Abstract
Platelet-derived growth factor BB (PDGF-BB) has been assigned a critical role in vascular stability by promoting the recruitment of PDGF receptor-β–expressing perivascular cells. Here we present data indicating that early hematopoietic/endothelial (hemangio) precursors express PDGFR-β based on coexpression with CD31, vascular endothelial growth factor receptor-2, and CD41 in 2 models: mouse yolk sac (embryonic day 8 [E8]) and differentiating mouse embryonic stem cells (embryoid bodies). Expression of PDGFR-β on hemangioprecursor cells in the embryoid bodies gradually disappeared, and, at E14, expression appeared on perivascular cells. Activation of the PDGFR-β on the hemangioprecursors accelerated the differentiation of endothelial cells, whereas differentiation of the hematopoietic lineage was suppressed. In E9.5 yolk sacs derived from recombinant mice expressing kinase-active PDGFR-β with an aspartic acid to asparagine (D894N) replacement in the kinase activating loop and from mice with ubiquitous expression of PDGF-BB driven by the Rosa26 locus, the number of CD41-expressing early hematopoietic cells decreased by 36% and 34%, respectively, compared with staged wild-type littermates. Moreover, enhanced vascular remodeling was evident in the Rosa26–PDGF-BB yolk sacs. We conclude that PDGFR-β is expressed on early hemangioprecursor cells, regulating vascular/hematopoietic development.
Introduction
Endothelial cells derive from mesodermal precursors that appear in the yolk sac and develop into structures denoted blood islands during embryonic day 7 (E7) to E7.5 in the mouse. Endothelial precursor cells, or angioblasts, make up the outer cell layer of the blood islands, whereas cells in the center mature into hematopoietic cells.1 In a process denoted vasculogenesis, angioblasts form a primitive vascular plexus2,3 that becomes remodeled and pruned through angiogenesis, which is the formation of new blood vessels from existing capillaries.
Growth factors direct the development of the vasculature. Vascular endothelial growth factor A (VEGF-A) and its receptor VEGFR-2 (alternatively denoted Flk-1 or KDR to indicate murine or human species, respectively) are known to be critical in vascular development. Ablation of genes encoding either VEGF-A or VEGFR-2 leads to early embryonic death because of lack of proper differentiation or proper localization of endothelial cells4-6 (for a review, see Olsson et al7 ). Embryonic stem (ES) cells expressing VEGFR-2 differentiate into endothelial cells when exposed to VEGF-A,8 whereas treatment with platelet-derived growth factor BB (PDGF-BB) promotes differentiation into mural cells.9,10 PDGF-BB has also been ascribed an important role in the maturation and remodeling of vessels during later stages of development and in adult angiogenesis through the recruitment of pericytes.11,12
PDGF is a heparin-binding growth factor13-15 of disulfide-bonded A, B, C, and D polypeptides that assemble into the homodimers PDGF-AA, -BB, -CC, and -DD.16 The A and B polypeptides also form heterodimers, denoted PDGF-AB. PDGF transduces cellular responses by binding to 2 related protein tyrosine kinase receptors, PDGF receptor-α and -β (PDGFR-α and -β). PDGF-BB binds to PDGFR-α and -β with similar affinity, whereas PDGF-AA binds only to PDGFR-α.17
Binding of PDGF to its receptors leads to activation of the receptor tyrosine kinase and to subsequent initiation of cytoplasmic signal transduction pathways, in turn leading to the migration, proliferation, and differentiation of PDGF-responsive cell types. PDGF receptors are present on connective tissue cells such as fibroblasts and smooth muscle cells, but they have also been detected on other cell types.18 Through the production of PDGF-BB, endothelial cells recruit PDGFR-β–expressing pericytes. Vessels in mice homozygous for gene inactivation, leading to loss of either PDGF-BB or PDGFR-β, essentially lack pericyte coating in organs such as the brain and adipose tissue at E16.5.19-22 Pdgf-b–/– embryos die at birth of cardiovascular dysfunction consisting of organ-specific hemorrhages and edema caused by microaneurysms and dilated vessels as a consequence of impaired pericyte coverage.20,22
In this report, we show that PDGF-BB/PDGFR-β activity regulates vascular development in differentiating ES cells and in yolk sacs from mice with an activating PDGFR-β mutation, D894N,23 or with ubiquitous Rosa26-driven expression of PDGF-BB. This effect is most likely exerted directly through PDGFR-β–expressing progenitor cells. In all models examined, enhanced PDGFR-β activity led to decreased numbers of hematopoietic precursor cells. Moreover, increased differentiation of endothelial cells and vascular remodeling was evident in PDGF-BB–treated embryoid bodies and in Rosa26–PDGF-BB yolk sacs. These data demonstrate a novel role for PDGF-BB/PDGFR-β in the differentiation of early hematopoietic/endothelial (hemangio) precursor cells.
Materials and methods
Cell culture
ES cell lines R124 and Pdgfr-β–/–19 were kind gifts from Dr A. Nagy (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON, Canada) and Dr P. Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA). ES cells were aggregated in hanging drops8 in the absence of leukemia inhibitory factor (LIF) (day 0), and at day 4, aggregates were seeded out on tissue culture plastic or in 8-chamber glass wells in the presence or absence of growth factors (30 ng/mL; Peprotech, Rocky Hill, NJ), as indicated. For all conditions, at least 6 to 8 bodies were analyzed in 3 or more independent experiments. Representative and highly reproducible results are shown.
Immunohistochemistry
Embryoid bodies were fixed in zinc buffer (200 mM Tris HCl, 150 mM NaCl, containing 37 mM zinc chloride, 23 mM zinc acetate, 3.2 mM calcium acetate, and 0.2% Triton X-100; pH 6.6). For chromogen staining, endogenous peroxidase activity was blocked by treating fixed cultures in 3% H2O2/methanol followed by incubation in blocking reagent (TNB; Perkin Elmer Life Sciences, Shelton, CT). After incubation with primary antibody against CD31 (platelet-endothelial cell adhesion molecule-1 [PECAM-1]; BD Biosciences, Franklin Lakes, NJ), cultures were washed several times with Tris-buffered saline (TBS)/75 mM NaCl/0.2% Tween-20 (TNT) followed by incubation with biotinylated goat anti–rat IgG (Vector Laboratories, Burlingame, CA) diluted in TNB. After several washes with TNT, cultures were incubated with streptavidin–horseradish peroxidase (HRP). Immune reactivity was visualized by standardized AEC kit (Vector laboratories). Slides were mounted with Ultramount aqueous mounting medium (DAKO, Carpinteria, CA).
For fluorescent staining, embryoid bodies were fixed in zinc buffer or in ice-cold ethanol/acetone 1:1 (vol/vol; for the PDGFR-β antibody) followed by blocking in TNT containing 10% goat serum (Sigma, Chicago, IL) and incubation with primary antibodies CD31, VEGFR-2, CD41 (BD Biosciences), vascular endothelial (VE)–cadherin (R&D Systems, Minneapolis, MN), PDGFR-β (97A; a kind gift from Dr Andrius Kazlauskas, Schepens Eye Research Institute, Harvard Medical School, Boston, MA),25 and fluorescein isothiocyanate (FITC)–conjugated mouse monoclonal antibody against α-smooth muscle actin (α-SMA; Sigma). After several washes with TNT, cultures were incubated with appropriate secondary antibodies diluted in blocking buffer (Alexa; Molecular Probes, Eugene, OR).
Immunoblotting
Cultures were lysed in Nonidet P40 (NP40) lysis buffer with protease inhibitors and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, followed by electroblotting to Hybond-C extra membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blocked in TBS/0.2% Tween-20 (TBS-T)/5% nonfat milk, and antibodies against α-SMA, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), CD31, and PDGFR-β were diluted in blocking solution and incubated with the membrane overnight. After washing, immune reactivity was visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech).
Neutralization of PDGFR-β
Embryoid bodies formed from wild-type ES cells were seeded on glass slides in the presence of PDGF-BB (30 ng/mL). At day 6, fresh medium was added, complemented with the PDGFR-β neutralizing antibody CD140b (BD PharMingen, San Diego, CA), or with control rabbit IgG (DAKO), each at 30 μg/mL. At day 8, the embryoid bodies were fixed and stained using goat anti–VE-cadherin (R&D Systems).
Embryoid body angiogenic sprouting assay
Embryoid bodies of wild-type or Pdgfr-B–/– origin, were placed in a solidified collagen I matrix composed of Ham F12 medium (PromoCell GmbH, Heidelberg, Germany), 6.26 mM NaOH, 20 mM HEPES, 0.117% NaHCO3, 1% Glutamax-I (Gibco, Grand Island, NY), and 1.5 mg/mL collagen I (Cohesion Technologies; Angiotech Pharmaceuticals, Vancouver, BC, Canada) at day 4. Medium containing 30 ng/mL VEGF-A165 was replaced at day 8. At day 10, cultures were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS). Samples were further blocked and permeabilized in 3% bovine serum albumin (BSA) and 0.2% Triton X-100 in PBS, followed by sequential overnight incubations with primary (anti-CD31 and anti–α-SMA) and secondary antibodies.
Magnetic cell sorting
Embryoid bodies (150-200) were dissociated at 37°C by incubation in 2.5 mg/mL collagenase and cell dissociation solution (Sigma), further dispersed by passage through an 0.8-mm needle, and cleared by passage through a 40-μm strainer (BD Biosciences), to remove cell clusters and debris. The flow-through was incubated on ice with a mixture of rat anti-CD31 FITC-conjugated antibody and rat anti–VEGFR-2 phycoerythrin (PE)–conjugated antibody (BD Biosciences) in PBS/2 mM EDTA, 0.5% BSA, and 5% goat serum (Sigma), before incubation with goat anti–rat IgG–coated magnetic microbeads (Miltenyi Biotec). CD31+ and VEGFR-2–positive cells were purified using MS separation columns (Miltenyi Biotec). To increase the quality and purity of the cell preparation, the column purification step was repeated. Homogeneity of the cell preparation for CD31 and VEGFR-2 expression was verified by flow cytometry and semiquantitative PCR.
Real-time and semiquantitative PCR analysis
Total RNA was extracted from embryoid bodies at day 8 with the RNeasy mini kit (Qiagen, Valencia, CA). Contaminating genomic DNA was digested with DNAse I (Amersham Pharmacia Biotech), and 1 μg total RNA was used for cDNA synthesis using oligo dT primers and the Advantage RT-for-PCR-Kit (Clontech, Palo Alto, CA). Primers were made for α-fetoprotein, α-SMA, β-actin, CD31, CD41, CD45, PDGF-B, PDGFR-α, PDGFR-β, RGS-5, Tal-1, VE-cadherin, VEGF-A, and VEGFR-2 (Table 1). β-Actin was used as an endogenous reference, and non–reverse-transcribed RNA was used as a negative control. Tenfold dilutions of target gene and β-actin PCR products were included to monitor amplification accuracy. PCR samples containing cDNA (6 individual preparations; Figure 1) primers (0.25 μM final concentration) and 2 × SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) were run in triplicate on an ABI Prism 7700 instrument (Applied Biosystems) with an initial 10-minute activation at 95°C, followed by 45 cycles at 95°C for 15 seconds and 60°C for 1 minute. Threshold cycle (CT) value was calculated, and transcript levels were then normalized against β-actin levels. Changes in transcript levels were expressed as mean ± SD fold induction compared with those left untreated. Real-time PCR analysis of PDGFR-β, VEGFR-2, and VE-cadherin transcript expression in purified endothelial cells derived from 6-, 8-, and 14-day-old embryoid bodies (PDGF-BB–treated or untreated) was performed on 3 different cell preparations. Mean ± SD fold inductions are shown. Identical conditions were used for semiquantitative PCR and were run for 30 cycles; thereafter, samples were separated by agarose gel electrophoresis. Real-time PCR analysis of Tal-1, CD41, and CD45 transcript expression was performed on embryoid bodies treated or not treated with PDGF-BB or VEGF-A for 6, 8, and 14 days, and PCR conditions for Tal-1 were set to an initial 10-minute activation at 95°C, followed by 45 cycles at 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 1 minute. Results of expression levels at day 8 are shown as mean ± SD fold induction for 3 individual preparations.
Yolk sac immunostaining
Yolk sacs from E8 mouse embryos, strain DBA, were freeze-sectioned, fixed in ice-cold methanol, and analyzed by immunofluorescent staining using the anti-CD31, anti–VEGFR-2, and anti–PDGFR-β antibody 97A described. A biotin step, followed by incubation with streptavidin-488 (Molecular Probes), was included for the PDGFR-β antibody.
E9.5 yolk sacs from SvJ129 Rosa26-Pdgf-b (A.A., P.L., J.N., C.B., manuscript in preparation) and C57BL/6 D849N Pdgfr-β mice23 were staged by somite counting and were processed by 30-minute fixation in 4% paraformaldehyde, followed by washes in PBS. Yolk sacs were blocked for 1 hour in TBS-T, 3% BSA followed by incubation overnight at 4°C with primary antibodies directed against CD41 (BD Biosciences) or VEGFR-2 (R&D Systems), diluted in TBS-T, 3% BSA. A detailed characterization of this recombinant mouse will appear elsewhere (A.A., P.L., J.N., C.B., manuscript in preparation). Briefly, Rosa26-PDGF-BB mice were generated by targeted mutagenesis of the Rosa26 locus, as described,26 using a Rosa26 targeting vector with an inserted human Pdgf-b cDNA sequence preceded by a loxP-flanked stop-sequence. Activation of PDGF-BB expression through deletion of the stop-sequence was achieved by crossing with Tie2-Cre mice.27 Endothelial cell–specific deletion was achieved in the first generation, and ubiquitous deletion of the stop sequence was achieved in a proportion of the animals of the second generation because of Tie2-Cre activity during oogenesis. Only embryos ubiquitously expressing PDGF-BB were used. After incubation with secondary antibodies in TBS-T/3% BSA (Alexa 488 and 568; Molecular Probes) and subsequent washes in TBS-T, yolk sacs were mounted in Fluoromount-G (Southern Biotechnology, Birmingham, AL). Incubation with secondary antibodies alone was used as a control for the specific reactivity of the primary antibodies. CD41+ cells were counted under 20 × magnification. Results are shown as mean ± SD fold induction, and P values are indicated.
Microscopy
Specimens were inspected under an Eclipse E1000 microscope (Nikon, Tokyo, Japan; Figures 1, 2, 3, 5) or an LSM 510 META confocal laser-scanning microscope with an inverted microscope (Carl Zeiss International, Oberkochen, Germany; Figures 6 and 7). The following objectives were used: Nikon Plan Apochromat 2×/0.1, 4×/0.2, 10×/0.45, 20×/0.75, 40×/0.95, and 60×/1.4 oil immersion; Zeiss confocal Plan Neofluar 40×/1.3 oil immersion 2 and Plan Apochromat 63×/1.4 oil immersion. Microphotographs were captured using a Nikon Eclipse DXM 1200 camera. The following software programs were used: Laser Scanning Microscope LSM 510 version 3.2 (Zeiss) and Easy Image 2000 Analysis version 2.7.4.03 (www.easyimagetools.com). Processing of microphotographs was done using Adobe Photoshop version 8.0.1 and Adobe Illustrator version 11.0.0 (Adobe Systems, San Jose, CA).
Flow cytometry
Embryoid bodies (E8) were dispersed into single-cell suspension by incubation in collagenase (2.5 mg/mL; Sigma) and cell dissociation solution (Sigma) and were fixed in zinc buffer at a concentration of 1 × 106 cells/mL. Fixed cultures were washed in TBS and then incubated in TBS-T, 10% goat serum, and 10 μg/mL antibodies against CD31 or VE-cadherin (kind gift from the late Rupert Timpl, Max-Planck Institute, Munich, Germany). After several washes in TBS-T, fixed cells were incubated with Alexa 568 goat anti–rat or Alexa 488 goat anti–rabbit IgG secondary antibodies (Molecular Probes) diluted in TBS-T. Cells were then washed and analyzed by flow cytometry (FACS Vantage SE; BD Biosciences).
Statistical analysis
Immunostainings and immunoblots show representative results from experiments repeated at least 3 times. Quantification of vascular area and length was performed on 10 embryoid bodies per condition and is given as mean ± SD. Real-time PCR data of transcript levels in Figure 1 are given as the mean ± SD induction level of PDGF-BB–treated embryoid bodies of 6 individual experiments compared with basal control (set to 1.0). Estimation of PDGFR-β and VEGFR-2 transcript levels in purified endothelial cells are given as mean ± SD induction level of PDGF-BB–treated cells compared with control of 3 individual experiments (Figure 4). Tal-1, CD41, and CD45 transcript analysis (Figure 5) are given as the mean ± SD induction level in PDGF-BB– or VEGF-A–treated embryoid bodies for 3 individual experiments compared with basal. CD41+ cells in yolk sacs were counted at 20 × magnification in at least 6 individual fields per yolk sac. Yolk sacs from 8 to 10 embryos derived from 3 different breedings were included for each recombinant and wild-type strain; all embryos were at a somite stage between 20 and 25 (compatible with E9.5). Results are shown as mean ± SD fold induction, and P values are indicated.
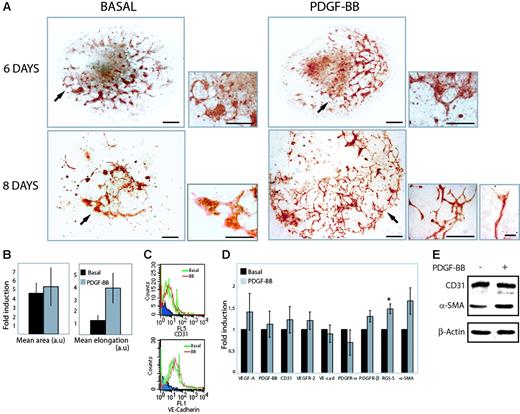
PDGF-BB promotes vascular development and sprouting angiogenesis. (A) Embryoid bodies were cultured in 15% FBS for 6 or 8 days in the absence (basal) or presence of 30 ng/mL PDGF-BB. Endothelial cells were visualized by immunohistochemical staining for CD31 (red). Arrows in the larger panels at the left (bars represent 300 μm) indicate areas shown at higher magnification in the smaller panels to the right (bars represent 100 μm). For the 8-day PDGF-BB–treated culture, a higher magnification is also shown at the bottom far right to demonstrate sprouting and a tip cell extending filopodia (bar represents 20 μm). For all conditions, 6 to 8 bodies were analyzed in 3 or more independent experiments. (B) Quantification of the mean area and length estimated in 10 individual embryoid bodies for each condition, stained immunohistochemically for CD31. Results are expressed as fold induction over basal (mean ± SD). (C) FACS analysis of CD31 and VE-cadherin–expressing cells in collagenase-dispersed embryoid bodies treated with PDGF-BB (BB) or not treated (basal). FL5 (CD31), FL1 (VE-cadherin). Blue indicates reactivity of the secondary antibody alone. (D) Real-time PCR analysis of VEGF-A, PDGF-B, CD31, VEGFR-2, VE-cadherin (VE-cad), PDGFR-α, PDGFR-β, RGS-5, and α-SMA expression in embryoid bodies cultured for 8 days in the absence (basal) or presence of PDGF-BB. Data are given as mean ± SD and are based on analyses of 6 individual RNA preparations. *P < .001, paired Student t test. (E) Immunoblotting for expression of CD31 and α-SMA in R1 embryoid bodies cultured for 8 days in the absence (–) and presence (+) of PDGF-BB.
PDGF-BB promotes vascular development and sprouting angiogenesis. (A) Embryoid bodies were cultured in 15% FBS for 6 or 8 days in the absence (basal) or presence of 30 ng/mL PDGF-BB. Endothelial cells were visualized by immunohistochemical staining for CD31 (red). Arrows in the larger panels at the left (bars represent 300 μm) indicate areas shown at higher magnification in the smaller panels to the right (bars represent 100 μm). For the 8-day PDGF-BB–treated culture, a higher magnification is also shown at the bottom far right to demonstrate sprouting and a tip cell extending filopodia (bar represents 20 μm). For all conditions, 6 to 8 bodies were analyzed in 3 or more independent experiments. (B) Quantification of the mean area and length estimated in 10 individual embryoid bodies for each condition, stained immunohistochemically for CD31. Results are expressed as fold induction over basal (mean ± SD). (C) FACS analysis of CD31 and VE-cadherin–expressing cells in collagenase-dispersed embryoid bodies treated with PDGF-BB (BB) or not treated (basal). FL5 (CD31), FL1 (VE-cadherin). Blue indicates reactivity of the secondary antibody alone. (D) Real-time PCR analysis of VEGF-A, PDGF-B, CD31, VEGFR-2, VE-cadherin (VE-cad), PDGFR-α, PDGFR-β, RGS-5, and α-SMA expression in embryoid bodies cultured for 8 days in the absence (basal) or presence of PDGF-BB. Data are given as mean ± SD and are based on analyses of 6 individual RNA preparations. *P < .001, paired Student t test. (E) Immunoblotting for expression of CD31 and α-SMA in R1 embryoid bodies cultured for 8 days in the absence (–) and presence (+) of PDGF-BB.
Results
PDGF-BB induces sprouting angiogenesis through PDGFR-β in differentiating ES cells
The effect of PDGF-BB treatment on vascular development in aggregated differentiating ES cells (embryoid bodies) was analyzed by immunohistochemical staining for the endothelial cell marker CD31 (Figure 1A). We have previously shown that the embryoid body model faithfully recapitulates early vascular development.8,28 Embryoid bodies were cultured in the absence or presence of PDGF-BB for 6 or 8 days. In embryoid bodies treated for 8 days, PDGF-BB promoted a marked acceleration of vascular development and sprouting angiogenesis at the periphery of the bodies (Figure 1A, PDGF-BB) that was lacking in parallel control cultures (Figure 1A, basal).
Treatment with PDGF-BB did not affect the area of CD31+ cells at day 8, but vessel length increased in the presence of PDGF-BB (Figure 1B), indicating that the morphologic changes induced by PDGF-BB mainly consisted of a reorganization of endothelial cells from clusters of cells into vessel-like structures. That PDGF-BB treatment did not lead to an increase in endothelial cell number was further underscored by fluorescence-activated cell sorting (FACS) analyses of dispersed embryoid bodies incubated with antibodies against CD31 or VE-cadherin (Figure 1C).
The effects of PDGF-BB on expression levels of endothelial and mural cell markers in differentiating embryoid bodies (E8) were estimated by real-time PCR (Figure 1D). PDGF-BB treatment did not significantly change VEGF-A, PDGF-BB, CD31, VEGFR-2, VE-cadherin, or PDGFR-α expression. In contrast, expression of the pericyte marker RGS-5 increased significantly. There was also a clear up-regulation of PDGFR-β and α-SMA transcripts. Thus, at this time point, we did not observe changes in expression of endothelial cell markers, whereas expression of mural cell markers was elevated in PDGF-BB–treated embryoid bodies. Immunoblotting for CD31 and α-SMA further underscored this conclusion (Figure 1E).
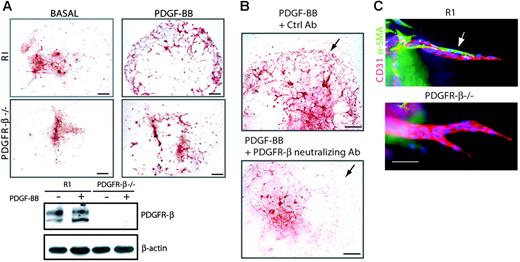
PDGF-BB promotes vascular development and sprouting angiogenesis by PDGFR-β. (A) Embryoid bodies derived from R1 or Pdgfr-β–/– stem cells were cultured for 8 days in the absence (basal) or presence of 30 ng/mL PDGF-BB. Endothelial cells were visualized by staining for CD31 (red; bars represent 300 μm). Immunoblotting for PDGFR-β was performed on embryoid bodies cultured in the absence (–) or presence (+) of PDGF-BB. (B) Embryoid bodies cultured for 8 days in the presence PDGF-BB were exposed to neutralizing PDGFR-β antibody CD140b or control nonimmune serum (Ctrl) between day 6 and day 8 of differentiation. Immunostaining for VE-cadherin showed the formation of a capillary plexus in cultures treated with the control antibody but not in cultures treated with the neutralizing antibody (arrows). Bars represent 300 μm. (C) Embryoid bodies of wild-type and Pdgfr-β–/– origin were placed in 3-D collagen gels at day 4 and cultured in the presence of VEGF-A165 (30 ng/mL) until day 10. Embryoid bodies were whole-mount stained for expression of CD31 (red), α–SMA (green), and Hoechst 33342 (blue). Bar represents 50 μm.
PDGF-BB promotes vascular development and sprouting angiogenesis by PDGFR-β. (A) Embryoid bodies derived from R1 or Pdgfr-β–/– stem cells were cultured for 8 days in the absence (basal) or presence of 30 ng/mL PDGF-BB. Endothelial cells were visualized by staining for CD31 (red; bars represent 300 μm). Immunoblotting for PDGFR-β was performed on embryoid bodies cultured in the absence (–) or presence (+) of PDGF-BB. (B) Embryoid bodies cultured for 8 days in the presence PDGF-BB were exposed to neutralizing PDGFR-β antibody CD140b or control nonimmune serum (Ctrl) between day 6 and day 8 of differentiation. Immunostaining for VE-cadherin showed the formation of a capillary plexus in cultures treated with the control antibody but not in cultures treated with the neutralizing antibody (arrows). Bars represent 300 μm. (C) Embryoid bodies of wild-type and Pdgfr-β–/– origin were placed in 3-D collagen gels at day 4 and cultured in the presence of VEGF-A165 (30 ng/mL) until day 10. Embryoid bodies were whole-mount stained for expression of CD31 (red), α–SMA (green), and Hoechst 33342 (blue). Bar represents 50 μm.
The effect of PDGF-BB was mediated by PDGFR-β, as demonstrated using ES cells established from Pdgfr-β–/– blastocysts (Figure 2A), which failed to respond to PDGF-BB with the formation of a peripheral capillary plexus. The lack of PDGFR-β expression was validated by Western blotting (Figure 2A). The involvement of PDGFR-β in the PGDF-BB–induced response was further indicated by results from treatment of PDGF-BB–induced wild-type embryoid body cultures with an anti–PDGFR-β neutralizing antibody. This treatment led to attenuated formation of the PDGF-BB–induced peripheral capillary plexus (Figure 2B). Pdgfr-β gene ablation did not impair the propensity of the ES cells to form PDGF-independent vascular structures, as inferred from the morphology of vessels in VEGF-A165–treated Pdgfr-β–/– embryoid body cultures on glass (data not shown) or from the capacity to form angiogenic sprouts in 3-D collagen gels (Figure 2C). Notably, angiogenic sprouts formed from wild-type embryoid bodies in 3-D collagen were coated with α-SMA–positive perivascular cells, whereas such perivascular cells were missing from the angiogenic sprouts formed from Pdgfr-β–/– embryoid bodies (Figure 2C).
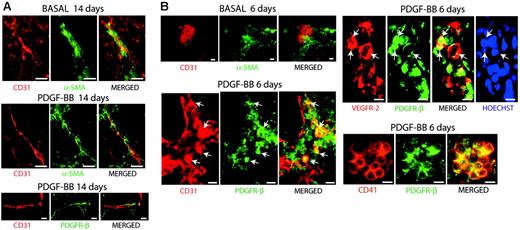
Colocalization of PDGFR-β with CD31, VEGFR-2, and CD41 in hemangioprecursor cells. (A) Embryoid bodies cultured for 14 days in the absence (top panel) or presence (middle and bottom panels) of 30 ng/mL PDGF-BB were analyzed by immunofluorescent staining for CD31 (red) and α-SMA (green) in the 2 upper panels and for CD31 (red) and PDGFR-β (green) in the bottom panel. At this late time point, PDGFR-β expression was confined to perivascular α-SMA–positive cells. (B) Embryoid bodies cultured for 6 days in the absence (top panel) or presence of 30 ng/mL PDGF-BB were analyzed for expression of CD31 (red) and α-SMA (green), CD31 (red) and PDGFR-β (green), VEGFR-2 (red) and PDGFR-β (green), and for CD41 (red) and PDGFR-β (green), as indicated. At day 6 in the presence of PDGF-BB, colocalization was evident in clusters of immature hemangioprecursor cells. Merged images are shown to the right in each panel. Where indicated, cells were treated with Hoechst 33342 to visualize nuclei. Bars represent 20 μm.
Colocalization of PDGFR-β with CD31, VEGFR-2, and CD41 in hemangioprecursor cells. (A) Embryoid bodies cultured for 14 days in the absence (top panel) or presence (middle and bottom panels) of 30 ng/mL PDGF-BB were analyzed by immunofluorescent staining for CD31 (red) and α-SMA (green) in the 2 upper panels and for CD31 (red) and PDGFR-β (green) in the bottom panel. At this late time point, PDGFR-β expression was confined to perivascular α-SMA–positive cells. (B) Embryoid bodies cultured for 6 days in the absence (top panel) or presence of 30 ng/mL PDGF-BB were analyzed for expression of CD31 (red) and α-SMA (green), CD31 (red) and PDGFR-β (green), VEGFR-2 (red) and PDGFR-β (green), and for CD41 (red) and PDGFR-β (green), as indicated. At day 6 in the presence of PDGF-BB, colocalization was evident in clusters of immature hemangioprecursor cells. Merged images are shown to the right in each panel. Where indicated, cells were treated with Hoechst 33342 to visualize nuclei. Bars represent 20 μm.
Hemangioprecursor cells express PDGFR-β during early vascular development in embryoid bodies
Immunofluorescent staining of embryoid bodies at day 14 of differentiation revealed perivascular α-SMA–positive cells adjacent to CD31+ vessel structures in the basal condition and to an increased extent wrapped around CD31+ vessel structures in PDGF-BB–treated embryoid bodies (Figure 3A, upper and middle panels). These perivascular cells expressed PDGFR-β (Figure 3A, bottom panel).
Next, we analyzed an early stage of differentiation (day 6; Figure 3B). In the absence of PDGF-BB, α-SMA–positive cells were excluded from the CD31+ cell clusters. Interestingly, in PDGF-BB–treated embryoid bodies, PDGFR-β expression was found on immature, CD31-expressing vascular precursor cells localized in blood island-like clusters (Figure 3B). Coexpression of PDGFR-β with VEGFR-2 further confirmed the identity of these cells as vascular progenitors. We also identified the coexpression of PDGFR-β with CD41, a cell surface protein corresponding to the α-chain of the platelet integrin αIIbβ3, which was recently identified as a novel marker for primitive and definitive hematopoiesis at E8.25 in the murine embryo.29 PDGFR-β expression was detected on CD41+ cells in primitive cell clusters and not in differentiated hematopoietic cells (data not shown).
Regulated expression of PDGFR-β on endothelial cells during vasculogenesis and angiogenesis
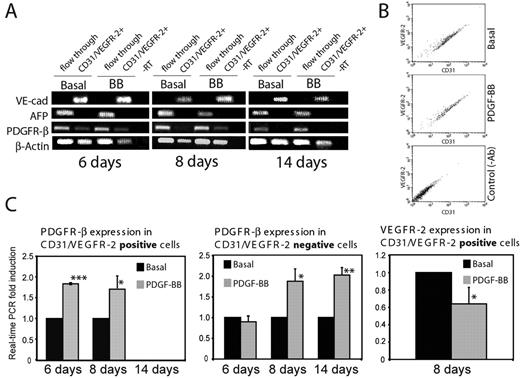
PDGFR-β levels and kinetics of expression in endothelial cells and perivascular cells were examined by RT-PCR (Figure 4A). Embryoid bodies were treated with PDGF-BB for different time periods (6, 8, or 14 days), followed by collagenase digestion and magnetic bead fractionation of cells using antibodies against CD31 and VEGFR-2. The identity of this cell population (CD31/VEGFR-2+) was verified and compared with that of the flow-through by semiquantitative PCR (Figure 4A). The purity of CD31/VEGFR-2+ cells was further demonstrated by FACS analysis, which also confirmed that CD31 and VEGFR-2 expression profiles were not affected by PDGF-BB (Figure 4B). As shown in Figure 4A, purified CD31/VEGFR-2+ cells expressed VE-cadherin but not α-fetoprotein (an endodermal marker) at all time points. PDGFR-β transcripts were present in the purified CD31/VEGFR-2+ cells at days 6 and 8 but not at day 14. PDGFR-β was expressed in nonendothelial cells (flow-through) at all time points.
Expression of PDGFR-β on CD31/VEGFR-2–positive cells at different time points. (A) Semiquantitative PCR analysis of VE-cadherin (VE-cad), αfetoprotein (AFP), PDGFR-β, and β–actin levels in immunopurified CD31/VEGFR-2–expressing cells and in the flow-through fractions derived from embryoid bodies cultured for 6, 8, or 14 days in the presence or absence of PDGF-BB. (B) Validation by FACS analyses of CD31 and VEGFR-2 expression in immunopurified fractions derived from embryoid bodies cultured for 6 days in the presence or absence of PDGF-BB. Unstained cells served as a control. (C) Real-time PCR analysis of PDGFR-β expression in CD31/VEGFR-2–positive cells and in flow-through and of VEGFR-2 expression in the CD31/VEGFR-2–positive cells derived from embryoid bodies treated or not treated with PDGF-BB, as described in panel A. Results are given as mean ± SD fold induction compared with basal of 3 separate experiments. *P < .05; **P < .008; ***P < .001.
Expression of PDGFR-β on CD31/VEGFR-2–positive cells at different time points. (A) Semiquantitative PCR analysis of VE-cadherin (VE-cad), αfetoprotein (AFP), PDGFR-β, and β–actin levels in immunopurified CD31/VEGFR-2–expressing cells and in the flow-through fractions derived from embryoid bodies cultured for 6, 8, or 14 days in the presence or absence of PDGF-BB. (B) Validation by FACS analyses of CD31 and VEGFR-2 expression in immunopurified fractions derived from embryoid bodies cultured for 6 days in the presence or absence of PDGF-BB. Unstained cells served as a control. (C) Real-time PCR analysis of PDGFR-β expression in CD31/VEGFR-2–positive cells and in flow-through and of VEGFR-2 expression in the CD31/VEGFR-2–positive cells derived from embryoid bodies treated or not treated with PDGF-BB, as described in panel A. Results are given as mean ± SD fold induction compared with basal of 3 separate experiments. *P < .05; **P < .008; ***P < .001.
We quantified PDGFR-β expression normalized to β-actin levels in the purified cell populations by real-time PCR. As seen in Figure 4C (left panel), PDGFR-β transcripts were readily detected in the CD31/VEGFR-2–expressing cells at day 6 and the expression level nearly doubled as a consequence of PDGF-BB treatment. The higher expression level of PDGFR-β in the presence of PDGF-BB remained at day 8. At day 14, we could no longer detect PDGFR-β expression in the CD31/VEGFR-2+ cell population. Similar analysis was performed on the nonendothelial cell population. In nonendothelial cells treated with PDGF-BB, the level of PDGFR-β expression did not change at day 6 compared with the basal condition, but it increased significantly at days 8 and 14 (Figure 4C, middle panel). These data demonstrate that PDGFR-β is expressed in an early vascular population in a manner regulated by PDGF-BB and that the expression decreases with time. In the nonvascular cell population, PDGF-BB also induced increased PDGFR-β expression levels that persist through day 14 of differentiation. Interestingly, PDGF-BB treatment reduced the expression of VEGFR-2 in endothelial cells on day 8 (Figure 4C, right panel). This was compatible with an accelerated differentiation process because VEGFR-2 expression is known to decrease during in vivo differentiation.30 Expression levels of VE-cadherin remained unchanged between days 6 and 14 and were not affected by PDGF-BB (data not shown).
PDGF-BB decreases the expression of markers for primitive and definitive hematopoiesis
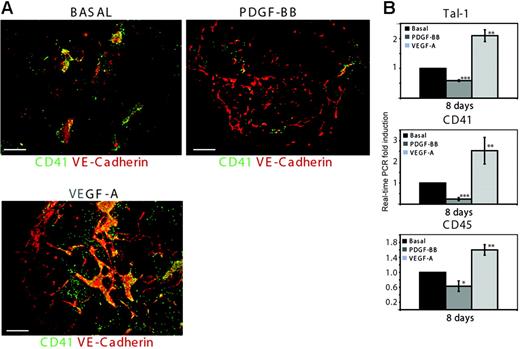
We detected PDGFR-β expression on CD41+ cells in primitive cell clusters (Figure 3B) and investigated the consequence of PDGF-BB treatment on hematopoietic development. In the basal condition in day 8 embryoid bodies, VE-cadherin–positive cells were associated with CD41-expressing hematopoietic cells (Figure 5A). In response to treatment with PDGF-BB for 8 days, CD41 expression markedly decreased while VE-cadherin–expressing cells formed a differentiated vascular plexus (Figure 5A). In agreement with the fact that VEGF-A promotes endothelial and hematopoietic development,6 treatment with VEGF-A for 8 days increased the number of CD41+ cells in the primitive VE-cadherin–positive cell structures in the center of the embryoid body. Thus, PDGF-BB appears to enhance endothelial cell differentiation whereas hematopoietic development is suppressed, as implied by the loss of CD41+ cells.
PDGF-BB induces the down-regulation of Tal-1, CD41, and CD45, markers for primitive and definitive hematopoiesis. (A) Embryoid bodies cultured for 8 days in the absence (basal) or presence of 30 ng/mL PDGF-BB or of VEGF-A165 were stained by immunofluorescence to visualize the expression of CD41 and VE-cadherin. Bars represent 300 μm. (B) Real-time PCR analysis of Tal-1, CD41, and CD45 expression in embryoid bodies cultured in the absence (basal) or presence of 30 ng/mL PDGF-BB or 30 ng/mL VEGF-A for 8 days. Results are expressed as mean ± SD fold induction of 3 separate experiments. *P < .05; **P < .005; ***P < .001.
PDGF-BB induces the down-regulation of Tal-1, CD41, and CD45, markers for primitive and definitive hematopoiesis. (A) Embryoid bodies cultured for 8 days in the absence (basal) or presence of 30 ng/mL PDGF-BB or of VEGF-A165 were stained by immunofluorescence to visualize the expression of CD41 and VE-cadherin. Bars represent 300 μm. (B) Real-time PCR analysis of Tal-1, CD41, and CD45 expression in embryoid bodies cultured in the absence (basal) or presence of 30 ng/mL PDGF-BB or 30 ng/mL VEGF-A for 8 days. Results are expressed as mean ± SD fold induction of 3 separate experiments. *P < .05; **P < .005; ***P < .001.
We further investigated the kinetics of Tal-1, CD41, and CD45 expression by real-time PCR analyses in embryoid bodies treated with PDGF-BB or VEGF-A compared with basal. Tal-1 is essential for the development of all hematopoietic lineages31-33 and is believed to be a marker for primitive and definitive hematopoiesis, whereas CD45 is expressed on all nucleated mature hematopoietic cells, such as leukocytes and monocytes, and constitutes a marker for definitive hematopoiesis.34 Real-time PCR analyses showed that PDGF-BB treatment suppressed expression levels of Tal-1 at days 6 and 8 compared with levels in parallel cultures in basal condition (Figure 5B and data not shown). CD41 and CD45 expression levels were also decreased at day 8 in response to PDGF-BB. In contrast, treatment with VEGF-A resulted in increased expression levels of all 3 hematopoietic markers. These data indicate that the commitment of progenitor cells to the hematopoietic lineage is restricted by PDGF-BB.
Role of PDGFR-β expression in early hemangioprecursor cells during vasculogenesis in the murine yolk sac
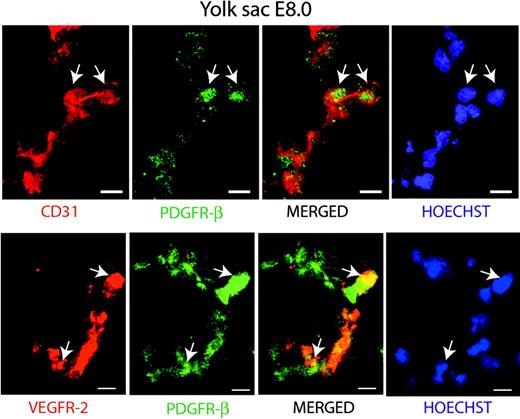
To validate the role of PDGF-BB/PDGFR-β in the guidance of early hemangioprecursor differentiation in vivo, we analyzed yolk sacs derived from E8 mouse embryos. As shown in Figure 6, we identified PDGFR-β coexpression on CD31+ (upper panel) or VEGFR-2+ (lower panel) cells in sections of wild-type yolk sacs.
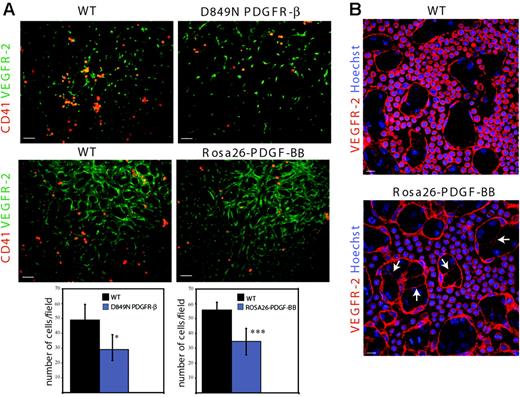
To study the consequence of PDGF-BB/PDGFR-β activity on hematopoietic/endothelial cell development in vivo, we used 2 recombinant mouse models. In one of these, an aspartic residue in the activating loop of PDGFR-β was exchanged for asparagine (D849N). In related receptor tyrosine kinases such as c-Kit and PDGFR-α, the corresponding conserved residue is known to regulate the folding of the activation loop, and mutation at this site leads to a ligand-independent increase in kinase activity.35 Indeed, biochemical analyses of D849N PDGFR-β–expressing mouse embryonic fibroblasts indicate increased kinase activity of the mutant receptor, resulting in ligand-independent activation of, for example, the phosphatidylinositol 3′-kinase pathway.23 In a second model, enhanced and ubiquitous expression of PDGF-BB in the embryo was driven by the Rosa26 locus. Immunostaining of whole-mount stage-matched E9.5 yolk sacs to visualize CD41 and VEGFR-2 expression (Figure 7A) allowed quantification of CD41+ cells in vascular structures. As shown in the lower part of Figure 7A, the number of CD41-expressing cells was significantly reduced by 36% and 34% in yolk sacs derived from D849N PDGFR-β and Rosa26-PDGF-BB mice, respectively, compared with their respective wild types. Moreover, VEGFR-2 staining of D849N PDGFR-β and Rosa26-PDGF-BB yolk sacs showed an increased number of cytoplasmic extensions indicative of vascular remodeling compared with their respective wild types (Figure 7B and data not shown). These data validate the effects of PDGF-BB we observed in the embryoid body model and strongly indicate that PDGF-BB regulates the fate of the hemangioprecursor pool by stimulating the differentiation of endothelial cells and by restricting the differentiation of hematopoietic cells.
Coexpression of PDGFR-β with CD31 and VEGFR-2 in the E8 yolk sac. Immunostaining of sections from yolk sacs derived from E8 embryos. Top panel: Visualization of cells coexpressing CD31 (red) and PDGFR-β (green) by confocal microscopy. Bottom panel: Coexpression of VEGFR-2 (red) and PDGFR-β (green) in the yolk sac. Nuclei are visualized by Hoechst 33342 staining (blue). Arrows indicate coexpressing cells. Bars represent 10 μm.
Coexpression of PDGFR-β with CD31 and VEGFR-2 in the E8 yolk sac. Immunostaining of sections from yolk sacs derived from E8 embryos. Top panel: Visualization of cells coexpressing CD31 (red) and PDGFR-β (green) by confocal microscopy. Bottom panel: Coexpression of VEGFR-2 (red) and PDGFR-β (green) in the yolk sac. Nuclei are visualized by Hoechst 33342 staining (blue). Arrows indicate coexpressing cells. Bars represent 10 μm.
Reduced number of CD41-expressing hematopoietic cells and increased remodeling in PDGF-BB/PDGFR-β–activated yolk sacs. (A) Whole-mount, somite stage–matched yolk sacs from E9.5 D849N PDGFR-β (top panels) and Rosa26-PDGF-BB (middle panels) embryos were compared with their respective stage-matched wild-type yolk sacs in immunofluorescent analyses of CD41 and VEGFR-2. Bars represent 50 μm. The number of CD41-expressing cells/field in 6 to 8 fields in the E9.5 yolk sacs (n = 8 for each of D849N PDGFR-β and wild-type C57BL/6; n = 10 for each of Rosa26-PDGF-BB and wild-type SvJ129) are shown as mean ± SD fold induction. ***P = .001. *P = .042. (B) VEGFR-2/Hoechst 33342 costaining of wild-type and Rosa26-PDGF-BB yolk sacs shows an increased number of cytoplasmic extensions (arrows) and branching of the vascular plexus. Bars represent 20 μm.
Reduced number of CD41-expressing hematopoietic cells and increased remodeling in PDGF-BB/PDGFR-β–activated yolk sacs. (A) Whole-mount, somite stage–matched yolk sacs from E9.5 D849N PDGFR-β (top panels) and Rosa26-PDGF-BB (middle panels) embryos were compared with their respective stage-matched wild-type yolk sacs in immunofluorescent analyses of CD41 and VEGFR-2. Bars represent 50 μm. The number of CD41-expressing cells/field in 6 to 8 fields in the E9.5 yolk sacs (n = 8 for each of D849N PDGFR-β and wild-type C57BL/6; n = 10 for each of Rosa26-PDGF-BB and wild-type SvJ129) are shown as mean ± SD fold induction. ***P = .001. *P = .042. (B) VEGFR-2/Hoechst 33342 costaining of wild-type and Rosa26-PDGF-BB yolk sacs shows an increased number of cytoplasmic extensions (arrows) and branching of the vascular plexus. Bars represent 20 μm.
Discussion
Endothelial cells develop in blood islands in the yolk sac from mesodermal precursor cells and form a primary endothelial plexus that later becomes remodeled into a mature network. The existence of a common endothelial and hematopoietic precursor cell, the hemangioblast, in blast colony–forming cell populations derived from differentiating ES cells and in blood islands in the yolk sac has been suggested.1,36-42 The basic helix-loop-helix factor stem cell leukemia/acute T-cell leukemia-1 (SCL/Tal-1) and VEGFR-2 have been proposed as markers for the hemangioblast.1 Tal-1 has a critical function in embryonal hematopoietic development, and ablation of Tal-1 expression leads to a developmental arrest at the hemangioblast stage.33,43 CD41 (also denoted glycoprotein IIB), a classic megakaryocyte/platelet-specific marker44,45 and a putative target for Tal-1,46 corresponds to the alpha subunit of the αIIbβ3 integrin complex. CD41 has recently also been identified on hematopoietic progenitors and has been shown to constitute a marker for primitive and definitive hematopoiesis in the mouse.29,46 VEGFR-2, on the other hand, is critical for endothelial cell function and is the earliest known marker for vascular endothelial cells.47 In the murine embryo, VEGFR-2 expression first appears in the proximal lateral mesoderm, giving rise to hematopoietic and endothelial lineages in the yolk sac.48 VEGFR-2 expression is down-regulated on hematopoietic cells29 but is retained on endothelial cells. Subsequently, other markers appear for the developing and mature vascular endothelial cells, such as VE-cadherin.49
Our data indicate that hemangioprecursor cells may express PDGFR-β based on coexpression with VEGFR-2, CD31, or CD41 on morphologically immature cells in clusters of vascular progenitors. VEGFR-2/PDGFR-β– and CD31/PDGFR-β–coexpressing cells were also identified in the yolk sac at E8 (Figure 6). The PDGFR-β–positive hemangioprecursor cell may be part of a heterogeneous, transient, hemangioblastic pool. Interestingly, the differentiation of such cells to the hematopoietic lineage was negatively regulated by PDGF-BB, reflected by the PDGF-BB–induced down-regulation of Tal-1, CD41, and CD45 expression in real-time PCR analyses of embryoid bodies (Figure 5). PCR data were validated by immunostaining, revealing that the number of CD41- and CD45-expressing cells was reduced in embryoid bodies treated with PDGF-BB. In addition, the number of CD41-expressing cells was decreased in yolk sacs of recombinant mice expressing either a ligand-independent PDGFR-β kinase or elevated levels of PDGF-BB (Figures 5, 7, and data not shown). Thus, it is possible that PDGF-BB/PDGFR-β acts as a switch toward endothelial cell development. Accordingly, endothelial cell differentiation increased in embryoid bodies, and there was increased vascular remodeling in yolk sacs from the 2 different types of in vivo models used in this study (Figures 1, 7). PDGF-BB–mediated vascular plexus formation was dependent on PDGFR-β, as demonstrated by the use of Pdgfr-β–/– ES cells and PDGFR-β–neutralizing antibodies. PDGF-BB–induced differentiation of endothelial cells could be exerted by stimulation of the PDGFR-β–expressing vascular precursor cell and possibly also by perivascular cells that are already committed to a vascular smooth muscle cell fate and that may produce factors such as VEGF-A acting on the endothelial cells. Real-time PCR analyses indicated enhanced differentiation of the perivascular smooth muscle cells by PDGF-BB, based on increased expression of RGS-5 (Figure 1). Quantification of the number of RGS-5–expressing perivascular cells by immunostaining at this point was not feasible because of the lack of reagents.
Our data demonstrated that PDGFR-β expression was lost from endothelial cells during development; we did not detect PDGFR-β expression on vascular endothelial cells in later development, either by immunostaining (Figure 3A) or by real-time PCR analysis of purified CD31/VEGFR-2–positive cells (Figure 4). During differentiation of the embryoid bodies, VEGFR-2 expression in endothelial cells decreased with time, in agreement with the in vivo situation,30 whereas VE-cadherin expression remained stable (Figure 4C and data not shown). The decrease in VEGFR-2 expression was promoted by PDGF-BB, concurring with our suggestion that PDGF-BB accelerates the differentiation of endothelial cells. This assumption is further supported by the fact that overexpression of the Shb adapter protein in ES cells causes increased vascularization of embryoid bodies by inducing an increased number of precursor cells coexpressing VEGFR-2 and PDGFR-β.50 It is conceivable that the effect of Shb overexpression is mediated to a large extent by augmented PDGFR-β signaling. Furthermore, PDGFR-β expression may be reinduced in endothelial cells under certain circumstances, such as in cultured endothelial cells, in the placenta, and in certain tumor vessels.51,52 The fact that tumor vessels remain unstable and immature might be compatible with the notion that the down-regulation of PDGFR-β is a prerequisite for the maturation of endothelial cells.
Gene inactivation of PDGF-B/PDGFR-β19,20,53 results in deficient pericyte coating of vessels. During early development, α-SMA–positive cells were excluded from clusters of hemangioprecursor cells (Figure 3B). However, at day 14, PDGFR-β–expressing pericytes were found in the close vicinity of, and sometimes wrapped around, differentiated vessel structures in the embryoid bodies (Figure 3A). Yamashita et al9 showed that VEGFR-2–expressing endothelial cell precursors, selected from ES cells by growing on OP-9 feeders and subsequently on collagen IV, differentiate into mural cells in the presence of PDGF-BB. This endothelial/mural switch may account for the increase in α-SMA and RGS-5 expression that we recorded in the PDGF-BB–treated embryoid bodies. PDGF-BB could also act directly on PDGFR-β–expressing pericyte precursors. The existence of bone marrow–derived pericyte precursors coexpressing PDGFR-β and stem cell antigen (Sca) was recently described.54 Through paracrine signaling involving PDGF-BB and other factors produced by endothelial cells, such precursors could mature to desmin-positive pericytes. Moreover, RGS-5, which is a guanosine triphosphatase (GTPase)–activating protein that negatively regulates certain α subunits of heterotrimeric G-proteins, has been identified as a marker for developing pericytes in the fetal vasculature, and Pdgf-b–/– embryos show reduced expression of RGS-5.55,56
In conclusion, our data indicate that the early development of hematopoietic, endothelial, and perivascular smooth muscle cells is intimately linked and that the differentiation of these lineages may be guided by PDGF-BB, which thereby is assigned a more critical role in mesodermal development than previously anticipated.
Prepublished online as Blood First Edition Paper, May 11, 2006; DOI 10.1182/blood-2006-04-014894.
Supported by grants from the Swedish Cancer Society (project 3820-B04-09XAC) (L.C.-W.), the Swedish Research Council (project K2005-32X-12552-08A) (L.C.-W.), the Novo Nordisk Foundation (L.C.-W.), and the Swedish Research Council (31X-10822) (M.W.).
I.N. and P.M. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issuse.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Andrius Kazlauskas for providing the antiserum against PDGFR-β and for critical reading of the manuscript, Dr Andras Nagy for providing the R1 ES cells, and Dr Philippe Soriano for providing the Pdgfr-β–/– ES cells. We posthumously thank Dr Rupert Timpl (deceased; Max-Planck Institute, Munich, Germany) for the anti–VE-cadherin antibodies. We thank Aive Åhgren (Ludwig Institute for Cancer Research) for technical assistance.