Mature megakaryocytes form structures called proplatelets that serve as conduits for platelet packaging and release at vascular sinusoids. Since the megakaryocyte expresses abundant levels of integrin αIIbβ3, we have examined a role for fibrinogen in proplatelet development and platelet release alongside that of other matrices. Primary mature murine megakaryocytes from bone marrow aspirates readily formed proplatelets when plated on fibrinogen at a degree that was significantly higher than that seen on other matrices. In addition, αIIbβ3 was essential for proplatelet formation on fibrinogen, as megakaryocytes failed to develop proplatelets in the presence of αIIbβ3 antagonists. Interestingly, inhibition of Src kinases or Ca2+ release did not inhibit proplatelet formation, indicating that αIIbβ3-mediated outside-in signals are not required for this response. Immunohistochemical studies demonstrated that fibrinogen is localized to the bone marrow sinusoids, a location that would allow it to readily influence platelet release. Further, thrombopoietin-stimulated αIIb-/- mice had a reduced increase in platelet number relative to controls. A similar observation was not observed for platelet recovery in αIIb-/- mice in response to antibody-induced thrombocytopenia, indicating the existence of additional pathways of regulation of proplatelet formation. These results demonstrate that fibrinogen is able to regulate proplatelet formation via integrin αIIbβ3.
Introduction
Megakaryocytes are the immediate cellular precursors to platelets, and are largely understood to give rise to nascent platelets by forming long, branching filaments called proplatelets (reviewed in Hartwig and Italiano1 and Schulze and Shivdasani2 ). Proplatelets have long been observed in bone marrow preparations via histological staining and electron microscopy3,4 and in cultures of primary megakaryocytes.5,6 Both the early studies and more current research have observed the consistent presence of small, platelet-sized swellings that reside along the length of the proplatelet, presumably representing the developing platelet. Recent studies have identified the underlying architecture of proplatelets, consisting of microtubules that comprise the shaft of the proplatelet and actin filaments that allow for extensive proplatelet branching.7 The microtubules also delineate the nascent platelet boundary and serve as a mechanism for delivery of granules and organelles to the distal end of the proplatelet, providing the forming platelets with the necessary components for hemostatic function.7,8
While the architecture of the proplatelet has been well established, the conditions that induce proplatelet formation are not yet well understood, particularly in vivo. A number of studies have begun to address this deficiency by investigating the signaling pathways that contribute to proplatelet formation. For example, plasma from thrombocytopenic rabbits stimulates proplatelet formation, suggesting that soluble factors are capable of inducing proplatelet growth in response to platelet depletion.9 In addition, the use of soluble inhibitors has indicated that protein kinase C, serine/threonine phosphatases, and mammalian target of rapamycin are necessary for normal proplatelet development.10-12 Genetic approaches have also provided valuable insight into the intracellular factors that contribute to proplatelet growth. Deletion of the megakaryocyte transcription factors NF-E2 and GATA-1 demonstrate that both are required for proplatelet formation and normal platelet counts.13-15 Further, proteins under NF-E2 control, such as 3β-hydroxysteroid dehydrogenase, β1-tubulin, Rab27b, and caspase-12 are implicated in the normal regulation of proplatelet formation and/or platelet release.16-20
There is mounting evidence to suggest that the bone marrow stroma contributes to proplatelet development and platelet release. Recent research has shown that the chemokine SDF-1 and the growth factor FGF-4 are necessary for megakaryocyte recruitment to sinusoid endothelial cells and can help to reconstitute platelet counts following myelosuppression.21 In addition, matrix proteins such as vitronectin and collagen have been shown to promote proplatelet formation,22,23 and antibodies directed against GPIbα and the integrin subunit αIIb inhibit proplatelets when added to cultured megakaryoytes.24 However, there are no studies to our knowledge that have looked at the role of fibrinogen itself in regulating proplatelet development.
In the present study, we demonstrate that fibrinogen supports robust proplatelet development from uncultured primary murine megakaryocytes in vitro and that, in vivo, fibrinogen is localized to vascular sinusoids. Further, we demonstrate that integrin αIIbβ3 is necessary for proplatelet formation on fibrinogen in vitro, and that the absence of αIIbβ3 leads to reduction in platelet counts in mice who received injections of thrombopoietin (TPO) relative to controls. We conclude that the interaction of fibrinogen with αIIbβ3 is an important component of proplatelet formation and platelet release from murine megakaryocytes.
Materials and methods
Reagents
Bovine serum albumin (BSA; fatty acid-free), apyrase (grade VII), PGI2, 10% formalin, human placental laminin, and human plasma vitronectin were from Sigma (Gillingham, United Kingdom). The Src family kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) was from Merck Biosciences (Nottingham, United Kingdom). Fibrinogen was from Enzyme Research Laboratories (Swansea, United Kingdom). Horm collagen was from Nycomed (Linz, Austria). Von Willebrand factor (VWF) and botrocetin were generous gifts from Michael Berndt (Monash University, Victoria, Australia). Fluorescein isothiocyanate (FITC)-conjugated antimouse fibrinogen and FITC-conjugated IgG control polyclonal antibodies were from Emfret Analytics (Würzburg, Germany). Anti-mouse αIIb integrin (clone MWReg30) and rat IgG1 control antibodies were from Serotec (Oxford, United Kingdom). Antimouse CD105 (clone MJ7/18) was from eBioscience (San Diego, CA). Texas Red-conjugated streptavidin was from Southern Biotechnology Associates (Birmingham, AL). Murine TPO was from Peprotech (London, United Kingdom). Lotrafiban was a generous gift from GlaxoSmithKline (King of Prussia, PA). Dimethyl BAPTA-AM was from Molecular Probes (Paisley, United Kingdom).
Mice, murine TPO injections, and induced thrombocytopenia
All mice were maintained using housing and husbandry in accordance with local and national regulations established by the UK Home Office. Mice deficient in αIIb and PLCγ2 were generated as previously described and bred on a C57BL6 background.25,26 Genotype was identified via genomic polymerase chain reaction.25,26 Where necessary, C57BL6 wild-type mice were purchased from Harlan (Oxon, United Kingdom). Littermate controls were used with genetically modified mice. Mice were given intraperitoneal injections of 0.3 μg of TPO in a solution of 20 mM Tris (pH 8.0), 0.9% NaCl, and 0.25% BSA for 4 consecutive days to boost megakaryocyte and proplatelet totals, with the megakaryocytes being harvested the day following the last TPO injection.27 Thrombocytopenia was induced by intraperitoneal injection of 2 μg/g polyclonal anti-GPIbα antibody (Emfret Analytics).
Observation of proplatelet formation
Murine femurs and tibias were isolated and the marrow was flushed with an isotonic buffer (143 mM NaCl, 5.6 mM KCl, 10 mM HEPES [pH 7.2], 10 mM glucose, 0.4% BSA, and 0.2 U/mL apyrase). The marrow aspirate was passed through a 21-gauge needle to disperse the tissue and spun at 200g for 10 minutes after the addition 2.67 μM PGI2. Following centrifugation, the aspirate was resuspended in the isotonic buffer without apyrase but supplemented with 2 mM MgCl2 and 0.2 mM CaCl2 at a total cellular concentration of 10 to 20 × 106 cells/mL. Resuspended aspirates were treated with various reagents and then added to glass coverslips that had been coated with various matrices overnight at 4°C. All cover slips were blocked in 5 mg/mL BSA for 1 hour prior to the addition of marrow aspirates. Cells were allowed to adhere for 3 to 4 hours at 37°C in a humidified atmosphere. The coverslips were then washed 3 times in PBS and fixed in 10% formalin. The cells were stained via alkaline phosphate-linked antibody binding using an antibody specific for αIIb, a biotinylated antirat secondary antibody, and an Alkaline Phosphatase Standard Staining kit with the Substrate Kit II (Vector Laboratories, Peterborough, United Kingdom) as per manufacturer's directions. The coverslips were mounted onto glass slides with Fluoromount-G (Southern Biotechnology Associates). Proplatelets were identified by locating large αIIb-positive cells that displayed at least 1 long filamentous structure that contained platelet-sized swellings. The percentage of megakaryocytes bearing proplatelets was determined by scanning the entire area (133 mm2) of the coverslips and tabulating the number of megakaryocytes with or without proplatelets. For real-time imaging, cell aspirate suspensions were allowed to incubate for 60 minutes on coated 25-mm coverslips to allow the megakaryocytes to adhere to the surface, then samples were transferred to a humidified incubation chamber at 37°C for video recording.
Microscopy
Differential interference contrast (DIC) microscopy on live-cell and fixed samples was performed on a Zeiss Axiovert 200 microscope (Welwyn Garden City, United Kingdom). Images were visualized using a Zeiss LD Plan Neofluar 20× objective (numeric aperture 0.4), captured with a CoolSnap ES charge-coupled device camera (Photometrics, Marlow, United Kingdom) and processed using SlideBook software (Intelligent Imaging Innovations, Denver, CO). Confocal microscopy of fixed tissue sections was performed on a Leica DMIRE2 microscope and visualized using a Leica HCX PL APO 40× objective (numerical aperture 1.25). Images were acquired and processed with Leica Confocal Software (Milton Keynes, United Kingdom). All microscope images were assembled and scaled using Adobe Photoshop software (Uxbridge, United Kingdom). Image adjustments were limited to brightness and contrast of the whole image.
Whole-blood platelet counts
Mouse blood was drawn from cardiac puncture or the hepatic portal vein into 100 μL acid citrate dextrose (85 mM sodium citrate, 111 mM glucose, 71 mM citric acid) and added to 200 μL Tyrode buffer (138 mM NaCl, 0.36 mM NaH2PO4, 2.9 mM KCl, 12 mM NaHCO3, 5 mM HEPES, 1 mM MgCl2, and 5 mM glucose, pH 7.3), or tail-bled with approximately 50 μL blood diluted into 100 μL PBS/20 mM EDTA. Blood was counted in a Pentra 60 cell counter (ABX Diagnostics, Shefford, United Kingdom). All blood counts were adjusted for total volume.
Histology
Murine femurs were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Zoeterwoude, The Netherlands) and gently frozen in liquid nitrogen. Frozen sections (8 μm) were made on a cryostat (Bright Instrument Company, Huntingdon, United Kingdom) and mounted onto X-tra Adhesive Microslides (Surgipath, Peterborough, United Kingdom). Slides were allowed to dry at room temperature for at least 1 hour, fixed in ice-cold acetone for 20 minutes, and subsequently allowed to dry for at least 15 minutes. Slides were stored at –80°C until analysis. Slides were stained with a premixed solution of 5 μg/mL FITC-fibrinogen antibody (or FITC-IgG control), 10 μg/mL biotinylated CD105 antibody, and 1:100 Texas Red-conjugated streptavidin in PBS with 2.5% BSA for 20 minutes and then rinsed gently 3 times in PBS. The samples were mounted and visualized using confocal microscopy.
Statistics
Student t test was used to analyze data, with a significant difference set at a P value less than .05.
Results
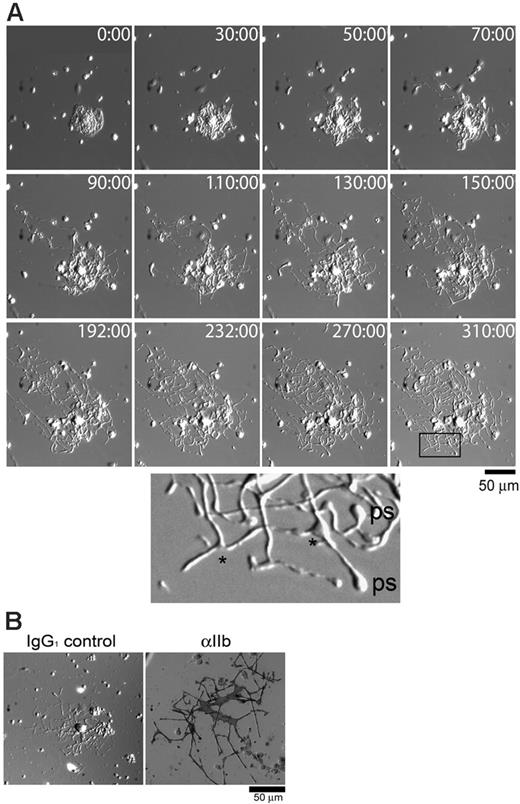
To assess whether fibrinogen could support proplatelet development on megakaryocytes ex vivo, freshly harvested bone marrow aspirates from murine femurs and tibias were plated on fibrinogen and the megakaryocytes were monitored for formation of proplatelets using real-time DIC microscopy. Proplatelets began to extend from the cell body within 30 to 50 minutes of commencement of recording and had usually reached their full extension within 200 minutes as exemplified in Figure 1A (the complete movie is available as Video S1 on the Blood website; see the Supplemental Video link at the top of the online article). Small swellings that are thought to be the nascent platelets and numerous branch points were observed along the length of the proplatelet and are highlighted in the enlarged section of Figure 1A. A similar morphology has been reported for proplatelet formation from primary cultures of megakaryocytes.7 Subsequent αIIb integrin subunit alkaline phosphatase staining of proplatelet-bearing structures from the bone marrow aspirates confirmed the megakaryocyte identity (Figure 1B).
Primary, uncultured megakaryocytes can rapidly produce proplatelets on fibrinogen. (A) Bone marrow aspirates were prepared as described in “Materials and methods” and plated onto fibrinogen-coated 25-mm glass coverslips. The cells were allowed to adhere for 1 hour at 37°C, then the coverslips were rinsed and transferred to a microincubation chamber. The chamber was placed into a humidified microscope chamber at 37°C and 5% CO2, and images were taken at the indicated time points (minutes). The enlarged area is from the box at time point 310:00 minutes. Branching points are indicated with an asterisk, and visible platelet swellings are labeled “ps.” Other cells, such as red blood cells and macrophages, are visible within the fields of view. The complete movie can be viewed on the Blood website (Video S1). (B) Proplatelets from primary megakaryocytes are αIIb-positive. Cells were allowed to adhere to fibrinogen-coated coverslips for 4 hours at 37°C, then fixed and stained for αIIb via alkaline phosphatase. Dark staining was observed only when the αIIb-specific antibody was used (right panel), and not the IgG1 control antibody (left panel).
Primary, uncultured megakaryocytes can rapidly produce proplatelets on fibrinogen. (A) Bone marrow aspirates were prepared as described in “Materials and methods” and plated onto fibrinogen-coated 25-mm glass coverslips. The cells were allowed to adhere for 1 hour at 37°C, then the coverslips were rinsed and transferred to a microincubation chamber. The chamber was placed into a humidified microscope chamber at 37°C and 5% CO2, and images were taken at the indicated time points (minutes). The enlarged area is from the box at time point 310:00 minutes. Branching points are indicated with an asterisk, and visible platelet swellings are labeled “ps.” Other cells, such as red blood cells and macrophages, are visible within the fields of view. The complete movie can be viewed on the Blood website (Video S1). (B) Proplatelets from primary megakaryocytes are αIIb-positive. Cells were allowed to adhere to fibrinogen-coated coverslips for 4 hours at 37°C, then fixed and stained for αIIb via alkaline phosphatase. Dark staining was observed only when the αIIb-specific antibody was used (right panel), and not the IgG1 control antibody (left panel).
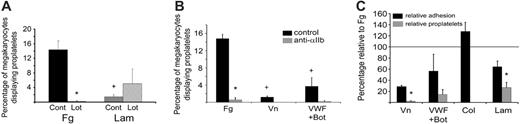
Inhibition of αIIbβ3 prevents proplatelet formation on fibrinogen, vitronectin, and VWF/botrocetin, but not on laminin. (A) Bone marrow aspirates from TPO-treated mice were plated on either 100 μg/mL fibrinogen (Fg) or 50 μg/mL laminin (Lam) in the presence of 10 μM lotrafiban, an αIIbβ3 antagonist. Cells were allowed to adhere for 3 hours at 37°C prior to PBS washing and fixation. Cells were visualized with αIIb-alkaline phosphatase staining, and the total number of adherent megakaryocytes and megakaryocytes with proplatelets was assessed for each coverslip. *Significant difference between control and lotrafiban treatment; + significant difference between fibrinogen and laminin. (B) Bone marrow aspirates were plated on fibrinogen, 10 μg/mL vitronectin (Vn), or 10 μg/mL VWF plus 3 μg/mL botrocetin (to ensure megakaryocyte/VWF binding under static conditions) in the presence or absence of an anti-αIIb antibody. The total number of adherent megakaryocytes and proplatelet-bearing megakaryocytes was determined as in panel A. *Significant difference between control and anti-αIIb treatment; + significant difference between fibrinogen and the other matrices. (C) Absolute cell numbers of adherent megakaryocytes and proplatelet-bearing megakaryocytes on vitronectin, VWF/botrocetin, 100 μg/mg collagen (Col), or laminin were normalized to the absolute amounts seen on fibrinogen in each individual experiment, with equal numbers equaling 100% relative to fibrinogen (solid line). Error bars indicate SEM, n = 3.
Inhibition of αIIbβ3 prevents proplatelet formation on fibrinogen, vitronectin, and VWF/botrocetin, but not on laminin. (A) Bone marrow aspirates from TPO-treated mice were plated on either 100 μg/mL fibrinogen (Fg) or 50 μg/mL laminin (Lam) in the presence of 10 μM lotrafiban, an αIIbβ3 antagonist. Cells were allowed to adhere for 3 hours at 37°C prior to PBS washing and fixation. Cells were visualized with αIIb-alkaline phosphatase staining, and the total number of adherent megakaryocytes and megakaryocytes with proplatelets was assessed for each coverslip. *Significant difference between control and lotrafiban treatment; + significant difference between fibrinogen and laminin. (B) Bone marrow aspirates were plated on fibrinogen, 10 μg/mL vitronectin (Vn), or 10 μg/mL VWF plus 3 μg/mL botrocetin (to ensure megakaryocyte/VWF binding under static conditions) in the presence or absence of an anti-αIIb antibody. The total number of adherent megakaryocytes and proplatelet-bearing megakaryocytes was determined as in panel A. *Significant difference between control and anti-αIIb treatment; + significant difference between fibrinogen and the other matrices. (C) Absolute cell numbers of adherent megakaryocytes and proplatelet-bearing megakaryocytes on vitronectin, VWF/botrocetin, 100 μg/mg collagen (Col), or laminin were normalized to the absolute amounts seen on fibrinogen in each individual experiment, with equal numbers equaling 100% relative to fibrinogen (solid line). Error bars indicate SEM, n = 3.
Although untreated mice produce sufficient numbers of megakaryocytes for single-cell analysis, it was necessary to boost megakaryocyte numbers for quantitative analysis of the effect of fibrinogen on proplatelet formation. To achieve this, mice were given injections of the cytokine TPO, which is the major regulator of megakaryocyte differentiation and maturation, for 4 days using the protocol developed by Kaushansky and colleagues.27 Following TPO injection, bone marrow aspirates were plated on fibrinogen and other matrices and allowed to adhere for 3 to 4 hours. Following adhesion, the cells were stained for αIIb and the entire coverslip was scanned to count both the total number of adherent megakaryocytes and those which had formed proplatelets. From this count, the percentage of megakaryocytes expressing proplatelets could be determined, serving as a way to normalize the assay and allow interexperimental comparison. In this time period, approximately 14% of the megakaryocytes on fibrinogen formed proplatelets, whereas significantly fewer proplatelet-bearing megakaryocytes were seen on laminin (P = .006), vitronectin (P < .001), or VWF plus botrocetin (P = .008; Figure 2A-B). No proplatelets were observed on collagen or on BSA (data not shown).
Fibrinogen-induced proplatelet formation was dependent on αIIbβ3, as treatment of megakaryocytes with either the αIIbβ3 antagonist lotrafiban (P = .004; Figure 2A) or the αIIb-blocking antibody MWReg30 (P < .001; Figure 2B) heavily reduced the percentage of proplatelet-expressing megakaryocytes on fibrinogen. In addition, no proplatelets were seen with αIIb-/- megakaryocytes plated on fibrinogen (data not shown). While proplatelet formation on laminin was not significantly affected in the presence of the αIIbβ3 antagonist lotrafiban (P = .43; Figure 2A), the levels of proplatelet formation on vitronectin and VWF/botrocetin were also reduced in the presence of an αIIbβ3 blocker (Figure 2B), suggesting that the interaction with αIIbβ3 is at least partially responsible for proplatelet formation on these matrices. The number of megakaryocytes on fibrinogen displaying proplatelets compares favorably with proplatelet development from megakaryocytes in tissue culture-dishes stimulated with serum,22 suggesting that fibrinogen is a powerful stimulator of proplatelet development.
In order to investigate whether the differences observed in proplatelet formation between matrices were dependent on the degree of megakaryocyte adhesion, the relative levels of megakaryocyte adhesion and proplatelet formation were analyzed in comparison with results for fibrinogen. These studies revealed that changes in the level of adhesion between fibrinogen and other matrices did not correspond to changes in numbers of megakaryocytes bearing proplatelets. For example, the level of megakaryocyte adhesion supported by vitronectin was approximately 29% of that on fibrinogen, whereas the degree of proplatelet formation was less than 3% of that seen on fibrinogen (P < .001). Further, a significant difference between the levels of adhesion and proplatelet formation were seen on laminin in comparison with fibrinogen (P = .04; Figure 2C). Strikingly, a significantly greater number of megakaryocytes adhered to collagen relative to fibrinogen, yet no proplatelets were observed (Figure 2C). Moreover, a similar analysis with fibrinogen adhesion and proplatelet formation showed that lotrafiban or anti-αIIb treatment reduced the number of megakaryocytes adhering on fibrinogen by 63% and 83%, respectively, while proplatelet formation was inhibited by 99% with each treatment (Table 1), further suggesting that adhesion levels are not directly correlated to proplatelet formation.
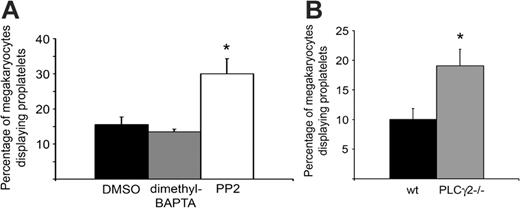
As integrin αIIbβ3 is a critical component of proplatelet formation on fibrinogen, we explored whether downstream signaling events from the integrin influence proplatelet formation. Strikingly, bone marrow aspirates treated with the Src family kinase inhibitor PP2 showed a significant increase in the percentage of megakaryocytes with proplatelets (P = .04; Figure 3A), despite the fact that Src kinases play a critical, proximal role in outside-in signaling by integrin αIIbβ3.28 Furthermore, potentiation of proplatelet formation on fibrinogen was also seen in murine megakaryocytes deficient in PLCγ2 (P = .03; Figure 3B), which also plays a role in outside-in signaling by the integrin.29,30 In both cases, there was no overall change in morphology of the megakaryocytes that were forming proplatelets in the presence of PP2 or absence of PLCγ2 relative to controls (not shown). Conversely, inhibition of intracellular Ca2+ elevation by integrin αIIbβ3 using the intracellular chelator dimethyl BAPTA-am had no significant effect on the number of megakaryocytes forming proplatelets on fibrinogen (Figure 3A) or on their morphology (not shown). Interestingly, the effect of PP2 is consistent with the findings of Lannutti and colleagues,31 who demonstrated that Src inhibition leads to megakaryocyte maturation. Together, these observations suggest that downstream signaling events through Src and PLCγ2 from αIIbβ3 may inhibit proplatelet formation independent of Ca2+ release.
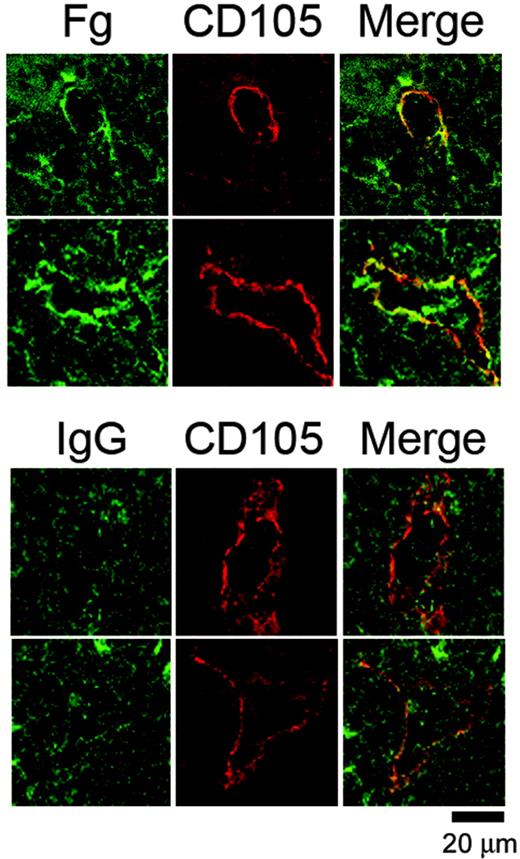
In view of the ability of fibrinogen to regulate proplatelet formation in vitro, we investigated whether megakaryocytes are likely to come into contact with the matrix protein by staining for fibrinogen in bone marrow sections. To our knowledge, there are no previous reports on the localization of fibrinogen within the bone marrow stroma, although the presence of laminin at bone marrow sinusoids has been described.32 Frozen femur sections (8 μm) were stained for fibrinogen and CD105, a marker for sinusoid endothelium.33 This revealed that fibrinogen and CD105 were colocalized at several places in the vascular sinusoids, whereas there was very little fibrinogen distributed through the rest of the bone marrow because both the control and fibrinogen-stained samples displayed comparable background staining (Figure 4). In comparison, there was no apparent colocalization of CD105 and an irrelevant IgG (Figure 4, lower panel). The localization of fibrinogen to vascular sinusoids indicates that it is ideally positioned to influence proplatelet formation and subsequent entry of new generated platelets into the vasculature.
αIIbβ3 downstream signaling events have variable contributions to proplatelet formation on fibrinogen. (A) Bone marrow aspirates from TPO-treated mice were treated with either DMSO, 20 μM dimethyl BAPTA-am, or 10 μM PP2, and allowed to adhere to fibrinogen for 4 hours at 37°C. Results were tabulated as a percentage of the total megakaryocytes that displayed proplatelets. Error bars represent SEM, n = 3. (B) Bone marrow aspirates from non-TPO-treated PLCγ2-/- mice or littermate wild-type controls were allowed to adhere to fibrinogen for 3 hours at 37°C. Results were tabulated as a percentage of the total megakaryocytes that displayed proplatelets. Error bars represent SEM, n = 4. *Significant difference compared with controls.
αIIbβ3 downstream signaling events have variable contributions to proplatelet formation on fibrinogen. (A) Bone marrow aspirates from TPO-treated mice were treated with either DMSO, 20 μM dimethyl BAPTA-am, or 10 μM PP2, and allowed to adhere to fibrinogen for 4 hours at 37°C. Results were tabulated as a percentage of the total megakaryocytes that displayed proplatelets. Error bars represent SEM, n = 3. (B) Bone marrow aspirates from non-TPO-treated PLCγ2-/- mice or littermate wild-type controls were allowed to adhere to fibrinogen for 3 hours at 37°C. Results were tabulated as a percentage of the total megakaryocytes that displayed proplatelets. Error bars represent SEM, n = 4. *Significant difference compared with controls.
Fibrinogen is localized at vascular sinusoids in the bone marrow. Representative images from frozen murine femur sections costained with an FITC-conjugated anti-mouse fibrinogen antibody or an IgG control antibody together with a biotin-linked anti-CD105 antibody preincubated with Texas Red-linked streptavidin. The tissue sections were visualized via confocal microscopy. Note that while sinusoidal gaps are visible in the control stain, there is no enrichment of green staining around the periphery of the sinusoid. Results are representative of 4 separate experiments.
Fibrinogen is localized at vascular sinusoids in the bone marrow. Representative images from frozen murine femur sections costained with an FITC-conjugated anti-mouse fibrinogen antibody or an IgG control antibody together with a biotin-linked anti-CD105 antibody preincubated with Texas Red-linked streptavidin. The tissue sections were visualized via confocal microscopy. Note that while sinusoidal gaps are visible in the control stain, there is no enrichment of green staining around the periphery of the sinusoid. Results are representative of 4 separate experiments.
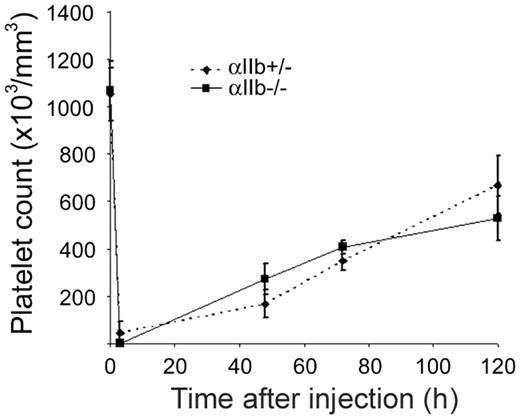
The physiologic significance of the present observations was investigated in mice deficient in integrin αIIbβ3. In agreement with previous studies,25,34 we found that αIIb-/- mice had a similar number of platelets compared with wild-type controls (Table 2). However, the injection of TPO caused a substantial increase in platelet counts in both wild-type and αIIb-/- mice, although the effect in the αIIb-/- mice was significantly lower than that in wild-type controls (Table 2). This study therefore reveals a novel role for integrin αIIbβ3 in supporting platelet formation in mice that have normal levels of platelets when treated with the cytokine, TPO. In order to investigate whether αIIb also contributes to platelet formation under thrombocytopenic conditions where TPO is also elevated,35 αIIb-/- mice and heterozygous littermate controls were given injections of anti-GPIbα to induce thrombocytopenia, and the recovery in platelet counts were monitored. Surprisingly, no effect on platelet recovery was observed after immune-induced thrombocytopenia (Figure 5). Additionally, platelet recovery in αIIb+/+ mice also did not demonstrate a change in the rate of platelet recovery (data not shown), thereby demonstrating that other factors regulate platelet formation and release into the vasculature under this condition.
Discussion
The present study demonstrates that the interaction of fibrinogen with αIIbβ3 is capable of stimulating proplatelet development in freshly harvested primary megakaryocytes, thereby building on recent observations that matrix proteins play important roles in regulating platelet formation. Importantly, within this group, the greatest proportion of proplatelet-forming megakaryocytes was seen on fibrinogen relative to a number of other surfaces, including laminin, vitronectin, and VWF/botrocetin. This observation is particularly striking compared with collagen, which stimulates a high level of megakaryocyte adhesion but is unable to support proplatelet formation under the conditions used in this study. The importance of the present observations is illustrated by the presence of fibrinogen in regions of the bone marrow that are associated with platelet release and by the reduction in platelet counts in αIIb-/- mice treated with TPO. On the other hand, the observation that mice deficient in the αIIb-/- integrin subunit have normal numbers of platelets in the absence of TPO treatment, and that platelet recovery in αIIb-/- mice following immune-induced thrombocytopenia is not significantly different from that of controls illustrates that other mechanisms mediate proplatelet formation in the absence of theαIIbβ3 integrin. Such mechanisms could be mediated by other matrix proteins. Overall, the present results demonstrate a novel role for the interaction of fibrinogen with integrin αIIbβ3 in supporting proplatelet formation, which is in addition to the role of the integrin in mediating platelet adhesion/aggregation and development of platelet progenitor cells.25
Although megakaryocytes can grow and differentiate in suspension, the bone marrow environment is rich in matrix proteins, and it is therefore not surprising that these proteins play a role in the stages of megakaryocyte development. Indeed, a number of earlier studies indicated that megakaryocytes form proplatelets upon adhesion to extracellular matrices, including reconstituted basement membrane,36 rehydrated collagen gels,37 and collagen-derived peptides.23 Furthermore, addition of matrix directly to existing megakaryocyte cultures also influences proplatelet development,22 suggesting that proplatelet formation may be regulated by matrix-receptor signaling in addition to providing a suitable surface for attachment. In support of this, Sabri et al23 have described signaling events by the collagen receptor α2β1 that inhibit proplatelet formation, suggesting that positive and negative regulation of proplatelets via surface contact is possible. Indeed, the appropriate spatial and temporal regulation of proplatelet formation may account for a number of differences in the present observations to those in the literature, which have been made primarily using megakaryocytes that have been purified from bone marrow and maintained in culture. Thus, for example, collagen has previously been proposed to support proplatelet formation in some22 but not all studies (Sabri et al23 and present study), although this could also be related to the type of collagen that has been used.
Platelet recovery after immune-induced thrombocytopenia is similar in αIIb+/- and αIIb-/- mice. Whole-blood platelet counts from αIIb+/- (♦, dashed line) or αIIb-/- (▪, solid line) mice were obtained prior to immune-induced thrombocytopenia (time point 0, n = 6), followed by injection of 2 μg/g anti-GPIbα antibody. Whole-blood platelet counts were then collected at the indicated time points after injection. Error bars represent SEM; n = 2 for 3-, 48-, and 72-hour time points, n = 4 for 120-hour time point.
Platelet recovery after immune-induced thrombocytopenia is similar in αIIb+/- and αIIb-/- mice. Whole-blood platelet counts from αIIb+/- (♦, dashed line) or αIIb-/- (▪, solid line) mice were obtained prior to immune-induced thrombocytopenia (time point 0, n = 6), followed by injection of 2 μg/g anti-GPIbα antibody. Whole-blood platelet counts were then collected at the indicated time points after injection. Error bars represent SEM; n = 2 for 3-, 48-, and 72-hour time points, n = 4 for 120-hour time point.
In the case of the present study, it is possible that the αIIbβ3 dependency is simply a consequence of matrix adhesion, as inhibiting activation of Src kinases and PLCγ2, which both play a proximal role in αIIbβ3 signaling,28-30 potentiates formation of proplatelets. On the other hand, the differences in the degree of proplatelet formation on fibrinogen relative to other surfaces implies that specific matrices are capable of inducing differing levels of proplatelets. It is therefore possible that the effect of PP2 (and perhaps also PLCγ2) is related to the observation of Lannutti et al31 that Src kinases play a role in inhibiting megakaryocyte maturation. Thus, the present observations are consistent with the possibility that a threshold of megakaryocyte maturation is necessary to achieve proplatelet extension.
Interestingly, in light of the present observations, there was no significant reduction in the present study of the number of platelets in αIIb-/- mice, a result that is in agreement with 2 previous reports.25,34 In addition, although fibrinogen-deficient mice showed a reduction in the number of circulating platelets, this value was not statistically significant.38 Glanzmann thrombocythemia patients also do not show evidence of reduced platelet counts,39 while cases of αIIb antagonism resulting in reduced platelet counts are more likely the result of immune-mediated clearance of platelets.40 Nevertheless, the present study has shown a reduction in platelet counts in αIIb-/- mice given injections of TPO. Although the full significance of this result is unclear, it may simulate a physiologic response to replenish circulating platelets, similar to the hematopoietic-promoting effects seen with mice given injections of TPO after cytotoxic cell treatment.27 Therefore, under normal physiologic circumstances, the importance of αIIbβ3-fibrinogen interactions may be evident only under certain conditions, possibly because of redundancy with other matrix proteins. This is further emphasized by the observation of normal recovery in platelet counts in response to immune-induced thrombocytopenia, thereby illustrating that several mechanisms can work toward a common outcome of platelet production. The present results strongly suggest that fibrinogen is one of these potential mechanisms, with the localization of fibrinogen to bone marrow sinusoids placing the matrix protein in an ideal position to support proplatelet formation and platelet release.
While our findings have shed new light on what factors influence proplatelet formation, an obvious drawback of the current study is its reductionist approach. While some matrices are clearly stimulating proplatelet growth, the complex stromal mixture of matrix proteins, soluble factors, and other cells are all likely to work in tandem to influence the transition of megakaryocytes to proplatelets. Further research using models that incorporate more of the complexity of the bone marrow stroma will help elucidate the environmental regulation of megakaryocytes into platelets, aided by our findings that suggest a differential contribution from individual matrix proteins.
Prepublished online as Blood First Edition Paper, May 2, 2006; DOI 10.1182/blood-2005-11-011957.
Supported by the British Heart Foundation (BHF). S.P.W. holds a BHF Chair.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Mammoud Khan, Dr Rudi Ganz, Ewan Ross, and Prof Chris Buckley for their assistance with histology; Dr Andrew Pearce, Craig Hughes, Shirley Peach, and Ian Ricketts for their assistance with mice; Dr Owen McCarty for microscope assistance, and Prof Jon Frampton and Dr Stephanie Dumon for valuable discussion.