline-treated lentivirus-infected Lin– cells were added to MS-5–containing wells (105 cells/500 μL) and adherent cells measured33 after 60 minutes (37°C, 5% CO2).
Limiting-dilution CAFC assay
Frequency of cobblestone area–forming cells (CAFCs) was determined.33,35 We used murine BM stroma–derived cell lines MS-5 and FBMD-1 to generate a confluent layer. MS-5 cells were plated in 96-well plates at 103 cells/well in 100 μL complete α-MEM (37°C, 5% CO2); FBMD-1 cells were plated similarly (33°C, 5% CO2) in MyeloCult 5300 (Stem Cell Technologies) with 10 μM l-hydrocortisone (Sigma) and antibiotics. Ten serial dilutions of untreated or tetracycline-treated lentivirus-infected Lin– cells were plated onto stromal-cell monolayers, in 24 wells per dilution, and examined weekly for 28 days for the presence of cobblestones. The number of CAFCs/105 cells plated was calculated from the number of cobblestone-negative wells for each dilution, using Poisson statistics.35
Statistics
All animal experiments were performed independently at least 5 times, using 8 mice per group and experiment. Statistical analyses were performed using GraphPad Prism software (GraphPad, San Diego, CA).
Results
bGHTg mice have increased numbers of circulating Sca-1+c-Kit+Lin–
Growth hormone (GH) participates in regulation of hematopoiesis and modulation of the immune response.36 In vivo GH treatment significantly increases the number of myeloid colony-forming units in BM and spleen.37 Certain hematopoietic subpopulations in bGHTg mice have a defect in migration toward CXCL12 gradients.23 As CXCL12 has a critical role in retention of precursors in BM,16 we evaluated this process in bGHTg mice.
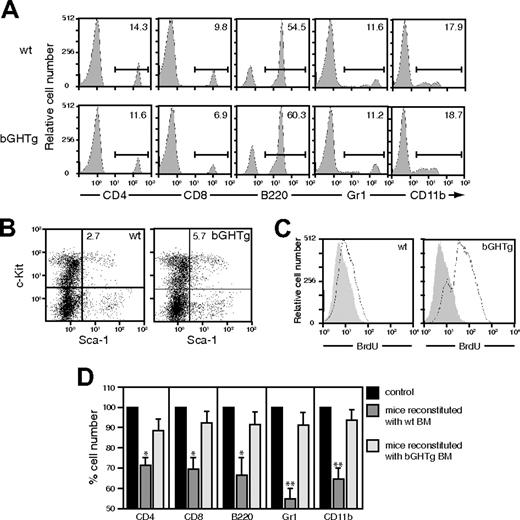
We analyzed PBLs from 3-month-old bGHTg and control mice by flow cytometry. There were no marked differences in T and B cells or in the different myeloid lineages between bGHTg and control mice (Figure 1A); however, the number of Sca-1+ c-Kit+Lin– cells in BM (Figure 1B) and in peripheral blood was significantly higher in bGHTg mice (0.60% ± 0.02% [6480 ± 216 cells/mL] vs 0.25% ± 0.01% [638 ± 25 cells/mL], P ≤ .01).
We analyzed BM-cell proliferative capacity by in vivo administration of 5-bromo-2′-deoxyuridine (BrdU) in 3-month-old bGHTg and control littermate mice, followed by flow cytometry analysis. bGHTg-derived Sca-1+c-Kit+Lin– cells proliferated more rapidly than controls (Figure 1C). Results were similar when we compared the clonogenic activity of BM cells from 3-month-old bGHTg and control mice; bGHTg cells showed increased in vitro colony formation for granulocytic-macrophage (CFU-GMs; bGHTg 1288 ± 12 colonies/femur vs control 501 ± 3 colonies/femur) and erythroid (BFU-Es; bGHTg 1178 ± 14 colonies/femur vs control 524 ± 4 colonies/femur) progenitors. To compare the in vivo reconstitutive capacity of these precursors, lethally irradiated 3-month-old C57BL/6 mice received an intravenous injection of total BM cells (106 in 300 μL) from bGHTg mice or control littermates. BM cells from both sources promoted complete reconstitution, although bGHTg precursors engrafted more efficiently, as indicated by hemogram (Table 1) and flow cytometry (Figure 1D) analysis of PBLs 28 days after transplantation. Comparative reconstitution efficiency was similar at 21 and 35 days after transplantation (not shown). These data confirmed a role for GH in maintaining immune-cell survival and proliferation.38
bGHTg mouse BM has large numbers of Sca-1+c-Kit+Lin– cells, which engraft more rapidly than those from control mice. (A) Flow cytometry analysis of T (CD4, CD8) and B-cell (B220) populations and myeloid lineages (CD11b, Gr1) from bGHTg and control (wt) mouse PBLs. Values shown represent the percentage of positive cells. (B) Flow cytometry analysis of Sca-1+c-Kit+Lin– BM cells from bGHTg and wt mice. (C) BrdU incorporation levels in BM cells from 3-month-old bGHTg and wt mice. Histograms correspond to representative animals (n = 6). (D) Flow cytometry analysis of mouse PBLs at 30 days after reconstitution with BM cells from bGHTg or wt mice. Data represent mean ± SD of 3 different experiments (n = 4 mice/assay).
bGHTg mouse BM has large numbers of Sca-1+c-Kit+Lin– cells, which engraft more rapidly than those from control mice. (A) Flow cytometry analysis of T (CD4, CD8) and B-cell (B220) populations and myeloid lineages (CD11b, Gr1) from bGHTg and control (wt) mouse PBLs. Values shown represent the percentage of positive cells. (B) Flow cytometry analysis of Sca-1+c-Kit+Lin– BM cells from bGHTg and wt mice. (C) BrdU incorporation levels in BM cells from 3-month-old bGHTg and wt mice. Histograms correspond to representative animals (n = 6). (D) Flow cytometry analysis of mouse PBLs at 30 days after reconstitution with BM cells from bGHTg or wt mice. Data represent mean ± SD of 3 different experiments (n = 4 mice/assay).
We characterized Sca-1+c-Kit+Lin– cells in the periphery by evaluating the clonogenic activity of PBL precursors from 3-month-old bGHTg and control mice. bGHTg peripheral blood showed increased capacity for in vitro colony formation of myeloid (CFU-GM; bGHTg 897 ± 9 colonies/mL vs control 140 ± 4 colonies/mL) and erythroid (BFU-E; bGHTg 816 ± 12 colonies/mL vs control 158 ± 5 colonies/mL) progenitors. The results indicate a larger number of circulating precursors in bGHTg mouse peripheral blood compared with controls.
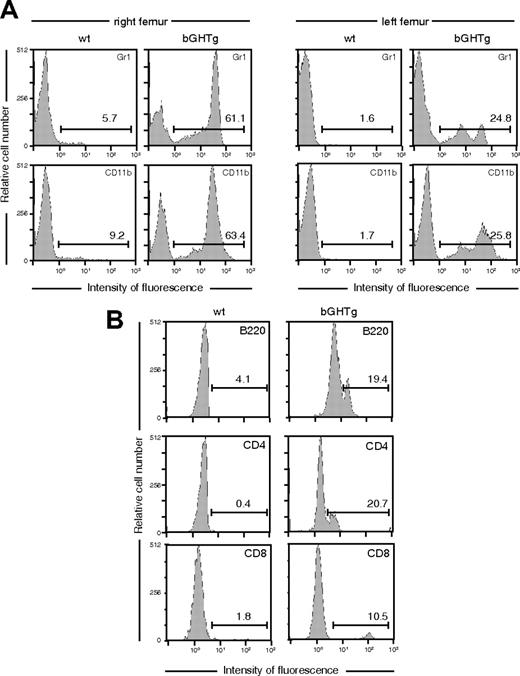
To evaluate the potential of these circulating cells to restore BM hematopoietic function, lethally irradiated 3-month-old female C57BL/6 mice received whole blood (300 μL, intravenous) from male bGHTg or control littermates. Neither treatment led to reconstitution, and all mice died during the first week after transplantation. Some HSC subpopulations are not detected after intravenous injection–based repopulation assays, as reconstitution requires cell incorporation into the circulation, recognition, and extravasation to the BM vasculature.39 Using intrafemoral injection (through the knee joint into the right femur), we injected whole peripheral blood (50 μL) from male bGHTg mice or controls into lethally irradiated 3-month-old female C57BL/6 mice. At 2 weeks after injection, bGHTg HSCs had engrafted BM in the right femur and in noninjected bones (left femur) (Figure 2A) and in periphery (Figure 2B), indicating rapid spread and unimpeded homing processes, whereas engraftment was poor and slower when control mouse blood was used (Figure 2A-B). Using the fluorescence in situ hybridization (FISH) technique, we detected the Y chromosome in more than 95% of Lin+ cells in reconstituted mice, confirming that they derived from the male donors (Figure S2).
bGHTg mice have a larger number of circulating Sca-1+c-Kit+Lin– cells than control mice. (A) Flow cytometry analysis of myeloid lineages (Gr1, CD11b) in the right (left panels) and left (right panels) femurs of mice reconstituted by injection in the right femur of whole blood from bGHTg and wt mice. Values (inset) represent the percentage of positive cells. (B) T (CD4, CD8) and B-cell (B220) population analysis in peripheral blood of mice reconstituted as in panel A. Values represent the percentage of positive cells. Histograms correspond to representative animals (n = 10).
bGHTg mice have a larger number of circulating Sca-1+c-Kit+Lin– cells than control mice. (A) Flow cytometry analysis of myeloid lineages (Gr1, CD11b) in the right (left panels) and left (right panels) femurs of mice reconstituted by injection in the right femur of whole blood from bGHTg and wt mice. Values (inset) represent the percentage of positive cells. (B) T (CD4, CD8) and B-cell (B220) population analysis in peripheral blood of mice reconstituted as in panel A. Values represent the percentage of positive cells. Histograms correspond to representative animals (n = 10).
Injection of rhGH mobilizes Sca-1+c-Kit+Lin– cells
To test the ability of exogenous GH treatment to reproduce the bGHTg mouse phenotype and to rule out possible influence of a local or systemic paracrine effect derived from bGHTg-cell use, we treated 3-month-old C57BL/6 mice with daily intramuscular rhGH injections for 7 days (short term) or 25 days (long term), and then analyzed Sca-1+c-Kit+Lin– cells in peripheral blood and BM.
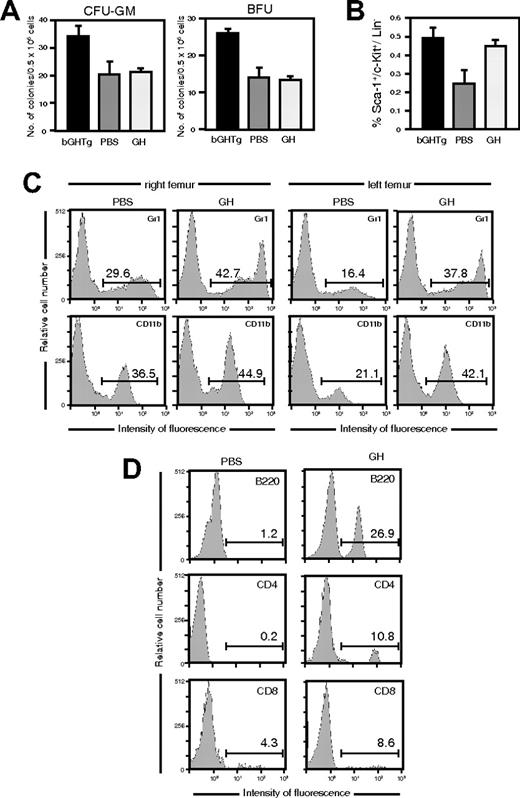
Short-term rhGH treatment altered neither the percentage of Sca-1+c-Kit+Lin– cells in BM compared with PBS-injected mice (4.0 ± 0.01 [12 744 ± 32 cells/femur] vs 3.0 ± 0.01 [8748 ± 29 cells/femur]) nor their clonogenic activity (Figure 3A). We nonetheless detected a Sca-1+c-Kit+Lin– cell increase in peripheral blood in rhGH-treated mice (Figure 3B). In clonogenic assay using total BM cells, long-term rhGH treatment induced an increase in CFU-GMs (726 ± 11 colonies/femur vs 216 ± 9 colonies/femur) and BFU-Es (663 ± 13 colonies/femur vs 173 ± 3 colonies/femur) compared with PBS-treated mice, confirming previous findings.40
Peripheral blood from long-term rhGH-treated mice showed an increase in Sca-1+c-Kit+Lin– cells compared with PBS-injected mice, which were similar to those in bGHTg mice (0.5% ± 0.005% [5320 ± 5 cells/mL] vs 0.25% ± 0.004% [580 ± 9 cells/mL]). rhGH thus not only triggers HSC proliferation but also mobilizes progenitors from BM to the periphery.
rhGH injection triggers HSC mobilization in mice. (A) Mice received a daily injection of rhGH or PBS (7 days), and the clonogenic activity of BM cells was evaluated. The mean ± SD is shown for 3 independent experiments. The clonogenic activity of BM cells from bGHTg mice is included as a control. (B) Flow cytometry analysis of Sca-1+c-Kit+Lin– cells in peripheral circulation of mice treated as in panel A. As control, Sca-1+c-Kit+Lin– staining is shown for bGHTg mouse blood. The mean ± SD is shown for 7 independent experiments. (C) Flow cytometry of myeloid lineages (Gr1, CD11b) in the right (left panels) and left (right panels) femurs of mice reconstituted by injection in the right femur with whole blood from mice treated for 7 days with rhGH or PBS. Values (inset) represent the percentage of positive cells. Histograms show representative animals (n = 8). (D) T (CD4, CD8) and B-cell (B220) population analysis in peripheral blood of mice reconstituted as in panel C. Values represent the percentage of positive cells. Histograms correspond to representative animals (n = 8).
rhGH injection triggers HSC mobilization in mice. (A) Mice received a daily injection of rhGH or PBS (7 days), and the clonogenic activity of BM cells was evaluated. The mean ± SD is shown for 3 independent experiments. The clonogenic activity of BM cells from bGHTg mice is included as a control. (B) Flow cytometry analysis of Sca-1+c-Kit+Lin– cells in peripheral circulation of mice treated as in panel A. As control, Sca-1+c-Kit+Lin– staining is shown for bGHTg mouse blood. The mean ± SD is shown for 7 independent experiments. (C) Flow cytometry of myeloid lineages (Gr1, CD11b) in the right (left panels) and left (right panels) femurs of mice reconstituted by injection in the right femur with whole blood from mice treated for 7 days with rhGH or PBS. Values (inset) represent the percentage of positive cells. Histograms show representative animals (n = 8). (D) T (CD4, CD8) and B-cell (B220) population analysis in peripheral blood of mice reconstituted as in panel C. Values represent the percentage of positive cells. Histograms correspond to representative animals (n = 8).
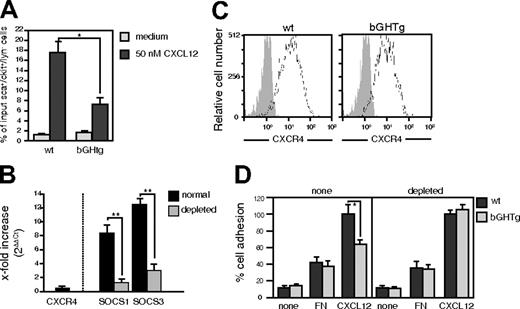
bGHTg Sca-1+c-Kit+Lin–cells show altered CXCL12 function. (A) Sca-1+c-Kit+Lin– cells from bGHTg and wt mice were allowed to migrate toward a CXCL12 gradient. The migration index was calculated as a percentage of input cells. Data represent the mean ± SD of quadruplicate determinations; *P ≤ .05. (B) Quantitative RT-PCR analysis of CXCR4, SOCS1, and SOCS3 mRNA from Sca-1+c-Kit+Lin– cells isolated from bGHTg or wt mouse BM. Results are expressed as x-fold increase (“RT-PCR analysis”). The mean ± SD is shown for 6 independent experiments; **P ≤ .01. (C) Flow cytometry measurement of CXCR4 levels in Sca-1+c-Kit+Lin– cells isolated from bGHTg or wt mouse BM. Histograms show representative experiments (n = 4). (D) Static adhesion of Sca-1+c-Kit+Lin– cells from bGHTg and wt mice, undepleted or GH-depleted in vitro, on FN alone or with CXCL12. Results are expressed as a percentage of maximum adhered cells. Data represent the mean ± SD of quadruplicate determinations; *P ≤ .05.
bGHTg Sca-1+c-Kit+Lin–cells show altered CXCL12 function. (A) Sca-1+c-Kit+Lin– cells from bGHTg and wt mice were allowed to migrate toward a CXCL12 gradient. The migration index was calculated as a percentage of input cells. Data represent the mean ± SD of quadruplicate determinations; *P ≤ .05. (B) Quantitative RT-PCR analysis of CXCR4, SOCS1, and SOCS3 mRNA from Sca-1+c-Kit+Lin– cells isolated from bGHTg or wt mouse BM. Results are expressed as x-fold increase (“RT-PCR analysis”). The mean ± SD is shown for 6 independent experiments; **P ≤ .01. (C) Flow cytometry measurement of CXCR4 levels in Sca-1+c-Kit+Lin– cells isolated from bGHTg or wt mouse BM. Histograms show representative experiments (n = 4). (D) Static adhesion of Sca-1+c-Kit+Lin– cells from bGHTg and wt mice, undepleted or GH-depleted in vitro, on FN alone or with CXCL12. Results are expressed as a percentage of maximum adhered cells. Data represent the mean ± SD of quadruplicate determinations; *P ≤ .05.
We tested the ability of rhGH-mobilized peripheral precursors to reconstitute lethally irradiated 3-month-old C57BL/6 mice. Mice received whole blood (50 μL; intrafemoral, right femur) from 7-day rhGH- or PBS-treated mice. By 2 weeks after reconstitution, precursors from rhGH-treated mice had engrafted BM in the right femur, in noninjected bones (left femur) (Figure 3C), and in periphery (Figure 3D), indicating rapid spread similar to that observed for bGHTg mouse precursors. Sca-1+c-Kit+Lin– cells from PBS-treated mice showed no engraftment (Figure 3C-D). The results confirm a role for GH not only in sustaining HSC proliferation but also in promoting precursor mobilization from BM.
Reduced CXCL12-mediated function in bGHTg mouse Sca-1+c-Kit+Lin– cells
As CXCL12/CXCR4 contribute to HSC confinement in BM,16,17 we tested the response of bGHTg mouse Sca-1+c-Kit+Lin– cells to CXCL12. Total BM cells from 3-month-old bGHTg and control mice were allowed to migrate toward a CXCL12 gradient; we measured Sca-1+c-Kit+Lin– cells before and after migration by flow cytometry analysis. Whereas Sca-1+c-Kit+Lin– cells from wt mouse BM migrated toward CXCL12, bGHTg mouse Sca-1+ c-Kit+Lin– cell migration was markedly reduced (Figure 4A). In bGHTg mice, CXCL12 failed to promote BM Sca-1+c-Kit+Lin– cell migration. RT-PCR analysis of Sca-1+c-Kit+Lin– cells sorted from 3-month-old bGHTg and control mice ruled out differences in CXCR4 mRNA expression (Figure 4B left), and 4-color flow cytometry analysis indicated no differences in CXCR4 expression (Figure 4C). RT-PCR analysis also ruled out changes in CXCL12 expression between bGHTg and control littermates' BM cells (x-fold increase 1.56 ± 0.02) (Figure S3).
GH-induced JAK/STAT activation triggers SOCS up-regulation and interference with chemokine signaling and function.23 Using RT-PCR, we evaluated SOCS mRNA levels in Sca-1+c-Kit+Lin– cells from bGHTg mice and controls. SOCS1 and SOCS3 mRNA were substantially increased in cells from bGHTg mice (Figure 4B right). As in bGHTg mouse immune system cells,23 SOCS1 and SOCS3 mRNA levels were down-regulated after GH depletion of HSCs (37°C, 2 hours in complete medium without GH) (Figure 4B right). In these conditions, Lin– cells recover the ability to respond to CXCL12, as shown in an in vitro adhesion assay (Figure 4D). Mouse reconstitution experiments were performed in conditions that simulate GH depletion (adult wt C57BL/6 mice), with a fully functional CXCR4; the greater engraftment using bGHTg mouse blood or BM cells would be attributable directly to the greater number of Sca-1+c-Kit+Lin– cells in these mice. We thus hypothesize a role for SOCSs in BM precursor mobilization.
To evaluate this possibility, BM Lin– cells from wt mice were transduced with retroviral vectors expressing SOCS1 (pEF-Flag-I/mSOCS1/ΔNGFR) or SOCS3 (pEF-Flag-I/mSOCS3/ΔNGFR), followed by Western blot confirmation of SOCS overexpression using anti-Flag antibodies (Figure 5A). Potential toxic effects of SOCS overexpression were ruled out by flow cytometry analysis of propidium iodide incorporation (not shown). Forced expression of SOCS1 or SOCS3 abrogated CXCL12-induced cell migration, confirming previous results in primary cells and cell lines23 ; cells transduced with empty/ΔNGFR migrated normally (Figure 5B).
We tested the ability of SOCSs to alter CXCL12-mediated Sca-1+c-Kit+Lin– cell function in an in vivo model. Lethally irradiated C57BL/6 mice received SOCS1/ΔNGFR-, SOCS3/ΔNGFR-, or empty/ΔNGFR-transduced Lin– cells (5 × 104 in 50 μL, intrafemoral, right femur), and we evaluated BM engraftment. Three days after injection, mice were bled and Sca-1+c-Kit+Lin–/NGFR+ cells analyzed by flow cytometry. We detected no positive cells in the blood of mice reconstituted with mock-transduced cells or empty vector/ΔNGFR, whereas mice reconstituted with SOCS1/ΔNGFR- or SOCS3/ΔNGFR-transduced cells showed large numbers of circulating Sca-1+c-Kit+Lin–/NGFR+ cells (Figure 5C). These results suggest that constitutive SOCS expression is sufficient to abrogate CXCL12-induced precursor retention in BM, confirming the relationship between SOCS up-regulation and lack of chemokine-mediated functions. By 2 weeks after reconstitution, empty/ΔNGFR-transduced precursors had engrafted BM of the right femur (Figure 5D) and noninjected bones (left femur), as well as periphery (not shown). This rapid spread was comparable with that found when bGHTg mouse precursors were used in a similar experiment. Anti-NGFR antibody staining showed that these populations derived from the injected cells (Figure 5D). Neither SOCS1-nor SOCS3-tranduced precursors engrafted (Figure 5D), indicating a role for SOCSs in regulating chemokine responses and HSC mobilization.
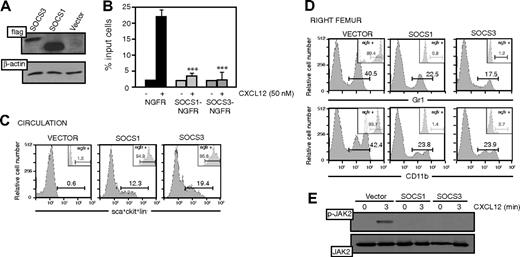
SOCS expression alters CXCL12-mediated Sca-1+c-Kit+Lin–cell mobilization. (A) pEF-Flag-I/mSOCS1/ΔNGFR (SOCS1)–, pEF-Flag-I/mSOCS3/ΔNGFR (SOCS3)–, or pEF-Flag-I/ΔNGFR (vector)–transduced Lin– cells were lysed and analyzed in Western blot with anti-Flag mAb. As control, the same membrane was developed with anti–β-actin. (B) pEF-Flag-I/mSOCS1/ΔNGFR–, pEF-Flag-I/mSOCS3/ΔNGFR–, or pEF-Flag-I/ΔNGFR–transduced Sca-1+c-Kit+Lin– cells were allowed to migrate toward a CXCL12 gradient. The migration index was calculated as a percentage of input cells. Data represent the mean ± SD of triplicate determinations; ***P ≤ .001. (C) Flow cytometry analysis of Sca-1+c-Kit+Lin– cells in peripheral circulation of mice reconstituted by intrafemoral injection with Lin– cells as in panel A. Insets show the analysis of NGFR+ cells in the Sca-1+c-Kit+Lin– population. Values represent the percentage of positive cells. Histograms show representative mice (n = 8). (D) Flow cytometry analysis of myeloid lineages (CD11b, Gr1) in BM from the right femur of mice reconstituted by intrafemoral injection with Lin– cells as in panel A. Insets show analysis of NGFR+ cells in CD11b+ and Gr1+ cells. Values represent the percentage of positive cells. Histograms show representative mice (n = 8). (E) Cells as in panel A were stimulated in vitro with CXCL12 (50 nM, 3 minutes), lysed, and analyzed by Western blot with anti–P-JAK2 mAb. As control, the membrane was developed with anti-JAK2.
SOCS expression alters CXCL12-mediated Sca-1+c-Kit+Lin–cell mobilization. (A) pEF-Flag-I/mSOCS1/ΔNGFR (SOCS1)–, pEF-Flag-I/mSOCS3/ΔNGFR (SOCS3)–, or pEF-Flag-I/ΔNGFR (vector)–transduced Lin– cells were lysed and analyzed in Western blot with anti-Flag mAb. As control, the same membrane was developed with anti–β-actin. (B) pEF-Flag-I/mSOCS1/ΔNGFR–, pEF-Flag-I/mSOCS3/ΔNGFR–, or pEF-Flag-I/ΔNGFR–transduced Sca-1+c-Kit+Lin– cells were allowed to migrate toward a CXCL12 gradient. The migration index was calculated as a percentage of input cells. Data represent the mean ± SD of triplicate determinations; ***P ≤ .001. (C) Flow cytometry analysis of Sca-1+c-Kit+Lin– cells in peripheral circulation of mice reconstituted by intrafemoral injection with Lin– cells as in panel A. Insets show the analysis of NGFR+ cells in the Sca-1+c-Kit+Lin– population. Values represent the percentage of positive cells. Histograms show representative mice (n = 8). (D) Flow cytometry analysis of myeloid lineages (CD11b, Gr1) in BM from the right femur of mice reconstituted by intrafemoral injection with Lin– cells as in panel A. Insets show analysis of NGFR+ cells in CD11b+ and Gr1+ cells. Values represent the percentage of positive cells. Histograms show representative mice (n = 8). (E) Cells as in panel A were stimulated in vitro with CXCL12 (50 nM, 3 minutes), lysed, and analyzed by Western blot with anti–P-JAK2 mAb. As control, the membrane was developed with anti-JAK2.
We analyzed the mechanism involved in SOCS blockade of CXCL12-induced responses. SOCS1 associates to JAK proteins, blocking their kinase activity, whereas SOCS3 binds the phosphorylated receptor.41-43 SOCS1/ΔNGFR-, SOCS3/ΔNGFR-, or empty/ΔNGFR-transduced Sca-1+c-Kit+Lin– cells were activated with CXCL12 (50 nM, 3 minutes, 37°C) and lysed, and cell extracts were tested in Western blot for phosphorylated JAK2 using anti–P-JAK2 antibody. CXCL12 induced JAK2 phosphorylation only in empty/ΔNGFR-transduced cells, confirming lack of CXCR4 function when SOCS1 or SOCS3 was constitutively up-regulated (Figure 5E).
To confirm these results in a ligand-independent, inducible approach, we used a tetracycline-regulated promoter (Tet-on system) to modulate lentiviral SOCS1 or SOCS3 gene delivery. Lin– cells from wt mouse BM were sorted and transduced with pLVTHM-tetO/SOCS1, -tetO/SOCS3, or -tetO/empty (Figure S1). We treated transduced cells with tetracycline to turn on SOCS gene expression and analyzed cell lysates in Western blot with appropriate anti-SOCS antibodies (Figure 6A). Whereas untreated precursors migrated normally toward a CXCL12 gradient, tetracycline treatment abrogated CXCL12-mediated Sca-1+c-Kit+Lin– cell chemotaxis in vitro in tetO/SOCS1- and tetO/SOCS3-transduced cells (Figure 6B).
Lethally irradiated C57BL/6 mice received tetO/SOCS1-, tetO/SOCS3-, or tetO/empty-transduced Lin– cells (5 × 104 in 50 μL, intrafemoral). We treated mice with the tetracycline analog doxycycline (Dox) at different times, and measured precursors in BM and periphery. Mice treated with Dox before injection showed no tetO/SOCS1- or tetO/SOCS3-transduced cell engraftment and injected cells were found in the periphery, whereas tetO/empty-transduced cells engrafted normally. Engraftment in untreated mice was normal for all cell types (Figure 6C).
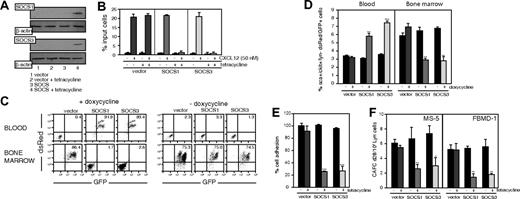
SOCS expression by a ligand-independent inducible approach alters CXCL12-mediated mobilization of Sca-1+c-Kit+Lin–cells. (A) pLVTHM-tetO/SOCS1 (top panels, lanes 3-4)–, tetO/SOCS3 (bottom panels, lanes 3-4)–, or tetO/mock (lanes 1-2)–transduced Lin– cells, untreated (lanes 1,3) or tetracycline treated (lanes 2,4), were lysed and analyzed in Western blot with anti-SOCS1 or -SOCS3 antibodies, as indicated. As control, membranes were developed with anti–β-actin mAb. (B) Cells as in panel A, untreated or tetracycline treated, were allowed to migrate toward a CXCL12 gradient. The migration index was calculated as a percentage of input cells. Data represent the mean ± SD of triplicate determinations. (C) Flow cytometry analysis of transduced Lin– cells in PBLs and BM of mice, untreated or doxycycline pretreated (48 hours), intrafemorally reconstituted with cells as in panel A. The cells are tracked by detection of dsRed/GFP-positive cells. Values represent the percentage of positive cells. Histograms correspond to representative animals (n = 8). (D) Flow cytometry analysis of Sca-1+c-Kit+Lin– cells in periphery (right) and in BM (left), untreated or doxycycline treated (15 days after reconstitution), intrafemorally reconstituted with cells as in panel A. Cells are tracked by detection of dsRed/GFP-positive cells and represented as a percentage of Sca-1+c-Kit+Lin– dsRed/GFP-positive cells versus total cells detected; **P ≤ .01; ***P ≤ .001. (E) Adhesion of cells as in panel A, untreated or tetracycline treated, to the stromal cell line MS-5. Results are expressed as a percentage of maximum adhered cells. Data represent the mean ± SD of quadruplicate determinations; ***P ≤ .001. (F) Cells as in panel A, untreated or tetracycline treated, were plated on confluent MS-5 or FBMD-1 monolayers in 96-well plates. We calculated the mean frequency ± SD CAFCs at culture day 28, using Poisson statistics; the data represent 1 of 2 independent experiments with similar results; *P ≤ .05,**P ≤ .01.
SOCS expression by a ligand-independent inducible approach alters CXCL12-mediated mobilization of Sca-1+c-Kit+Lin–cells. (A) pLVTHM-tetO/SOCS1 (top panels, lanes 3-4)–, tetO/SOCS3 (bottom panels, lanes 3-4)–, or tetO/mock (lanes 1-2)–transduced Lin– cells, untreated (lanes 1,3) or tetracycline treated (lanes 2,4), were lysed and analyzed in Western blot with anti-SOCS1 or -SOCS3 antibodies, as indicated. As control, membranes were developed with anti–β-actin mAb. (B) Cells as in panel A, untreated or tetracycline treated, were allowed to migrate toward a CXCL12 gradient. The migration index was calculated as a percentage of input cells. Data represent the mean ± SD of triplicate determinations. (C) Flow cytometry analysis of transduced Lin– cells in PBLs and BM of mice, untreated or doxycycline pretreated (48 hours), intrafemorally reconstituted with cells as in panel A. The cells are tracked by detection of dsRed/GFP-positive cells. Values represent the percentage of positive cells. Histograms correspond to representative animals (n = 8). (D) Flow cytometry analysis of Sca-1+c-Kit+Lin– cells in periphery (right) and in BM (left), untreated or doxycycline treated (15 days after reconstitution), intrafemorally reconstituted with cells as in panel A. Cells are tracked by detection of dsRed/GFP-positive cells and represented as a percentage of Sca-1+c-Kit+Lin– dsRed/GFP-positive cells versus total cells detected; **P ≤ .01; ***P ≤ .001. (E) Adhesion of cells as in panel A, untreated or tetracycline treated, to the stromal cell line MS-5. Results are expressed as a percentage of maximum adhered cells. Data represent the mean ± SD of quadruplicate determinations; ***P ≤ .001. (F) Cells as in panel A, untreated or tetracycline treated, were plated on confluent MS-5 or FBMD-1 monolayers in 96-well plates. We calculated the mean frequency ± SD CAFCs at culture day 28, using Poisson statistics; the data represent 1 of 2 independent experiments with similar results; *P ≤ .05,**P ≤ .01.
Groups of tetO/SOCS1-, tetO/SOCS3-, and tetO/empty-transduced Sca-1+c-Kit+Lin– cell recipients were maintained for 15 days to allow complete engraftment before initiation of Dox treatment. After 7 days of treatment, mice reconstituted with tetO/SOCS1- and tetO/SOCS3-transduced cells had larger numbers of circulating Sca-1+c-Kit+Lin– cells than mice reconstituted with tetO/empty-transduced cells (Figure 6D, left). Mice reconstituted with tetO/SOCS1- and tetO/SOCS3-transduced cells had lower Sca-1+c-Kit+Lin– cell numbers in BM than controls (Figure 6D, right). In untreated mice, Sca-1+c-Kit+Lin– cell numbers in BM and periphery were comparable for all mouse groups (Figure 6D). We ruled out differences in BM-cell proliferative capacity by administering BrdU to mice before Dox treatment, followed by flow cytometry analysis (Figure S4). Using an RT-PCR before and after Dox treatment, we ruled out differences in CXCL12 BM mRNA levels between mice reconstituted with tetO/SOCS1- or tetO/SOCS3-tranduced cells and controls (Figure S3).
To evaluate the mechanism involved in this SOCS-mediated HSC egress from BM, we determined HSC precursor frequency in stromal cell–dependent, limiting-dilution CAFC assays. These experiments require coordinated HSC adhesion and migration beneath a layer of supporting stromal cells. pLVTHM-tetO/SOCS1–, -tetO/SOCS3–, or -tetO/empty–transduced Lin– cells were plated and cocultured on MS-5 and FBMD-1 stromal cells, and CAFC frequency was determined weekly for 28 days in untreated or tetracycline-treated cultures. Tetracycline-induced SOCS1 and SOCS3 up-regulation triggered more than 50% decrease in CAFC frequency compared with untreated cells (Figure 6E). Cell-cycle evaluation by BrdU incorporation ruled out proliferation differences in the cells under these experimental conditions (Figure S5). Together, these data suggest defective HSC interaction with the hematopoietic environment. To measure this interaction directly, we determined adhesion of untreated and tetracycline-treated pLVTHM-tetO/SOCS1–, -tetO/SOCS3–, or -tetO/empty–transduced Lin– cells to MS-5 cells; SOCS1- and SOCS3-expressing Lin– cells showed a 4-fold reduction in adhesion compared with controls (Figure 6F). These results confirm that SOCS1 or SOCS3 expression in BM HSCs abolishes CXCL12-mediated retention and triggers mobilization.
Discussion
Although peripheral and cord blood contain a substantial HSC fraction,44 the primary source of transplantable hematopoietic precursors is BM. Mobilized HSCs are currently the preferred source of stem and progenitor cells for autologous and allogeneic transplants, as their use leads to rapid engraftment and entails fewer procedural risks than harvested BM cells.45 HSC mobilization techniques involve interference with the physiologic interplay between stromal and hematopoietic cells. The most common mobilization agent in clinical use is G-CSF46 ; following chemotherapy, repeated G-CSF stimulation results in SCF (stem-cell factor) shedding and release, progenitor-cell proliferation, as well as activation and/or degradation of adhesion molecules, leading to HSC mobilization.45 A substantial number of patients are poor mobilizers,47 however, and must be treated with high G-CSF doses, which can cause hematologic toxicity.48 The search for alternative precursor mobilization approaches is thus of maximum importance.45
One mechanism that retains HSCs in BM is the attraction of CXCR4-expressing precursors to CXCL12-expressing BM stromal cells.49,50 CXCL12 signaling is essential for the HSC motility required for BM repopulation and development in the hematopoietic system.47,49,51 CXCL12 overexpression in murine circulation leads to stem-cell mobilization.52 The use of a selective CXCR4 antagonist, AMD3100, induces rapid mobilization of mouse and human hematopoietic precursors, and augments G-CSF–induced precursor-cell mobilization synergistically.53,54
Cytokines and chemokines activate the JAK/STAT pathway, enabling crosstalk between these 2 protein families23,55 through up-regulation of specific SOCS, the family of intracellular proteins that have emerged as key physiologic regulators of cytokine responses.41,56,57 In bGHTg mice, we detected altered CXCL12-induced migration of B cells and granulocytes, in vitro and in vivo. Previous data demonstrate that this is due to GH-induced up-regulation of SOCS3, which in turn associates phosphorylated CXCR4, blocking its signaling cascade.23 Similar effects are induced by SOCS1, which binds JAK to abrogate later signaling events.55
The higher levels of circulating Sca-1+c-Kit+Lin– cells in bGHTg than in wt mice suggested that these effects were due to SOCS-mediated blockade of CXCR4 function. Administration of rhGH to wt mice reproduced the bGHTg mouse phenotype. In well-characterized in vivo murine models, GH induces increased CFU-GM and BFU-E numbers in BM and spleen, and triggers HSC mobilization, although the mechanism remains unclear.58 The bGHTg mouse model cannot show whether the precursor increase in periphery is the result of a larger number of these cells in BM, of a direct effect on their mobilization, or a combination of the 2. Our results in wt mice after short-term GH treatment indicated an increase in blood Sca-1+c-Kit+Lin– cells, whereas proliferation of these precursors was unaffected in BM. Although GH thus affects Sca-1+c-Kit+Lin– cell proliferation in BM, it also restricts their retention in this niche, promoting mobilization to blood. In this process, GH abrogates CXCL12-induced Sca-1+c-Kit+Lin– cell retention in BM by up-regulating SOCS1 and SOCS3, which might thus bind JAK2 and CXCR4, respectively, and block CXCR4 function, as observed in other cell types.23 Moreover, when we evaluated bGHTg HSC reconstitution of lethally irradiated adult wt mice (an in vivo GH depletion equivalent), SOCS1 and SOCS3 were down-regulated and CXCR4 was fully functional. This result, and the large number of HSCs in bGHTg BM and blood explain why we observed greater engraftment compared with controls.
Stem-cell mobilization is a multifactorial process in which cell-to-cell and cell–to–extracellular matrix interactions have a key role. Our data confirm that SOCSs alter CXCL12 function and indicate the potential therapeutic interest of modulating SOCS levels. A recently described therapy is based on the use of recombinant forms of SOCS3 to inhibit inflammation and apoptosis.59,60 Our results show that expression of either SOCS1 or SOCS3 is sufficient to mobilize HSCs, as SOCS1/ΔNGFR or SOCS3/ΔNGFR transduction of Sca-1+c-Kit+Lin– cells altered their ability to migrate toward a CXCL12 gradient in vitro and to engraft in irradiated mouse BM. Furthermore, whereas tetO/SOCS1- or tetO/SOCS3-transduced cells engrafted normally in irradiated mice, doxycycline treatment triggered rapid precursor-cell mobilization. RT-PCR analysis showed no differences in CXCL12 mRNA levels in BM from bGHTg, control littermates, and tetO/SOCS mice. Our in vitro experiments demonstrated that SOCS1 and SOCS3 expression abrogated CXCL12-induced HSC adhesion to fibronectin, suggesting that SOCSs modulate CXCL12-mediated HSC retention in BM. Recent evidence indicates that SOCS3 up-regulation by heat-shock protein 60 blocks CXCR4-mediated responses in human T cells.61 CXCR4 and the small GTPase Rac1 must be incorporated into membrane lipid rafts for HSC homing,62 and deletion of murine Rac1 or Rac2 alleles leads to massive HSC egress from BM into blood.63 Rac1 is required for the engraftment phase of hematopoietic reconstitution, and interaction of Rac1–/– HSCs with the BM microenvironment was altered in vitro.64 Our data show that SOCS expression promotes rapid HSC egress in vivo, alters CXCL12-induced HSC adhesion on fibronectin in vitro, and provokes defective interactions with the hematopoietic microenvironment, including reduced adhesion to stromal cells and reduced CAFC frequency. Although further experiments are needed to clarify this point, SOCS silencing of CXCR4 may directly affect CXCL12 activation of Rac in HSCs.
We cannot rule out that SOCSs might alter HSC self-renewal. Data in the literature are contradictory; whereas some authors report that lack of SOCS3 reduces self-renewal and promotes murine stem-cell differentiation,65 others indicate a role for SOCS3 in promoting murine stem-cell differentiation.66 Although experiments are under way to evaluate these possibilities, our data support the use of SOCS-based therapies in situations that require BM hematopoietic precursor mobilization. This is especially important for patients with non-Hodgkin lymphoma, relapsed Hodgkin lymphoma, or multiple myeloma, whose management can require high-dose sequential chemotherapy and autologous stem-cell transplantation.67 Administration of repeated chemotherapy cycles is associated with decreased CD34+ cell mobilization.40 In addition to the known SOCS functions in inflammation, allergy, and homeostasis,49 our observation that they modulate HSC mobilization corroborates the importance of SOCSs in human pathologies. Our results also underline the implications of crosstalk between cytokine and chemokine signaling, and show how SOCS expression may modify the equilibrium between the generation and maintenance of hematopoietic precursors and their mobilization.
Authorship
O.M.P., M.d.C.M.-O., J.M.R.-F., L.M.-M., D.L., L.G., P.L., and E.S. performed research; J.M.R.-F., C.M.-A., A.B., and M.M. designed research; M.A. and A.B. contributed reagents; and M.M. wrote the paper.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 15, 2006; DOI 10.1182/blood-2006-02-006353.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Drs S. Kunkel and S. Lira for critical reading of the paper; J. P. García-Ruiz and E. Delgado for bGHTg mice; J. A. Cancelas for FBMD-1 stromal cells; T. Willson for pEF-FLAG-I/mSOCS1 and mSCOS3 constructs; D. Trono for controllable lentiviral vectors; and A. Zaballos for help with RT-PCR analysis; we also thank C. Bastos and C. Mark for secretarial and editorial assistance, respectively.
O.M.P. is supported by a grant from the Fundación Ramón Areces. This work was supported in part by grants from the Lilly Foundation, the Spanish Ministry of Education and Science (SAF 2005-03388), the Comunidad de Madrid (CAM 200520M025), and the European Union (Innochem project UE-518167). The Department of Immunology and Oncology was founded by and is supported by the CSIC and Pfizer.