Abstract
There is conflicting information about the influence of body mass index (BMI) on the pharmacokinetics, toxicity, and outcome of chemotherapy. We compared pharmacokinetics, outcome, and toxicity data across 4 BMI groups (underweight, BMI ≤ 10th percentile; normal; at risk of overweight, BMI ≥ 85th and < 95th percentile; overweight, BMI ≥ 95th percentile) in 621 children with acute lymphoblastic leukemia (ALL) treated on 4 consecutive St Jude Total Therapy studies. Chemotherapy doses were not adjusted to ideal BMI. Estimates of overall survival (86.1% ± 3.4%, 86.0% ± 1.7%, 85.9% ± 4.3%, and 78.2% ± 5.5%, respectively; P = .533), event-free survival (76.2% ± 4.2%, 78.7% ± 2.1%, 73.4% ± 5.5%, and 72.7% ± 5.9%, respectively; P = .722), and cumulative incidence of relapse (16.0% ± 3.7%, 14.4% ± 1.8%, 20.6% ± 5.1%, and 16.7% ± 5.1%, respectively; P = .862) did not differ across the 4 groups. In addition, the intracellular levels of thioguanine nucleotides and methotrexate polyglutamates did not differ between the 4 BMI groups (P = .73 and P = .74, respectively). The 4 groups also did not differ in the overall incidence of grade 3 or 4 toxicity during the induction or postinduction periods. Further, the systemic clearance of methotrexate, teniposide, etoposide, and cytarabine did not differ with BMI (P > .3). We conclude that BMI does not affect the outcome or toxicity of chemotherapy in this patient population with ALL.
Introduction
The prevalence of obesity has increased substantially over the past few decades and is now about 15% among children and adolescents in the United States1 ; therefore, it is a factor frequently encountered in pediatric oncology. Reports of the influence of adult obesity on the outcome of cancer treatment2-6 and treatment-related toxicity4,6,7 are contradictory, and there are few reports for pediatric patients. The Children's Cancer Group (CCG) found that overweight children with acute myeloid leukemia (AML) are at greater risk of treatment-related complications8 and that overweight children with acute lymphoblastic leukemia (ALL) aged 10 years or older have a significantly greater risk of relapse and a lower probability of event-free survival (EFS).9 There is little information about the influence of underweight on the outcome of ALL,10-13 and most available reports10-12 are from developing countries. In children with AML, the CCG8 reported that underweight patients have a lower chance of survival and a greater risk of toxicity.
Chemotherapy dosage for obese patients is often empirically reduced on the basis of ideal body weight because of concerns about excessive toxicity; however, dose reduction may compromise the outcome of treatment.14 There are very few reports15,16 comparing the pharmacokinetics of various drugs in either adult or pediatric obese patients and normal-weight controls, and these studies were done in very small patient groups. None of the studies found pharmacokinetic differences between overweight and non-overweight patients to be associated with clinical effects. We therefore retrospectively studied the influence of body mass index (BMI) on the outcome, toxicity, and, when available, pharmacokinetics of chemotherapy agents in children with ALL treated on 4 consecutive studies at St Jude Children's Research Hospital.
Patients, material, and methods
Patients
The Institutional Review Board of St Jude Children's Research Hospital approved this retrospective study. We reviewed the records of 653 patients with newly diagnosed ALL treated on St Jude Total Therapy studies (Total XII, XIIIA, XIIIB, and XIV) conducted from 1988 through 2000. Twenty-six patients younger than 1 year of age at the time of diagnosis and 6 patients with Down syndrome were excluded from this analysis, leaving 621 patients. The details of these treatment regimens have been reported elsewhere.17-20 The chemotherapy doses in the protocols were to be based on actual body weight or body surface area in all protocols, and there were no recommendations to adjust for abnormal body composition, although half of the patients treated on the Total XII protocol had their dosage of methotrexate, teniposide, and cytarabine adjusted on the basis of their drug clearance.17 In addition, mercaptopurine doses were adjusted on the basis of thiopurine methyltransferase (TPMT) activity and toxicity.21 Toxic effects and their grades were defined by National Cancer Institute (NCI) toxicity criteria (Version 2). BMI was calculated by using weight and height as previously reported.22 For patients older than 2 years of age, underweight was defined as a BMI-for-age at or below the 10th percentile; normal was defined as a BMI-for-age above the 10th and below the 85th percentile; risk of overweight was defined as a BMI-for-age at or above the 85th percentile and less than the 95th percentile23 ; and overweight was defined as a BMI-for-age at or above the 95th percentile. For patients aged 1 to 2 years, we used the weight-for-length percentile instead of the BMI-for-age percentile. The SAS Program for the CDC Growth Charts (downloaded from http://www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm) was used to calculate the percentiles of BMI-for-age and weight-for-length; for children 2 years of age or older whose length was less than 77 cm or greater than 120 cm, the weight-for-length charts were used to determine the weight-for-length percentile, as the above SAS program does not cover these 2 groups of patients.
Pharmacokinetics
Pharmacokinetic data and confirmed actual administered doses were available for high-dose methotrexate, etoposide, teniposide, cytarabine, and oral mercaptopurine (dosing was available but concentration time data were not). In addition, intracellular concentrations of thioguanine nucleotides and methotrexate polyglutamates were available in the Total XII study. All specimens were collected prospectively for pharmacokinetic analysis, and some results have been reported previously.17,24-26 The population pharmacokinetics were determined by using the 2-stage approach.27 First, the pharmacokinetic parameters for each individual were estimated for each of the courses by using compartmental analysis. A 2-compartment model was used for methotrexate, etoposide, and teniposide, and a 1-compartment model was used for cytarabine. In all cases, the systemic clearance was determined by the equation CL = ke/V, where ke is the elimination rate constant and V is the systemic volume. Second, the population pharmacokinetics was analyzed by using linear mixed-effects modeling as implemented in S-Plus (Version 7.0; Insightful Corporation, Seattle, WA) using the following model:
where CLij is the clearance of the drug for patient i course j; θ1 is the logarithm of the population mean clearance; θ2 is the coefficient that describes the effects of the obesity grouping on the clearance; θi are the coefficients that describe the effects of the confounding factors (study, course, age); and η and ϵ represent the interpatient and intrapatient (residual) variability, both of which values are assumed to have a mean of zero. By taking the logarithm of the clearance, we assume that it is log-normally distributed. The ability of the obesity group covariate to significantly improve the model fit (as determined by a reduction of 3.84 [P < .05] in the negative 2 log likelihood, based on the F-test) was investigated.
Statistical analysis
The distribution of presenting features and of the rate of remission at the end of induction were compared across the groups by the chi-square test. Multivariable logistic regression28 analysis was used to further compare the rate of remission at the end of induction after adjusting for other prognostic factors at diagnosis: age, white blood cell (WBC) count, immunophenotype, presence of Philadelphia chromosome, DNA index, and NCI risk category.
The main outcome measures were estimates of event-free survival (EFS), overall survival (OS), and cumulative incidence of relapse (CIR). The duration of EFS was measured from the date of complete remission to the date of first treatment failure of any kind (relapse, death, ALL lineage switch, or second malignancy) or to the date of most recent follow-up. Failure to induce remission was considered an event at zero time. The duration of OS was calculated from the date of diagnosis to the date of death due to any cause or the date of most recent follow-up. Data for patients who were alive at the most recent contact date were censored at the time of that contact. EFS and OS distributions were estimated by the method of Kaplan and Meier29 and compared by using the log-rank test.30 Multivariable Cox proportional-hazards regression models31 adjusted for age at diagnosis, sex, WBC count at diagnosis, immunophenotype, DNA index, presence of the Philadelphia chromosome, the TEL-AML1 or MLL-AF4 fusion genes, and NCI risk criteria were used to compare EFS and OS estimates among the 4 groups.
Any leukemia relapse was included in the analysis of the CIR; second cancer or death due to any cause were regarded as competing events. Patients who did not enter remission were excluded, and patients who remained alive and in remission were censored at the time of last follow-up. The risk of relapse and competing risks were estimated as described by Kalbfleisch and Prentice32 and compared by the method described by Gray.33 Multiple-regression modeling of subdistribution functions34 in competing risks, with adjustment for other prominent risk factors in childhood ALL, was used to further assess the association between obesity and leukemic relapse.
The chi-square test was used to compare the number of patients who experienced grade 3 or 4 toxicity among the 4 BMI groups during and after remission induction. Furthermore, for the toxicity after remission induction, time at risk of toxicity and multiple episodes were taken into account by weighted logistic regression. The weights were generated according to the cumulative incidence of episodes of toxicity. More specifically, each episode of toxicity was weighted according to the following formula:
where Zi is the cumulative incidence of toxicity episodes at the time of the ith toxicity episode or the time when a patient who had no toxicity was censored; tox = 1if Zi corresponds to a toxicity episode, and tox = 0 otherwise. Data were censored at the end of therapy for patients who did not experience toxicity after induction (consolidation and continuation periods). Data for patients who went off therapy after induction before any toxicity was observed were censored at the off-therapy time.
All analyses were stratified by protocol. A probability value of .05 or less (P ≤ .05) was prospectively selected as the criterion of statistical significance. This analysis reflects the data as of January 31, 2006.
Results
Patients
Of 621 patients included in this analysis, 102 (16.4%) were underweight at the time of diagnosis, 400 (64.4%) were of normal weight, 64 (10.3%) were at risk of overweight, and 55 (8.9%) were overweight. There was no significant difference among the 4 groups in the distribution of presenting features (Table 1). Relative to the normal-weight group, there were minimal differences (≤ 8%) in the administered drug dose (per unit of body surface area) among the 3 other weight groups, with the exception of the dose at day 5 in the targeted group for cytarabine and teniposide (Table 2), but there were only 12 patients that received this dose.
Outcome of treatment according to BMI
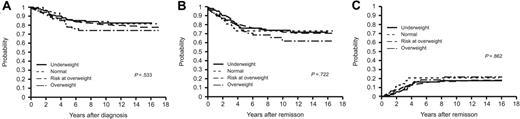
As of January 31, 2006, the median duration of follow-up was 10.5 years (range, 2.4-16.9 years). The rate of complete remission (CR) in the underweight (n = 102), normal weight (n = 400), at risk of overweight (n = 64), and overweight (n = 55) groups was 99.0%, 97.0%, 98.4%, and 98.2%, respectively (P = .626). OS estimates did not differ significantly across the 4 weight groups (P = .533; Figure 1A). The 5-year OS estimates of these groups were 86.1% ± 3.4%, 86.0% ± 1.7%, 85.9% ± 4.3%, and 78.2% ± 5.5%, respectively. The EFS curves (Figure 1B) showed the 5-year EFS estimates to be 76.2% ± 4.2%, 78.7% ± 2.1%, 73.4% ± 5.5%, and 72.7% ± 5.9%, respectively (P = .722). CIR did not differ significantly between the 4 groups (P = .862; Figure 1C). The 5-year CIR was 16.0% ± 3.7%, 14.4% ± 1.8%, 20.6% ± 5.1%, and 16.7% ± 5.1% for patients in the underweight, normal weight, at risk of overweight, and overweight groups.
Because the CCG has reported an increased incidence of relapse in obese patients 10 years of age or older,9 we compared OS, EFS, and CIR in patients younger than 10 years old versus those 10 years of age or older in each of the 4 BMI groups. We found no difference in any of these parameters among the 4 BMI groups in patients younger than 10 years old (P = .628, P = .449, and P = .321 for OS, EFS, and CIR, respectively) or in patients 10 years of age or older (P = .474, P = .103, and P = .341 for OS, EFS, and CIR, respectively).
To assess whether the BMI thresholds used to define the BMI groups had affected our results, we also compared the above parameters across patient groups defined according to the following thresholds: (1) 10th percentile or below, 11th to 94th percentile, and 95th percentile or above; (2) 11th to 84th percentile and others (10th percentile or below, or 85th percentile or above); (3) 10th percentile or below, 11th to 84th percentile, and 85th percentile or above; (4) 95th percentile or above, and below 95th percentile. No significant difference in outcome was observed between the groups as defined in any of these categories (data not shown).
Cox proportional-hazards regression models adjusted for patients' characteristics at diagnosis (age, race, sex, WBC count, immunophenotype, DNA index, Philadelphia chromosome, TEL-AML1, MLL-AF4, and protocol) were used to separately assess the effect of BMI on EFS, OS, and CIR. Age, sex, WBC count, immunophenotype, DNA index, the Philadelphia chromosome, TEL-AML1, and protocol were independent prognostic factors (P < .05), as expected. BMI was not an independent predictor of EFS, OS, or CIR in any analysis (data not shown).
Toxicity during the induction and postinduction periods
The frequency of grade 3 or 4 toxicity in the BMI groups during the induction and postinduction periods is summarized in Table 3. The 4 groups did not differ significantly in the number of patients who experienced grade 3 or 4 toxicity during the induction (P = .537) or postinduction (P = .739) periods. When the incidence of toxicity in the postinduction period was evaluated with adjustment for the time at risk and multiple episodes, there was also no significant difference (P = .887). The number of episodes of toxicity during induction was not compared because of the brief duration of induction. The frequency of the type of toxicity (allergic, cardiovascular, endocrine, gastrointestinal, hematologic, hepatic, metabolic, neurologic, pulmonary, renal, skin, infections, coagulation, pain, syndromes, and constitutional symptoms) during induction or the postinduction period was also compared among the 4 BMI groups. No significant difference was observed with the exception of a lower incidence of allergy in overweight patients during the postinduction period (0% vs 1.0% in underweight, 20% in normal-weight, and 7.8% in at-risk patients; P = .039), a finding that may occur by chance due to multiple testing.
Clinical outcomes of patients according to BMI category. BMI categories are as follows: underweight (BMI ≤ 10th percentile, n = 102), normal weight (BMI > 10th and < 85th percentile, n = 400), at risk of overweight (BMI ≥ 85th and < 95th percentile, n = 64), or overweight (BMI ≥ 95th percentile, n = 55). Shown are Kaplan-Meier estimates of (A) overall survival; (B) event-free survival; and (C) cumulative incidence of relapse.
Clinical outcomes of patients according to BMI category. BMI categories are as follows: underweight (BMI ≤ 10th percentile, n = 102), normal weight (BMI > 10th and < 85th percentile, n = 400), at risk of overweight (BMI ≥ 85th and < 95th percentile, n = 64), or overweight (BMI ≥ 95th percentile, n = 55). Shown are Kaplan-Meier estimates of (A) overall survival; (B) event-free survival; and (C) cumulative incidence of relapse.
Pharmacokinetics according to BMI
Table 4 lists the population average systemic clearance of methotrexate, teniposide, etoposide, and cytarabine in the 4 BMI groups. We adjusted the analysis of each drug for known confounding factors, including age (> 10 vs ≤ 10 years), course, and study. The mean systemic clearance of the underweight, risk of overweight, and overweight groups differed from that of the normal-weight group by less than 17% (P > .3). In addition, the intracellular levels of thioguanine nucleotides and methotrexate polyglutamates did not differ between the 4 BMI groups (P = .73 and P = .74, respectively).
Discussion
We observed no association between BMI and outcome or toxicity in children with ALL. Our results should be interpreted with caution because of the relatively small number of patients and the use of different treatments, compared to those of the CCG study.9 Nonetheless, the major strength of this study is that we correlated the BMI, clinical outcome, and pharmacokinetics in a large number of patients. Previous studies had suggested that increased toxicity7,8 or decreased survival2,9 were associated with altered drug metabolism in obese patients, but there were conflicting reports. Moreover, most previously reported pharmacokinetic studies were single-agent studies in relatively small patient groups.15 In our large cohort, we found no difference in systemic clearance across the BMI categories. This result is consistent with the absence of a significant difference among these groups in outcome or toxicity.
Some studies have found an increased risk of relapse and a reduced probability of survival in underweight patients with ALL.10-12 Almost all such reports have come from developing countries, where underweight patients are likely to be profoundly malnourished and where malnutrition is usually accompanied by low socioeconomic status, lack of education, and other factors that contribute to poor outcome. In contrast, our patients were generally not malnourished or mildly malnourished based on our observation, although nutritional status of our patients was not systemically evaluated. Although malnutrition has been suggested to alter pharmacokinetics, such an effect appears to apply only to severe cases in which important parameters (eg, serum protein levels or hepatic drug metabolism) are altered.35 Our results are consistent with a prior report from a developed country13 indicating that underweight status did not affect pharmacokinetics, outcome, or toxicity in children with ALL.
We found that drug clearance normalized to body surface area of 4 drugs (high-dose methotrexate, etoposide, teniposide, and cytarabine) did not differ among the weight groups. In addition, the intracellular levels of thioguanine nucleotides and methotrexate polyglutamates did not differ between the 4 BMI groups (P = .73 and P = .74, respectively). This finding suggests that these agents should be dosed based on actual body surface area. We acknowledge that pharmacokinetic data were available for only 4 drugs. Some studies in smaller groups of patients have suggested that other chemotherapeutic agents commonly used for ALL may have lower (cyclophosphamide,36 doxorubicin,16 methylprednisolone37 ) or higher (mercaptopurine38 ) drug clearance in overweight patients.
We focused on the BMI at the time of diagnosis in our analysis. When we examined the serial BMI data available for 279 patients, a majority had an increase in BMI over the entire treatment period (data not shown). Nonetheless, this change appears to be of little importance in light of the lack of difference among the BMI groups in pharmacokinetics or in outcome. BMI at diagnosis was not associated with the CR rate, the drug clearance, or the risk of toxicity during induction.
We conclude that the clearance of the chemotherapeutic agents we tested was not affected by abnormal BMI. Furthermore, we found no evidence that abnormal BMI adversely affected the outcome or toxicity of treatment in children with ALL. Therefore, we recommend that patients with ALL receive doses of methotrexate, cytarabine, and epipodophyllotoxins calculated on the basis of actual body surface area.
Authorship
Contribution: N.H. provided the study concept; N.H., J.C.P., and Y.Z. provided the study design and drafted the manuscript; J.C.P. and Y.Z. provided statistical expertise; N.H., J.C.P., Y.Z., E.P.K., B.I.R., R.C.R., J.E.R., M.M.H., J.T.S., C.-H.P., S.C.H., S.J., and M.V.R. acquired the data; N.H., J.C.P., Y.Z., C.-H.P., and M.V.R. analyzed and interpreted data; all authors reviewed the manuscript; C.-H.P. conducted the Total studies; and N.H. and M.V.R. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 17, 2006; DOI 10.1182/blood-2006-05-024414.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by grants CA-21765, CA-51001, CA-36401, CA-78224, CA-71907, CA-60419, and GM-61393 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC). C.-H.P. is the American Cancer Society F. M. Kirby Clinical Research Professor.
We thank Sharon Naron and Donald Samulack, PhD, for expert editorial review; Tad McKeon, Elaine Entrekin, and Annette Stone for data collection; Cheng Cheng, PhD, and Michael Hancock for suggestions on statistical analysis; Terezie Mosby, MS, RD, IBCLC, for discussion on nutrition; Julie Groff for assistance with the figures; Jeana Cromer for administrative assistance; and Imella Herrington for secretarial assistance.