Comment on Lan et al, page 487
Enhancing human hematolymphopoiesis and especially T-lymphopoiesis remains an important goal for optimizing the current humanized immunodeficient mouse models. Yang's team reports an improved human fetal thymus/liver–implanted immunodeficient mouse model in which a striking increase of the overall hematolymphopoiesis with significantly enhanced immune functions can be achieved via a simple intravenous infusion of autologous human fetal liver CD34+ cells.
Xenograft models provide a valuable system for evaluating physiologic functions of human cells in both basic research and preclinical settings. In contrast to large animals, mice with a severe combined immunodeficiency (Prkdcscid, or SCID) mutation1 resulting in lack of both T and B cells are much more commonly used due to their easy accessibility, cost-effectiveness, and feasibility of backcrossing with other immunogenetic mutations to further knock down innate immunity. Since the initial generation of SCID mice, a number of improved strains, such as nonobese diabetic (NOD)/SCID, NOD/SCID/β2mnull, NOD/SCID/γcnull, and Rag2null/γcnull have been derived to better adopt human lymphohematopoietic cells.2 Although the inability to differentiate human T cells was overcome in the γcnull SCID strains3,4 as well as the human fetal thymus/liver (Thy/Liv)–implanted model,5 following transplantation of human hematopoietic cells, the overall yield or rate of expansion of de novo regenerated human T cells was still low. Therefore, further enhancing human hematolymphopoiesis and especially the production of functional T cells remains an important task in the field.
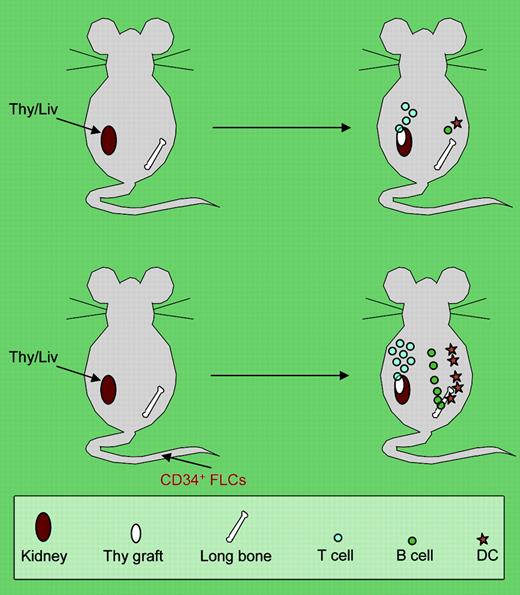
In this issue of Blood, Lan et al report a small modification but a big improvement over the previous Thy/Liv model. The authors cleverly combined the infusion of autologous CD34+ human fetal liver cells with human fetal Thy/Liv implants5 in NOD/SCID mice (see the figure). In this model, they were able to achieve a very high degree of engraftment (up to 40% in blood) of human T cells along with other hematopoietic lineages (B and myeloid cells). Moreover, the human cells massively populated the secondary lymphoid organs, and the formation of white pulplike structures was observed in the spleen. In particular, human dendritic cells (both myeloid and plasmacytoid) were present only in the spleen and lymph nodes of CD34+ cell-infused Thy/Liv mice, and human immunoglobulin was also significantly increased in these mice. Most impressively, the human T-cell immunity in vivo was evidenced by rejection to a porcine skin xenograft in the CD34+ cell–infused Thy/Livgroup but not the Thy/Liv-only group. Notably, the infusion of the human CD34+ cells did not seem to just serve as“add-on” engraftment to the basal human hematopoiesis and lymphopoiesis achieved by the human fetal thymus/liver implants alone. Rather, the infused CD34+ cells, even with a limited number (1 to 5 × 105 per animal), had a profound synergistic effect on the establishment of human lymphohematopoiesis in the mice. One possible cause behind the dramatic effects, as the authors point out, is that the infused CD34+ cells may equip the microenvironment with some essential immune cellular elements such as dendritic cells in the secondary lymphoid organs for a robust T-cell response to antigens.FIG1
The top panel shows the traditional human fetal Thy/Liv-grafted model with poor in vivo immunity due to inefficient human dendritic-cell (DC) and B-cell development. The bottom panel shows that intravenous injection of CD34+ fetal liver cells (FLCs) leads to multilineage repopulation of human lymphohematopoietic cells, formation of secondary lymphoid organs, and development of a functional human immune system in human fetal Thy/Liv-grafted NOD/SCID mice.
The top panel shows the traditional human fetal Thy/Liv-grafted model with poor in vivo immunity due to inefficient human dendritic-cell (DC) and B-cell development. The bottom panel shows that intravenous injection of CD34+ fetal liver cells (FLCs) leads to multilineage repopulation of human lymphohematopoietic cells, formation of secondary lymphoid organs, and development of a functional human immune system in human fetal Thy/Liv-grafted NOD/SCID mice.
Given the heterogeneous nature of the infused CD34+ cells, however, mechanisms for the high T-cell yield need to be further elucidated. The effective process is likely multifactorial: for example, besides hematopoietic stem/progenitor cells, some facilitating cells in the bulk CD34+ cells may also play important roles in contributing to the engraftment. In addition, an optimal cell dosage or ratio between the infused cells and implanted tissues required for the maximal effect remains to be determined. Nonetheless, although further improvements are needed and the biologic basis of the model remains to be determined, these authors have clearly made an important step toward optimal human hematolymphopoiesis in the mice, thereby allowing investigators to approach a broader set of biologic and preclinical issues on human hematopoietic stem cell repopulation, immune reconstitution, infection, vaccine development, autoimmunity, and transplant tolerance. ▪