Abstract
This study assessed the incidence of cytomegalovirus (CMV) infection after transplantation of cord blood (CB) from unrelated donors and evaluated strategies for screening CB donors. Posttransplantation CMV infection, reported in 23% of 1221 CB recipients, was associated with patient pretransplantation CMV serology (P < .001), but not with CMV serology in CB donors or their mothers. A total of 26 988 infant CB donors were evaluated by viral culture of saliva. Subgroups were evaluated by polymerase chain reaction in CB (CB-PCR) in 2 case-control studies. In the first study, 33 of 47 saliva culture–positive CB donors were confirmed by CB-PCR. All mothers of the 33 infants with confirmed CMV infection were CMV–total antibody positive, but only 1 of 3 had CMV-IgM antibody. The second study evaluated infants born to mothers with CMV-IgM antibody. Of these, 5 of 170 saliva culture–negative infants were positive by CB-PCR. The incidence of congenital CMV infection in CB donors was low (0.12%). Maternal serology had poor predictive value for CMV infection in their infant CB donors and bore no detected relationship to CMV infection in CB recipients. Saliva culture for CMV had both false-positive and -negative results. CB-PCR was a useful alternative for detecting CMV in CB donors.
Introduction
Despite effective antiviral therapy, cytomegalovirus (CMV) remains one of the leading causes of morbidity and mortality after hematopoietic stem cell (HSC) transplantation.1,2 CMV infection in transplant recipients can occur by reactivation of a latent infection or from newly acquired infections with donor blood, tissue, or organs as a source of the virus.3,4 Thus, U.S. Food and Drug Administration (FDA) rules on donor eligibility require testing donors of viable, leukocyte-rich blood and HSCs for “evidence of infection due to cytomegalovirus.”5 For donors of bone marrow or peripheral blood stem cells, screening relies on traditional serologic methods to detect CMV–total antibody since their presence (found in more than half of adult bone marrow donors in the United States) is indicative of active or latent infection, either of which can be transmitted to graft recipients.6,7 Umbilical cord blood (CB), an increasingly accepted alternative to bone marrow grafts, is presumed to have a low risk of transmitting CMV because of the low rate of congenital CMV infection (reported in 0.2% to 2.5% of U.S. births).8-10 No study to date, however, has systematically assessed the risk of CMV infection in CB transplant recipients or has evaluated the effectiveness of methods to screen CB donors in this context.
Options for CMV screening for CB donors include (1) serologic testing of the donor infant's mother to identify a risk of infection in the infant, (2) serologic testing of the infant's CB, (3) detection of infection in the infant by viral culture or CMV DNA–specific polymerase chain reaction (PCR) to detect CMV nucleic acid sequences, or (4) a combination of serologic testing and detection of infection in the infant. Interpretation of data obtained by serologic methods alone is complicated in CB donors because of active transport of maternal IgG antibody across the placenta. Thus, positivity for CMV–total antibody in the mother or in CB generally reflects the mother's lifetime exposure to CMV and a potential risk, but not necessarily infection in the infant. CMV–IgM antibody (IgM anti-CMV) in the mother at the time of the infant's birth, as a marker of a recent active CMV infection, should indicate an increased risk of infection in the infant. Some studies report, for example, that as many as one-third of mothers with primary CMV infection during pregnancy transmit the virus to the fetus.11 IgM anti-CMV remains detectable for only 4 to 6 months, however, so maternal testing at the time of delivery would routinely miss infections that occurred early in pregnancy. Moreover, infants may acquire CMV when a latent maternal infection reactivates during pregnancy and anti-CMV IgM in these mothers may remain negative or be positive only briefly. Given these limitations, it may not be justified that some CB banks accept or exclude CB units based on CMV-IgM status of the mother.12 Detection of CMV infection in the infant might be attempted more directly, by testing the infant's blood for IgM antibody, culturing urine or saliva for the virus, or testing blood, urine, or saliva for CMV DNA by nucleic acid amplification. IgM antibodies, however, are not detectable in most infected infants.10 Although isolation of virus by culture of urine or saliva is considered the gold standard for detecting congenital CMV infection, viral culture can be cumbersome and expensive and therefore, may not be practical for large-scale screening.13 Polymerase chain reaction (PCR) can detect CMV DNA in congenitally infected infants, but has not been evaluated extensively for screening CB.14,15
We undertook the current study to assess the incidence of posttransplantation CMV infection in recipients of CB donated to the New York Blood Center (NYBC) National Cord Blood Program (NCBP) and the relevance of donor maternal serologic tests as indicators of a risk of CMV transmission to recipients, and to evaluate possible strategies for identifying CMV-infected CB donors.
Materials and methods
Cord blood program
In 1992, we established the NYBC National Cord Blood Program (NCBP) as a public, full-service CB bank to collect, test, type, process, cryopreserve, store, and provide CB units for transplant to unrelated recipients.16 The program operates as a tissue bank licensed by the New York State Department of Health and under a U.S. FDA investigational new drug (IND) exemption. Maternal consent to donate CB to our program was obtained under procedures approved by the NYBC institutional review board (IRB) as well as the IRBs of each participating hospital. Mothers were not eligible to donate CB if they or their infants were known to have a blood-transmissible infection at the time of the infant's birth.
Cord blood collection
Blood remaining in the umbilical cord of the delivered placenta was collected using sterile technique and, with maternal consent, donated to the NCBP as previously described.16 Maternal blood samples (7 mL in 1.5 mL acid citrate dextrose anticoagulant solution) were taken routinely on admission to the hospital or within 1 to 2 days after delivery. Infant medical records were reviewed for signs of illness and specific diagnoses were made prior to discharge. Infants were not followed after discharge. Through September 2, 2005, 31 762 CB units had been donated and accepted by the program for use in clinical transplantation.
Culture of saliva for CMV
Starting in March 1995, infant's saliva was routinely collected for CMV culture. Samples were taken by wiping sterile cotton-tipped swabs around the infant's gums.17 Whenever possible, the sample was collected after the infant was more than 12 hours old (to minimize the chance of contamination with maternal vaginal secretions) and 2 hours or more after feeding to minimize interference with the culture by milk in the infant's mouth. Swabs were placed in transport media, stored at room temperature, and shipped within 72 hours of the donor's birth to the testing laboratory (Virology Laboratory, University of Alabama). Detection of CMV early-antigen fluorescent foci (DEAFF) identified CMV-positive cultures.18 A total of 26 988 infants whose mothers donated CB had their saliva cultured for CMV (96.5% of the 27 969 donations in the testing period). CB units that had positive saliva culture were not excluded from the inventory of units made available for transplantation.
Maternal serologic screening
IgM antibody to CMV (IgM anti-CMV) was detected by a microparticle enzyme immunoassay (MEIA, IMx system; Abbott Laboratories, Abbott Park, IL). Results were categorized as negative, equivocal, or positive according to manufacturer instructions. Maternal plasma collected through March 2002 was routinely tested for IgM anti-CMV (n = 11 726). Total antibody to CMV (total anti-CMV) in maternal plasma was detected by a solid-phase enzyme immunoassay (EIA) for IgG, IgM, and IgA (ABBOTT CMV TOTAL AB EIA; Abbott Laboratories). All mothers who delivered between February 1993 and November 1997 and from June 2001 through September 2005 were tested for total anti-CMV (n = 19 757). In addition to routine testing, samples from mothers whose infants were culture positive or were selected as controls in case-control studies as well as mothers of infants whose CB was used for transplantation were tested retrospectively for IgM and total anti-CMV, if not already tested routinely. Mothers who were positive for total anti-CMV, but negative for IgM anti-CMV were presumed to have predominantly IgG antibody and are referred to hereafter as “IgG positive.” Those with IgM anti-CMV detected were referred to as “IgM positive.” Anti-CMV titer was determined based on end-point positivity on 2-fold serial dilutions. CB units from mothers with IgM or IgG anti-CMV were not excluded from the inventory.
Cord blood transplant recipients
Between August 1993 and December 2004, 1564 patients underwent transplantation with single CB units provided by the NCBP. Transplant centers have reported patient characteristics and outcome data on 1490 (95%), with 1279 having data reported on both pretransplantation anti-CMV status and posttransplantation CMV infection. Outcome reports, including information on posttransplantation infection, are routinely obtained at 3, 6, and 12 months after transplantation, and annually thereafter. Among these, 1221 had results of the donor's maternal anti-CMV and were evaluated for evidence of their relationship to the incidence of posttransplantation CMV infection in CB recipients. Recipient pretransplantation anti-CMV status was classified as positive, negative, or unevaluable. The latter category included patients who had inherited immune deficiency diseases or who underwent transplantation in the first months of life when antibody positivity was most likely of maternal origin. Patients whose CMV antibody was unevaluable before transplantation were assumed to have not been infected previously with CMV and were grouped together with antibody-negative patients in transplantation outcome analyses. Most transplant centers monitored for CMV infection by testing recipients weekly for antigenemia or, more recently, for CMV DNA by PCR until day +100 and for longer, if the recipient was diagnosed as having acute graft versus host disease (GVHD). For this study, posttransplantation CMV infection was defined as any positive test for antigenemia or CMV DNA with onset as the date of first detection (reported on 90% of cases). CMV infection was further classified as mild when detected only in blood or as more severe when detected in other tissues or organs or associated with disease.
Plasma and white blood cell (WBC) samples
Aliquots of cord and maternal blood plasma were stored at –70°C. WBC fractions were derived either from whole blood or from the remnant RBC pellet obtained after centrifugation of the CB and separation of mononuclear cells (MNCs) by Ficoll-Hypaque.16
CMV DNA extraction
For DNA extraction from plasma, 1.0 mL was centrifuged at 9655 g. The supernatant was removed and the pellet resuspended in 200 μL Tris-HCl 50 mM buffer (Gibco, Grand Island, NY). For DNA extraction from WBCs, 1 × 106 or more cells were resuspended in 200 μL Tris-HCl 50 mM buffer. DNA was extracted by attachment to Qiagen columns (Qiamp; Qiagen, Boston, MA) as instructed by the manufacturer, and eluted from the column with 50 μL DNAase-free water.
In-house PCR for CMV
Selected primers for an “in-house” PCR were directed to a 280-bp region of the glycoprotein B (GlyB) of the human CMV genome.19 The extracted DNA (10 μL) was added to a PCR mix (25 pmol of each primer, 1× PCR buffer [10 mM Tris-HCl, pH = 8; 50 mM KCl; 10 mM NaCl], 6 mM MgCl2, Taq Gold, 5U [Perkin Elmer, Norwalk, CT], dUTP 1× [Sigma, St Louis, MO], bovine serum albumin [BSA; 0.2 mg/mL; Roche, Indianapolis, IN] and Uracil DNA glycosylase [0.25 U]) to give a final volume of 50 μL. Amplification was carried out in a Gene Amp 9600 Thermal Cycler (Perkin Elmer, Shelton, CT) as follows: hold at 25°C and 94°C for 10 minutes, respectively, followed by 40 cycles at 94°C, 56°C, and 72°C for 40 seconds each. A hold cycle at 72°C for 5 minutes was performed at the end of the reaction.
Negative, positive, and blank controls were included in each reaction. Negative controls were healthy adults who had negative CMV serology, and a blank control consisted of standard PCR reagents without a DNA sample. High and low viral copy (vc) CMV-positive controls were prepared from stock (10 000 vc/μL; Advanced Biotechnologies; Columbia, MD). PCR products were visualized by ethidium bromide gel electrophoresis through 2% agarose in 1× TBE buffer (1 M Tris, 0.9 M boric acid, 0.01 M EDTA; Invitrogen, Carlsbad, CA) and confirmed by hybridization with specific probes. To detect samples containing PCR inhibitors, a DNA sequence (generic HLA-DRB1; 240 bp) that does not share homology with CMV was co-amplified as a control target using a multiplex PCR together with the primers for CMV. For a run to be considered valid, the DRB1 control band had to be present. A sample with no amplification of CMV primers in a valid run was interpreted as CMV-PCR negative.
Nested PCR for CMV
For nested PCR, 2 μL PCR product was added to a mix containing 0.36 μM of each primer, 1× PCR buffer, 6 mM MgCl2, Taq Gold, 5U (Perkin Elmer, Norwalk, CT), BSA (0.2 mg/mL; Roche) and dUTP 1× (Sigma) in a 50 μL final volume. Specific primer sequences, internal to the original 280-bp GlyB sequences, were as follows: forward 5′aggtgctgcgtgatatgaacg3′ and reverse 5′-gacaacgaaatcctgttgggc-3′. DNA amplification was carried out as follows: hold at 94°C for 10 minutes, followed by 25 cycles of 94°C, 56°C, and 72°C for 30 seconds, and a hold cycle at 72°C for 5 minutes.
PCR product was evaluated by hybridization using 5′ biotinylated specific probes (Qiagen, Boston, MA) with the signal developed using a chemiluminescent system (ECL; Amersham Pharmacia Biotech, Buckinghamshire, England). Each membrane contained 10-fold dilutions from the commercial positive CMV DNA control (range, 1-5000 vc), as well as negative and blank controls, as described above.
Amplicor CMV-PCR
Using the same DNA samples, DNA (10 μL) was diluted in 40 μL Tris-HCl (pH = 7.4), 100 nM and MgCl2 (9.37 nM) and added to 50 μL PCR mix. PCR and product detection were performed with the CMV-Amplicor-Roche kit according to the manufacturer's instructions (Roche).
PCR assay sensitivity
Several dilutions from a commercial positive CMV control (ABI, Columbia, MD) comparable to a range of 1 vc to 5000 vc in 10 μL Tris-HCl (pH = 7.4) buffer were tested in duplicate in 4 separate experiments. The lowest concentration that could be accurately and consistently detected by the in-house PCR and the Amplicor-Roche PCR assay was equivalent to 25 vc.
Case-control studies of CMV infection in CB donors
In the first case-control study (case-control study 1), we evaluated whether CMV DNA could be detected in the CB of infants whose saliva culture was positive for CMV. Control CB samples were selected (2 controls: 1 case) from among culture-negative infants born in the same hospital on approximately the same date (Figure 1A). The second case-control study (case-control study 2) was designed to evaluate whether CMV infection could be detected by PCR among infants whose saliva culture was negative, with cases and controls chosen according to maternal CMV serology. Infants whose mothers had IgM anti-CMV or borderline reactivity were presumed to be at highest risk of congenital infection and served as cases. Controls were culture-negative infants whose mothers were total anti-CMV positive and IgM negative or infants whose mothers had no detectable CMV-specific antibody. Controls (1:1 to cases in each of the 2 categories) were selected from among infants born in the same hospital on approximately the same date as cases (Figure 1B).
Schematic representation of case-control studies 1 and 2, respectively. (A) Case-control study 1: detection of CMV infection by saliva culture and confirmation by polymerase chain reaction (PCR). (B) Case-control study 2: detection of possible CMV infection by PCR in saliva culture–negative infants.
Schematic representation of case-control studies 1 and 2, respectively. (A) Case-control study 1: detection of CMV infection by saliva culture and confirmation by polymerase chain reaction (PCR). (B) Case-control study 2: detection of possible CMV infection by PCR in saliva culture–negative infants.
Statistics
Fisher exact test and chi squared test (both 2-sided) were used to estimate the significance of differences in contingency tables. The incidence of posttransplantation CMV infection was estimated by Kaplan-Meier with relative risk (RR) in multivariate analyses estimated by Cox regression. Analyses used the Statistical Package for Social Sciences (SPSS, version 11.0; Chicago, IL).
Results
CB recipients and incidence of posttransplantation CMV infection
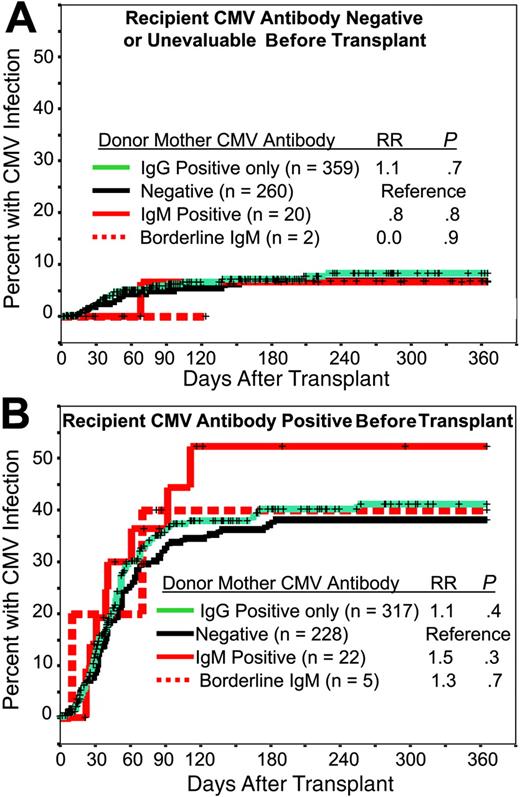
Transplant recipient characteristics along with their pretransplantation CMV serologic status are shown in Table 1. CMV-antibody prevalence increased with patient age and was more common in recipients that were not white and in patients who underwent transplantation in non-U.S. centers or who underwent transplantation recently. The lower prevalence among patients with genetic diseases reflected their younger age whereas the higher antibody prevalence among patients who recently underwent transplantation reflected an increased proportion of older recipients in recent years. There was no association between patient pretransplantation CMV serology and the CB donor's maternal CMV serology (data not shown). A total of 221 CMV infections were reported in the first year after transplantation. The incidence of CMV infection was 23% overall, 8% among patients who had no detected CMV antibody or were unevaluable before transplantation, and 41% among patients who were antibody positive (P < .001). Within these 2 patient categories, there was no association between the CB donor's maternal CMV antibody status and incidence of posttransplantation CMV infection (Figure 2A-B). The incidence of posttransplantation CMV infection was associated with recipient pretransplantation CMV serology, ethnicity, age, HLA mismatch, transplant center, and year of transplantation, but not with the donors' maternal CMV serology. In multivariate analysis, recipient age was no longer significant and donor maternal CMV serology remained insignificant (Table 2). Of transplanted CB units, 2 were positive by CMV-PCR: in 1 case the donor had a positive saliva culture and maternal total anti-CMV was positive but IgM was negative. The seronegative recipient had a CMV infection on day +45 after transplantation that resolved with gancyclovir treatment. In the second case, maternal IgM anti-CMV was positive but saliva culture had not been done. The recipient in this case was seropositive before transplantation and died of bacterial sepsis on day +52 with no CMV infection recognized up to the time of death. All other CB units given for transplantation to study recipients were PCR negative. Removal of the 2 recipients who received CB that was CMV-PCR positive did not change substantially the result of the multivariate analysis (data not shown).
Incidence of CMV infection after transplantation. Cumulative incidence of posttransplantation CMV infection in recipients who were CMV-antibody negative or unevaluable (A) or CMV-antibody positive (B) before transplantation: relationship to the cord blood donor's maternal anti-CMV antibody status. Relative risk (RR) and significance (P) were estimated by Cox regression.
Incidence of CMV infection after transplantation. Cumulative incidence of posttransplantation CMV infection in recipients who were CMV-antibody negative or unevaluable (A) or CMV-antibody positive (B) before transplantation: relationship to the cord blood donor's maternal anti-CMV antibody status. Relative risk (RR) and significance (P) were estimated by Cox regression.
Posttransplantation CMV infection was associated with severe (grade 3-4) acute GVHD (RR = 1.7, 95% confidence interval [CI] = 1.3-2.2; P < .001). Additionally, among patients who engrafted (n = 855), those with a posttransplantation CMV infection (n = 194) had a higher risk of secondary graft failure than those without a CMV infection (7.2% vs 2.2%, respectively; P = .002). Among the reported CMV infections, 63% of these were manifest only by detection of the virus in blood. Overall, CMV was reported as a cause or a contributing cause of death in 6.3% of the 693 recipients who died and had a reported cause of death (38 not reported).
Cord blood donors
CMV was detected by saliva culture in 47 of 26 988 donor infants tested (0.17%), none of whom were diagnosed clinically in the newborn period as having a congenital CMV infection. Of the 19 757 mothers evaluated in the period of routine testing, total anti-CMV was positive in 11 649 (59.0%). Among antibody-positive mothers, 30 of these had infants with CMV-positive saliva culture (0.26%). Two infants born to antibody-negative mothers also were culture positive. Of the 11 726 mothers tested routinely for IgM anti-CMV, 184 (1.6%) were positive and 76 (0.6%) had equivocal reactivity. Among the IgM-positive mothers, 8 had infants who were culture positive (4.3%), a rate more than 10-fold higher than in infants whose mothers were positive for total antibody (P < .001). None of the mothers with an equivocal CMV-IgM result had an infant who was culture positive.
Case-control study 1: detection of CMV by PCR in cord blood of CMV culture–positive infants
Thirty-three of the 47 saliva-culture positive infants had a PCR-positive CB by in-house PCR (cases 1-33, Table 3), all confirmed on repeat testing (20 also tested by the Roche PCR assay were confirmed positive). CMV DNA was usually detected in both plasma and WBC-derived DNA. In-house PCR (including nested PCR) was negative in 14 culture-positive infants (cases 34-47; 9 cases also tested and found negative by the Roche assay). One sample that was initially PCR positive only with the Roche assay in plasma could not be retested for confirmation. Three of the culture-positive, PCR-negative infants were born to CMV antibody–negative mothers (cases 35-37). One PCR-negative infant (case 40) had an identical twin (single placenta) whose saliva culture and CB-PCR were negative. Thus, a total of 33 infants were considered to have a congenital CMV infection that was confirmed by CB-PCR (0.12% of study infants). A PCR-confirmed CMV infection was found in 22 of the 11 649 infants from the period of routine testing whose mothers were total-antibody positive (0.19%) and 7 of the 184 infants from the period of routine IgM antibody testing whose mothers were IgM anti-CMV positive (3.8%, P < .001). CB-PCR (including nested) was negative in both WBC and plasma from all 94 culture-negative control infants.
All mothers of the 33 infants with PCR-confirmed CMV infection were positive for total anti-CMV, 79% with a high titer (≥ 1:80). Only 11 of them were IgM anti-CMV positive. Among control mothers, in contrast, 63 (67%) were anti-CMV positive (P < .001), with only 30% having high titers (P < .001) and only one with IgM (P < .001). The 14 case mothers whose infants' CMV infection was not confirmed by PCR were similar to control group mothers in their CMV antibody prevalence, titer, and IgM anti-CMV prevalence. All mothers in this study were tested for CMV DNA with the in-house PCR assay. CMV DNA was detected (by nested PCR only) in WBC-derived DNA from 6 mothers: 5 had an infant with a PCR-confirmed CMV infection and the sixth was culture positive but negative by CB-PCR.
Case-control study 2: possible CMV infection in saliva culture–negative infants
Among the 184 mothers with IgM anti-CMV, 176 had infants who were saliva culture–negative and among the 76 mothers with equivocal IgM reactivity, all infants were culture negative. CB was tested for CMV DNA by PCR on 246 of these culture-negative cases (170 of those 176 with an IgM-positive mother and all 76 with an equivocal IgM) and on 492 controls (246 from mothers with IgG but no IgM anti-CMV and 246 from mothers with no antibody detected). CMV DNA was detected by PCR in 5 CB samples, 2 by both the in-house PCR and Roche assays and 3 with the Roche assay alone (all confirmed on repeat testing). All 5 PCR-positive samples were from case infants born to mothers who had IgM anti-CMV, 4 with a high total anti-CMV titer (≥ 1:80). If these PCR-positive infants had a congenital CMV infection, despite their negative saliva culture, then up to 13 infections actually occurred among infants born to IgM-positive mothers, 7 detected by culture and PCR, 2 to 5 by PCR alone (for a total of 9-12 detected by PCR), and 1 by culture alone. If so, then 22% to 42% of PCR-positive infections among infants of IgM-positive mothers in this study were missed by saliva culture.
Discussion
This is the first study that evaluated whether there is an association between CB donor serology for CMV antibody and posttransplantation CMV infection in the recipient and, in addition, assessed alternative strategies to screen CB donors for CMV. As in previous studies of bone marrow (BM) or peripheral blood HSC transplant recipients, CMV infection was a significant cause of posttransplantation morbidity and mortality. Patients with a CMV infection, for example, had an increased incidence of secondary graft failure, possibly due, at least in part, to myelosuppression associated with antiviral therapy.20,21 Also as reported in previous studies, CMV infection was strongly associated with recipient pretransplantation CMV-antibody status. Recipient ethnicity and HLA mismatch were independently associated with the risk of CMV infection after transplantation, as were transplant center and year of transplantation. The association with HLA mismatch may be related to the higher incidence of acute GVHD in recipients of mismatched CB units.10 The association with year of transplantation may reflect improved diagnosis with the recently implemented PCR-based assays.
Unlike studies of BM transplantation, posttransplantation CMV infection in CB recipients had no detected association with donor serology (ie, the donor mother's CMV antibody status).22,23 Even when the donor's mother was IgM anti-CMV positive (those at highest risk of having a CMV-infected infant based on saliva culture and CB-PCR), there was no detected association with posttransplantation CMV, although this group was relatively small in our study. Thus, donor maternal CMV serology was a poor predictor of CMV transmission to recipients in CB transplantation, supporting the view that CB units need not be excluded for transplantation based on the donor's maternal CMV serologic results alone.
Our study revealed the expected low incidence of congenital CMV infection in CB donors (0.12% by positive saliva culture and confirmed CB-PCR), at the low end of ranges previously reported. The low incidence of congenital CMV infection might predict a lower overall incidence of posttransplantation CMV infection in CB recipients than in recipients of BM/peripheral blood grafts. Indeed, incidence in antibody-negative recipients was only 8% (presumably nearly all acquired from some source other than the CB graft). The incidence among anti-CMV–positive recipients (41%) also was lower than reported in some previous studies of BM transplant recipients.1,20,23 Comparison with historical rates, however, must be made with caution because of possible differences in diagnostic methods, CMV prophylaxis, or whether the marrow grafts were T-cell depleted. In a recent study for example, Chalandon and colleagues23 reported a high incidence of posttransplantation CMV in CMV-antibody–positive recipients of unmanipulated BM grafts from anti-CMV–negative donors (81%). In contrast, the incidence was only 33% in recipients of T-cell–depleted grafts from negative donors. The number of recipients in their study, however, was relatively small and they were selected for absence of severe posttransplantation complications. Nevertheless, their observation of failure to transfer CMV-specific memory T cells from these donors and consequent difficulty in mounting the primary immune response necessary to protect the patient raises the question of how T-cell function in CB might affect the risk of posttransplantation CMV infection. Parallel prospective studies with standardized case definitions would best answer such questions.
Most of the CB donor infants in this study who had a positive saliva culture had their infection confirmed by detection of CMV DNA in CB by PCR. Some presumed infections, however, were not confirmed by PCR and our data suggest that the culture-positive infants with a PCR-negative CB most likely had a false-positive saliva culture. One such infant, for example, had an identical twin who was saliva culture and CB-PCR negative. In 3 other cases, the mother had no detectable anti-CMV. Moreover, the fact that maternal serology (IgM prevalence and total antibody titer) for this group of infants was similar to that of culture-negative controls, supports this conclusion. A false-positive saliva culture could occur if there were contamination of the saliva sample by maternal vaginal secretions or for more trivial reasons such as labeling, technical, or clerical errors.24 Saliva culture also may have missed some congenital CMV infections. Among culture-negative infants who were presumed to be at high risk (IgM anti-CMV–positive mother), we found 2 to 5 possible infections detected by PCR (depending on the assay) and, like infants with confirmed infections, all but one had high maternal antibody titer. A false-negative culture could occur for technical reasons such as inadequate sample collection, recent feeding of the infant, loss of CMV viability during storage or transport, or for trivial reasons such as labeling, technical, or clerical errors.25 The possibility of additional false-negative cultures among infants whose mothers were total antibody positive but IgM negative cannot be excluded, even though none were detected by PCR, from either control group from study 2. Even if as many as 40% of congenital infections were missed by culture (as may have occurred in infants of IgM-positive mothers), the predicted prevalence of false-negative cultures in study infants whose mothers are IgG positive is estimated to be below 0.1%. The sample size of our IgG-positive/IgM-negative control group, however, was only sufficient to detect a PCR positivity rate 1.5% or higher with 95% confidence.
The positive predictive value for congenital CMV infections confirmed by CB-PCR was 3.8% (7/184) for infants born to IgM-positive mothers, somewhat lower than the 8.3% rate reported by Roback et al,12 and only 0.2% when the mother was total anti-CMV positive (regardless of IgM status). These data fit with our observation that maternal total and/or IgM positivity per se also were poor predictors of CMV transmission from CB to transplant recipients. Thus, a screening strategy based on antibody positivity in the donor's mother would unnecessarily exclude a large number of CB units, the vast majority of which have no risk of CMV transmission to recipients. Importantly, although infants of IgM anti-CMV–positive mothers had a higher prevalence of CMV infection than infants of IgG-positive mothers, our data demonstrate that screening for maternal IgM anti-CMV alone would miss two-thirds of infections. This finding is not surprising since IgM is only transiently positive after an acute CMV infection and CMV also can be transmitted from mother to infant when a latent infection reactivates during pregnancy, often without production of IgM anti-CMV.10,26-28
If testing CB donors for evidence of CMV infection remains mandated by the FDA, despite its low prevalence in newborns and despite the fact that traditional serologic screening methods are not very informative in CB, a practical strategy to detect the virus would be needed that takes into account specificity and sensitivity as well as cost and logistics. Although CMV culture of the infant's saliva or urine is considered the “gold standard” for detecting congenital CMV infection, it requires collection of a specimen that is separate from the CB product, thereby increasing the risk of errors in specimen collection, a possible explanation for some of the false-positive and false-negative cultures in our study. Testing the CB graft itself eliminates this source of error. Detection of CMV DNA in CB by nucleic acid–based methods, as shown in this study, can be reliable, sensitive, and specific with the advantage that they use DNA, a relatively stable molecule, rather than depending on collection of a specimen that accurately obtains and preserves viable virus. Additionally, PCR can be done retrospectively on stored samples prior to release of a CB unit for transplantation. The fact that confirmed congenital CMV infections were limited to infants of antibody-positive mothers suggests a potential screening strategy based on testing mothers for antibodies to CMV followed by prerelease testing of CB units for CMV DNA by PCR when the mother is antibody positive, regardless of her IgM anti-CMV status. At present, any strategy to detect infection per se in the CB donor, whether by culture or DNA-based assays, however, is hampered by the lack of an FDA-approved assay to screen CB donors.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 22, 2006; DOI 10.1182/blood-2006-04-020313.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We wish to thank Diana G. Daniels, George Chen, Armando Hernandez, and Shang Tei Yu for their excellent technical support and NYBC-NCBP Hospital staff who collected the CB and saliva specimens. We also thank the obstetricians and obstetrical nursing staff that supported the program in our collaborating hospitals and the physicians and staff of the 146 transplant centers that provided follow-up data on transplantation outcome for their patients who received cord blood units from our program. M.S.A. is a PhD candidate from Universidad de San Luis, Argentina.