Abstract
Plasma fibronectin enhances platelet thrombus formation on surfaces coated with collagen. We investigated the role of fibronectin assembly in this process. Platelets adherent to fibrillar type I collagen, but not platelets adherent to von Willebrand factor (VWF), supported assembly of plasma fibronectin under static conditions. At a shear rate of 1250 s–1, platelets adherent to collagen assembled coperfused plasma fibronectin and formed larger thrombi in a fibronectin-concentration–dependent manner, with a maximum effect at 250 μg/mL. Enhanced thrombus formation on collagen was blocked by a peptide that binds to the N-terminal region of fibronectin and inhibits fibronectin assembly. Cross-linking of fibronectin to collagen prior to exposure to platelets had no effect on thrombus formation. Collagen-induced platelet thrombus formation at a shear rate of 5000 s–1 required coperfusion with VWF and did not result in assembly of coperfused fibronectin. VWF-mediated increase in platelet thrombi on collagen was not enhanced and indeed was somewhat attenuated by coperfused fibronectin at a shear rate of 5000 s–1. These results indicate that, at moderately high but not very high shear rates, fibronectin assembly in platelet aggregates that form in response to collagen enhances thrombus formation and serves as an alternative to VWF-mediated enhancement.
Introduction
Fibronectin is a glycoprotein dimer of 250-kDa subunits that exists in a soluble form in plasma and other body fluids and in an insoluble multimeric form in tissues due to deposition around fibroblastic cells.1,2 Soluble fibronectin is also assembled into fibrillar arrays by adherent platelets by a process that, as with fibroblasts,3,4 is blocked by the N-terminal 70-kDa fragment of fibronectin or functional upstream fibronectin binding domain (FUD) of the protein F1 of Streptococcus pyogenes.5,6 Assembly of soluble fibronectin by adherent platelets is regulated by substrate-coated surfaces, for example, platelets adherent to fibronectin-, fibrin-, laminin-, or collagen-coated surfaces support fibronectin assembly, whereas platelets adherent to vitronectin- or fibrinogen-coated surfaces do not.5-7 Intravital videomicroscopy using plasma fibronectin knock-out mice demonstrated that in the absence of plasma fibronectin, unstable thrombi form after vascular injury that continuously shed platelets.8 Recently, we found that plasma fibronectin enhances platelet thrombi forming on fibrin as a consequence of its presence in fibrin matrices and assembly in developing platelet thrombi.9 These findings provide a possible explanation for the unstable thrombi observed in plasma fibronectin knock-out mice.8
The initial step of hemostasis is mediated by interaction of platelets with matrix proteins, especially von Willebrand factor (VWF) and collagen.10 Various collagen molecules, which consist of 3 chains in a collagen triple-helical conformation, are found in the vascular wall.11 Collagen types I, III, IV, and VI are effective for adhesion and activation of platelets via α2β1 and glycoprotein VI.10 However, interaction of collagen with its platelet receptors cannot initiate and propagate thrombus formation at high shear rates (> 1500 s–1) unless platelets are initially tethered by interaction between VWF and glycoprotein Ib/IX/V complex.12
Platelet accumulation on collagen at shear rates of 300 s–1 to 1250 s–1 is known to be enhanced by coperfusion with plasma fibronectin.13-15 Because platelets adherent to collagen assemble plasma fibronectin under static conditions,5,16 we hypothesized that this enhancement is due to assembly of fibronectin. We therefore undertook studies of effects of plasma fibronectin on platelet behavior under shear conditions on surfaces coated with fibrillar type I collagen. Platelets adherent to collagen assembled plasma fibronectin under static and moderately high shear (1250 s–1) conditions. Thrombus formation at 1250 s–1 was enhanced by perfused fibronectin in a concentration-dependent manner and accompanied by deposition of fibronectin in developing thrombi. The enhancement of platelet thrombus formation was diminished by blocking of fibronectin assembly with FUD. Additional experiments demonstrated, surprisingly, that platelets adherent to VWF do not assemble plasma fibronectin. These results indicate that assembly of plasma fibronectin is responsible for enhancement of platelet thrombus formation on collagen and that this mechanism is independent of and, indeed, opposed by effects of VWF.
Materials and methods
Materials
Acid-soluble rat tendon fibrillar type I collagen was from Upstate (Lake Placid, NY). Purified human VWF17 was a kind gift from Robert Montgomery (Blood Center of Wisconsin, Milwaukee, WI). Sources and preparations of other reagents were described previously.7 Washed platelets were prepared by centrifugation of sodium citrate–treated human blood at 200g for 20 minutes and then by centrifugation of platelet-rich plasma at 2000g for 10 minutes as described previously.5 After washing with HEPES buffer (50 mM HEPES acid, 136 mM NaCl, 5.5 mM dextrose, and 10% sodium citrate solution, pH 7.6), platelets were resuspended in HEPES-Tyrode buffer (136 mM NaCl, 3 mM KCl, 2 mM MgCl2 6 H2O, 3.3 mM NaH2PO4 H2O, 15 mM HEPES salt, 4.4 mM HEPES acid, and 5.5 mM dextrose, pH 7.6) and supplemented with 0.1% fatty acid–free BSA and 2 mM CaCl2. A suspension of washed platelets, 2 to 3 × 108/mL, and 40% red blood cells was prepared as described previously.9 Approval to obtain blood samples was obtained from the University of Wisconsin–Madison review board. Informed consent was provided according to the Declaration of Helsinki.
Deposition of fibronectin on platelets adherent to collagen or VWF under static conditions
Collagen or VWF, 20 μg/mL, or a mixture of 10 μg/mL collagen and VWF in Tris-buffered saline (20 mM Tris-HCl, pH 7.4, and 150 mM NaCl) was coated on a 1.2-cm diameter glass coverslip or a well of a 24-well plate overnight at 4°C. Acid-soluble rat tendon collagen treated in this way forms fibrils with a periodicity of 67 nm as detected (eg, by atomic force microscopy).18 In some experiments, collagen, 20 μg/mL, was coated on a coverslip or well for 8 hours and then VWF, 20 μg/mL, was coated. Protein-coated surfaces were postcoated with 1% fatty acid–free BSA. To assay fibronectin assembly, 1 × 108 platelets in 0.5 mL HEPES-Tyrode buffer containing 0.1% fatty acid–free BSA and 2 mM CaCl2 in the absence or presence of 1 μM 1-oleoyl-lysophosphatidic acid (LPA) were plated on protein-coated surfaces in the presence of 25 μg/mL (50 nM) FITC-labeled fibronectin (FITC-fibronectin) for 1 hour at 37°C. After washing, adherent platelets were processed for epifluorescence microscopy as described previously.7 Images were captured and prepared for publication as described previously.9
Platelet adhesion assay under static conditions
Platelets, 1 × 107, in 100 μL HEPES-Tyrode buffer including 2 mM CaCl2 and 0.1% fatty acid–free BSA in the absence or presence of 1 μM LPA were plated in protein-coated wells in an area of 28 mm2 of a 96-well plate and incubated without or with 50 μg/mL soluble fibronectin for 30 minutes at 37°C. Number of adherent platelets was estimated based on luminescence signal due to platelet ATP as described previously.7
Adhesion and aggregation of platelets and incorporation of soluble fibronectin into platelet thrombi under shear conditions
The parallel plate flow chamber was performed as described previously.9 Briefly, a 3.5-cm diameter coverslip or 6-cm diameter Petri dish was coated overnight with 20 μg/mL collagen or VWF. In experiments to study the effect of fibronectin-collagen cross-linking, 20 μg/mL collagen was incubated on a coverslip or Petri dish with 50 μg/mL fibronectin, 2 mM CaCl2, and 1 U/mL thrombin in the absence or presence of 5 μg/mL factor XIII (FXIII). To verify the presence of cross-linked complexes, the protein mixture was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (a 3.3% stacking gel and 5% separating gel) under reduced conditions and stained for proteins.19
The protein-coated coverslip or Petri dish was assembled in the flow chamber. The inlet was connected to a buffer reservoir by silicone tubing, and the outlet was connected with a peristaltic pump to draw fluid through the flow chamber. Then, the apparatus was perfused with a prewarmed suspension of platelets and red blood cells in HEPES-Tyrode buffer containing 0.1% fatty acid–free BSA and 2 mM CaCl2 in the presence or absence of 5 μM LPA. In different experiments, the perfusate contained FITC-fibronectin or unlabeled fibronectin, 0 to 500 μg/mL; VWF, 10 μg/mL; 1 to 2 μM FUD; or various combinations of these proteins. The perfusion was for 5 minutes at a wall shear rate of 300 s–1, 1250 s–1, or 5000 s–1 at 37°C. The coverslip was taken out and processed for epifluorescence or confocal microscopy as described previously.9 In some experiments to assay the localization of perfused FITC-fibronectin vis-à-vis secreted fibrinogen, nonpermeabilized platelets were incubated with mouse anti–human fibrinogen followed by rhodamine-conjugated anti–mouse IgG antibody. Images were captured and processed as described previously.9 In addition, confocal images, taken with the 100 × objective, of secreted fibrinogen and FITC-fibronectin were merged and rotated at 90 degrees using Image J 1.34 (http://rsb.info.nih.gov/ij/).
The surface coverage and thrombus volume in an area of 58 000 μm2 were calculated from confocal microscopic images of platelets stained by rhodamine-phalloidin, obtained at 1.0-μm intervals in the z-axis using an excitation wavelength of 585 nm, as described previously.9 Images were analyzed using Metamorph software (version 4.5; Universal Imaging, West Chester, PA). The number of adherent platelets in thrombi in a flow field area of 38 mm2 was estimated as described previously.9 To calculate the number of adherent platelets, luminescence was measured by dilutions of standard suspensions of platelets. The comparison between platelet thrombi and the standard suspension took into account loss of ATP content due to secretion from dense granules,20 which we estimated as 25% and 40% based on the decrease in luminescence signals detected in platelets washed before and after aggregation in suspension stimulated by 5 μM LPA (for experiments done on VWF-coated surfaces) and 10 μg/mL soluble collagen (for experiments done on collagen-coated surfaces), respectively.
Effect of perfused VWF on thrombogenesis by fibronectin deposition and localization of VWF and fibronectin into platelet thrombi under shear conditions
A suspension of platelets and red blood cells was premixed with or without 50 μg/mL FITC-fibronectin plus 200 μg/mL unlabeled fibronectin and/or 10 μg/mL VWF in the presence of 5 μM LPA and perfused over collagen surfaces at a wall shear rate of 1250 s–1 or 5000 s–1 for 5 minutes. Coverslips were taken out of the chamber, and nonpermeabilized platelets were incubated with mouse anti-VWF followed by rhodamine-conjugated anti–mouse IgG antibody. Confocal images were processed using Image J.
Replication of results and statistical analysis
All experimental permutations were tested in 3 to 5 separate experiments carried out using blood of different volunteer donors. Data were expressed as mean ± SD. ANOVA with the Dunnett test was used for comparison of many groups. Differences were taken to be significant at P values of less than .05.
Results
Fibrillar type I collagen but not VWF supports assembly of plasma fibronectin by adherent platelets under static conditions
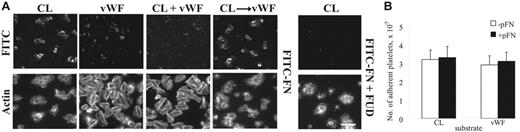
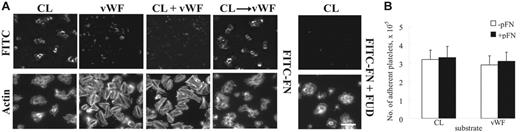
Assembly of plasma fibronectin by adherent platelets is dependent on the adhesive protein surfaces under both static and flow conditions.5-7 Consistent with previous findings,5 LPA-unstimulated platelets adherent to collagen-coated surfaces supported fibrillar deposition of FITC-fibronectin under static conditions (Figure 1A). LPA activation did not enhance platelet adhesion and fibronectin deposition by platelets adherent to collagen (not shown). Fibronectin assembled by platelets adherent to collagen was in platelet aggregates and difficult to image by regular epifluorescence microscopy (Figure 1A). When the sample was examined by confocal microscopy, FITC-fibronectin was found in a fibrillar pattern throughout platelet aggregates (not shown). VWF-coated surfaces supported adhesion and spreading of platelets but did not support deposition of plasma fibronectin by adherent platelets (Figure 1A). When a mixture of both proteins was tested, the mixed substrate acted like VWF (Figure 1A). When surfaces were coated with collagen and then VWF, the effect on fibronectin assembly was that of a collagen-coated surface (Figure 1A). Addition of LPA did not enhance fibronectin deposition by platelets adherent to VWF or VWF-collagen.
Deposition of plasma fibronectin on platelets adherent to collagen or VWF under static conditions. (A) Coverslips were coated with fibrillar type I collagen (CL), VWF, both proteins simultaneously, or collagen and then VWF. Platelets and 25 μg/mL (50 nM) FITC-fibronectin (FITC-FN) without or with 1 μM FUD and without LPA were added and incubated for 1 hour. Platelets were stained with rhodamine-phalloidin and then examined by epifluorescent microscopy for distribution of FITC-fibronectin and filamentous actin cytoskeleton. Platelets bound to collagen as aggregates. The images were obtained at the level of the coverslip surface. Bar = 10 μm. (B) Platelets were plated on protein-coated wells of a 96-well plate and incubated with or without 50 μg/mL soluble fibronectin (pFN) for 30 minutes without LPA. After 3 rinses, adherent platelets were quantified as described in “Materials and methods.” Values represent the mean ± SD (n = 3).
Deposition of plasma fibronectin on platelets adherent to collagen or VWF under static conditions. (A) Coverslips were coated with fibrillar type I collagen (CL), VWF, both proteins simultaneously, or collagen and then VWF. Platelets and 25 μg/mL (50 nM) FITC-fibronectin (FITC-FN) without or with 1 μM FUD and without LPA were added and incubated for 1 hour. Platelets were stained with rhodamine-phalloidin and then examined by epifluorescent microscopy for distribution of FITC-fibronectin and filamentous actin cytoskeleton. Platelets bound to collagen as aggregates. The images were obtained at the level of the coverslip surface. Bar = 10 μm. (B) Platelets were plated on protein-coated wells of a 96-well plate and incubated with or without 50 μg/mL soluble fibronectin (pFN) for 30 minutes without LPA. After 3 rinses, adherent platelets were quantified as described in “Materials and methods.” Values represent the mean ± SD (n = 3).
Coincubation of FITC-fibronectin with FUD inhibited fibronectin assembly without affecting platelet adhesion (Figure 1A). To see whether deposition of soluble fibronectin enhances platelet adhesion under static conditions, a luminescence-based platelet adhesion assay was performed.7 The number of platelets adherent to collagen or VWF was approximately 320 000 or 290 000, respectively, in an area of 28 mm2, in the absence of soluble fibronectin (Figure 1B). The number of platelets adherent to each substrate was not enhanced by coincubation of soluble fibronectin (Figure 1B).
Deposition of plasma fibronectin by adherent platelets depends on substrates to which platelets adhere and on shear conditions
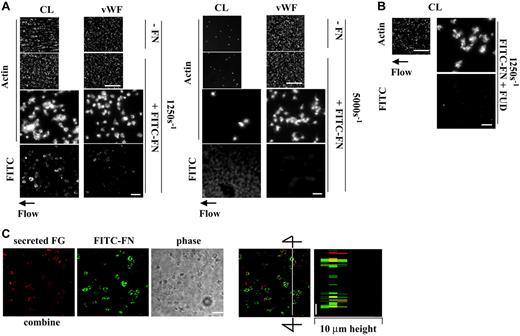
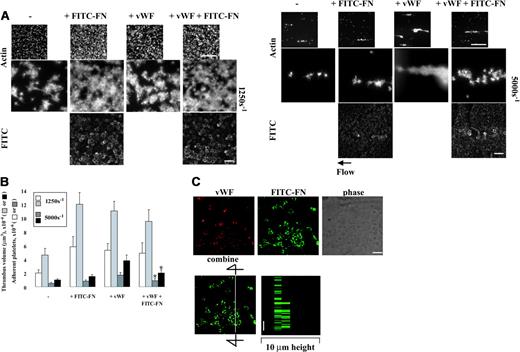
At a shear rate of 1250 s–1, deposition of plasma fibronectin in platelet thrombi is associated with several-fold enhancement of thrombus volume, surface coverage, and number of adherent platelets on surfaces coated with fibrin or fibrin cross-linked to fibronectin.9 To determine whether collagen or VWF causes deposition of plasma fibronectin by adherent platelets under shear conditions concomitant with enhancement of thrombus formation, a suspension of platelets and red blood cells was coperfused without or with 50 μg/mL FITC-fibronectin over surfaces coated with the proteins at various shear rates. Both collagen and VWF supported platelet adhesion in the absence of plasma fibronectin at a shear rate of 1250 s–1, with interesting differences between the 2 coatings (Figure 2A, left panel; Table 1). Compared with VWF surfaces, somewhat more platelets were found on collagen surfaces, but the platelets were in aggregates and covered less of the surfaces. The platelets adherent and aggregated on collagen assembled plasma fibronectin, whereas platelets adherent to VWF did not (Figure 2A, left panel). Formation of platelet thrombi on collagen was enhanced approximately 1.5-fold by coperfusion with 50 μg/mL plasma fibronectin (Figure 2A, left panel; Table 1). Formation of platelet thrombi on VWF was not enhanced by plasma fibronectin, and little FITC-fibronectin was deposited (Figure 2A, left panel; Table 1). Coperfusion of 5 μM LPA with platelet suspensions containing soluble plasma fibronectin did not result in fibronectin assembly or enhance platelet thrombus formation on surfaces coated with VWF (not shown). Similar results on each substrate were observed in parallel experiments done at a shear rate of 300 s–1 (not shown).
The wall shear rate in rabbit mesenteric arterioles in vivo ranges from 500 to 5000 s–1.21 Interaction of VWF or collagen with platelet glycoprotein Ib/IX/V or glycoprotein VI, respectively, is important for initial platelet adhesion to the injured vessel wall under high shear conditions.22,23 To test a role of fibronectin assembly at a high shear rate, the experiments were repeated at 5000 s–1. Compared with a shear rate of 1250 s–1, collagen supported platelet adhesion poorly at 5000 s–1 (Figure 2A right panel). Faint fluorescence due to binding of FITC-fibronectin to collagen-coated surfaces was detected, but there was no binding of FITC-fibronectin to platelets adherent to collagen and no enhancement of platelet adhesion and thrombus formation by coperfusion of plasma fibronectin (Figure 2A, right panel). VWF supported adhesion and aggregation of platelets at the high shear rate, but, as under static and lower shear conditions, perfused FITC-fibronectin was not deposited by platelets adherent to VWF (Figure 2A, right panel).
We then tested whether the N-terminal region of fibronectin mediates deposition of plasma fibronectin on platelets adherent to collagen at a wall shear rate of 1250 s–1 that supports fibronectin deposition in platelet thrombi. A suspension of platelets and red blood cells was coperfused over collagen-coated surfaces with FITC-fibronectin and FUD, which binds the N-terminal region of fibronectin24 and inhibits fibronectin assembly by platelets adherent to fibrin matrices.9 Coperfusion with 1 μM FUD diminished deposition of FITC-fibronectin and thrombogenesis on collagen (Figure 2B), indicating that assembly of fibronectin within platelet thrombi that formed on collagen is important for enhancement of thrombus formation. The microscopic observation was corroborated by measurement of surface coverage, thrombus volume, and number of adherent platelets (Table 1); coperfusion with FUD decreased all these measures of platelet thrombus formation on collagen to a control level.
Fibrinogen secreted from platelets and FITC-fibronectin deposited from the perfusate were localized in platelet thrombi formed on collagen at a shear rate of 1250 s–1 (Figure 2C). Deposited fibronectin partially colocalized with secreted fibrinogen in the middle of platelet thrombi as shown in a 1-μm slice from the middle of thrombi and a computer-generated vertical slice.
The ability of collagen to support thrombogenesis is enhanced by coperfusion of fibronectin with LPA under shear conditions
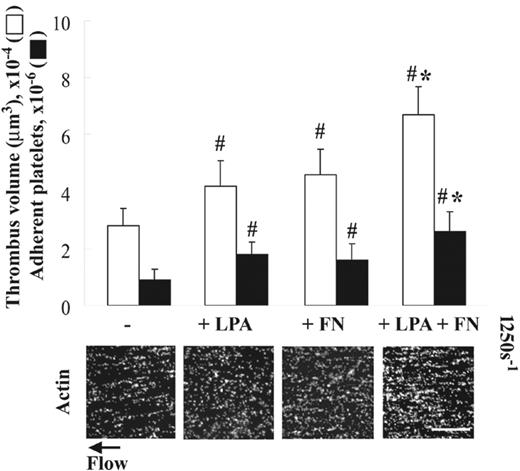
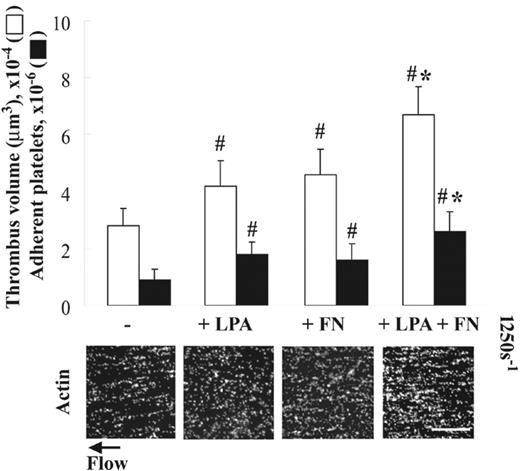
As reported previously,5 platelets adherent to collagen assemble plasma fibronectin equally in the absence (Figure 1A) or the presence (not shown) of LPA under static conditions. Platelet activators such as LPA, however, are required for enhancement of thrombogenesis due to increased deposition of plasma fibronectin by platelets adherent to fibrin matrices under shear conditions.9 To test whether LPA also enhances thrombogenesis on collagen under shear conditions, a suspension of platelets and red blood cells was coperfused without or with 5 μM LPA and/or 50 μg/mL plasma fibronectin over collagen-coated surfaces at 1250 s–1. Coperfusion with LPA or plasma fibronectin alone increased thrombogenesis on collagen surfaces by 1.5-fold compared with a control (Figure 3). Coperfusion of plasma fibronectin and LPA had an additive effect on enhancement of thrombogenesis compared with perfusion with either LPA or fibronectin alone (Figure 3). The similar enhancement of thrombogenesis on collagen surfaces by LPA and/or perfused fibronectin was observed under a shear rate of 300 s–1, whereas little enhancement by coperfusion with LPA and fibronectin was detected at a shear rate of 5000 s–1 (not shown).
Effect of incorporation of plasma fibronectin on platelet thrombus formation on collagen or VWF under shear conditions. (A) A suspension of platelets and red blood cells was mixed with or without 50 μg/mL FITC-fibronectin (FITC-FN) and perfused over surfaces coated with collagen (CL) or VWF at a wall shear rate of 1250 s–1 or 5000 s–1 for 5 minutes without LPA. Coverslips were taken out of the chamber and processed for epifluorescence microscopy. The small and large pictures were taken with 10 × (bar = 100 μm) and 100 × (bar = 10 μm) objectives, respectively. (B) FUD, 1 μM, was coperfused with FITC-FN. The images of phalloidin-stained platelets should be compared with images on collagen of panel A. (C) After perfusion, nonpermeabilized platelets were incubated with mouse anti–human fibrinogen (FG) followed by rhodamine-conjugated anti–mouse IgG antibody. Confocal microscopy of FITC-fibronectin and secreted fibrinogen was performed as described in “Materials and methods.” The images represent a 1-μm slice taken from 4-μm height of the coverslip. The right panel shows a perpendicular section through the line. Bar = 10 μm.
Effect of incorporation of plasma fibronectin on platelet thrombus formation on collagen or VWF under shear conditions. (A) A suspension of platelets and red blood cells was mixed with or without 50 μg/mL FITC-fibronectin (FITC-FN) and perfused over surfaces coated with collagen (CL) or VWF at a wall shear rate of 1250 s–1 or 5000 s–1 for 5 minutes without LPA. Coverslips were taken out of the chamber and processed for epifluorescence microscopy. The small and large pictures were taken with 10 × (bar = 100 μm) and 100 × (bar = 10 μm) objectives, respectively. (B) FUD, 1 μM, was coperfused with FITC-FN. The images of phalloidin-stained platelets should be compared with images on collagen of panel A. (C) After perfusion, nonpermeabilized platelets were incubated with mouse anti–human fibrinogen (FG) followed by rhodamine-conjugated anti–mouse IgG antibody. Confocal microscopy of FITC-fibronectin and secreted fibrinogen was performed as described in “Materials and methods.” The images represent a 1-μm slice taken from 4-μm height of the coverslip. The right panel shows a perpendicular section through the line. Bar = 10 μm.
Dependence of enhancement of platelet thrombus formation on concentration of perfused fibronectin under shear conditions
To relate our results to previous studies in which plasma fibronectin was re-added into fibronectin-depleted plasma at various concentrations prior to flow experiments,13-15 different concentrations of plasma fibronectin were coperfused with the cell suspension over collagen surfaces. Platelet thrombus formation was enhanced up to a physiologic concentration of 250 μg/mL in a concentration-dependent manner (Figure 4A-B). When 250 μg/mL fibronectin was coperfused with 2 μM FUD, the maximum thrombogenesis was decreased (Figure 4A), and thrombus volume and number of adherent platelets were also decreased by 60% to 70% (Figure 4B).
Effect of LPA on platelet thrombus formation on collagen under shear conditions. A suspension of platelets and red blood cells was premixed without or with 50 μg/mL soluble fibronectin (FN) and/or 5 μM LPA, and perfused over surfaces coated with 20 μg/mL collagen at a wall shear rate of 1250 s–1 for 5 minutes. Microscopy was performed as in Figure 2. Bar = 100 μm. Thrombus volume and number of adherent platelets were calculated as described in “Materials and methods.” Values represent the mean ± SD (n = 3-5). #P < .05 compared with a control (the absence of LPA and fibronectin). *P < .05 compared with LPA or fibronectin alone.
Effect of LPA on platelet thrombus formation on collagen under shear conditions. A suspension of platelets and red blood cells was premixed without or with 50 μg/mL soluble fibronectin (FN) and/or 5 μM LPA, and perfused over surfaces coated with 20 μg/mL collagen at a wall shear rate of 1250 s–1 for 5 minutes. Microscopy was performed as in Figure 2. Bar = 100 μm. Thrombus volume and number of adherent platelets were calculated as described in “Materials and methods.” Values represent the mean ± SD (n = 3-5). #P < .05 compared with a control (the absence of LPA and fibronectin). *P < .05 compared with LPA or fibronectin alone.
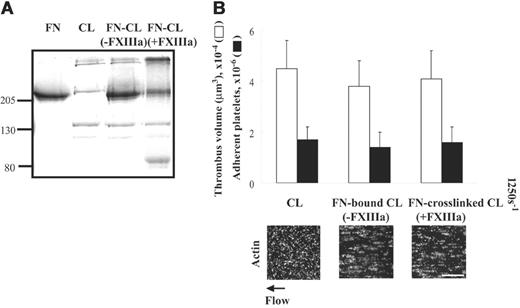
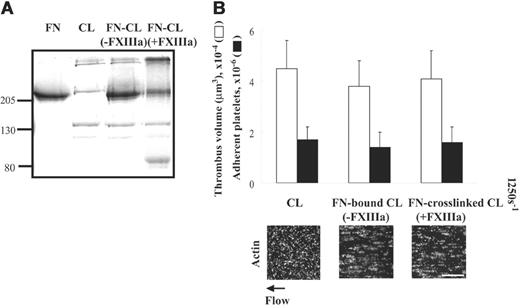
The ability of collagen to support thrombogenesis is not affected by cross-linking of fibronectin-collagen by activated factor XIII under shear conditions
Cross-linking of fibronectin to fibrin matrices by thrombin-activated FXIII (FXIIIa) enhances thrombogenesis several-fold under shear conditions compared with fibrin matrices alone.9 Collagen type I also can be cross-linked to fibronectin by FXIIIa.19 We therefore tested whether cross-linking of fibronectin to collagen by FXIIIa affects platelet thrombus formation at a shear rate of 1250 s–1. As previously described,19 high-molecular-weight complexes compatible with cross-linking between fibronectin and collagen were detected by a protein-stained SDS-PAGE gel (Figure 5A lane 4) but were not present when FXIII was absent during incubation (Figure 5A lane 3). Surfaces coated with fibronectin–cross-linked collagen did not affect thrombogenesis in the absence (not shown) or presence of plasma fibronectin compared with surfaces coated with collagen alone (Figure 5B). Similar results were observed in surfaces coated with collagen bound to fibronectin in the absence of FXIII (Figure 5B).
Effects of plasma fibronectin concentration in the perfusate on platelet thrombus formation on collagen under shear conditions. A suspension of platelets and red blood cells was premixed with or without 50, 100, 250, or 500 μg/mL soluble fibronectin (FN) and 5 μM LPA in the absence or presence of 2 μM FUD over surfaces coated with 20 μg/mL collagen at a wall shear rate of 1250 s–1 for 5 minutes. (A) Microscopy was performed as described in Figure 2. Bar = 100 μm. (B) Thrombus volume and number of adherent platelets were calculated as described in Figure 3. Squares indicate plasma fibronectin alone at various concentrations. The triangles indicate 250 μg/mL plasma fibronectin plus 2 μM FUD. Values represent the mean ± SD (n = 3).
Effects of plasma fibronectin concentration in the perfusate on platelet thrombus formation on collagen under shear conditions. A suspension of platelets and red blood cells was premixed with or without 50, 100, 250, or 500 μg/mL soluble fibronectin (FN) and 5 μM LPA in the absence or presence of 2 μM FUD over surfaces coated with 20 μg/mL collagen at a wall shear rate of 1250 s–1 for 5 minutes. (A) Microscopy was performed as described in Figure 2. Bar = 100 μm. (B) Thrombus volume and number of adherent platelets were calculated as described in Figure 3. Squares indicate plasma fibronectin alone at various concentrations. The triangles indicate 250 μg/mL plasma fibronectin plus 2 μM FUD. Values represent the mean ± SD (n = 3).
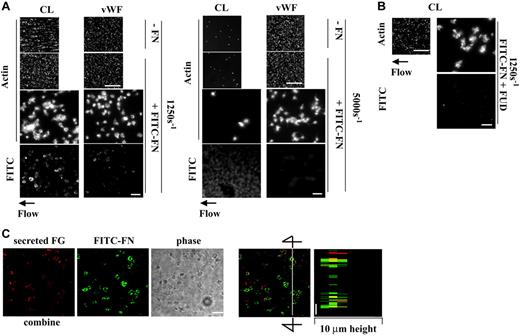
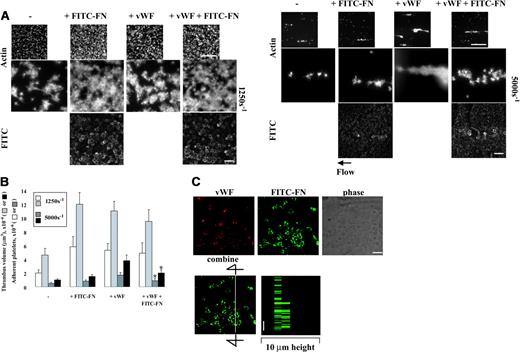
Coperfusion of VWF and fibronectin has no additive effect on platelet thrombogenesis on collagen under shear conditions
Plasma VWF plays a key role in initial adhesion of platelets to collagen under shear conditions via interaction with glycoprotein Ibα and αIIbβ3.12 We therefore compared effects of perfused VWF or fibronectin alone or the 2 together on platelet thrombogenesis on collagen at shear rates of 1250 s–1 and 5000 s–1. Similar to previous reports,12 perfused VWF, 10 μg/mL, enhanced thrombogenesis on collagen at a moderate high shear rate of 1250 s–1 by 2.5-fold (Figure 6A left panel and 6B). When VWF was coperfused with FITC-fibronectin, 50 μg/mL, and unlabeled fibronectin, 200 μg/mL, no additive effect on thrombogenesis was found (Figure 6A left panel and 6B). VWF did not inhibit fibronectin deposition in platelet thrombi (Figure 6A left panel). Perfused VWF enhanced platelet thrombi on collagen at a shear rate of 5000 s–1 by 3-fold, whereas perfused fibronectin, 250 μg/mL, only slightly increased platelet thrombi (Figure 6A right panel and 6B). FITC-fibronectin was associated mostly with collagen matrices, although there was weak staining on small platelet aggregates. When VWF and fibronectin were coperfused, thrombogenesis was slightly but significantly decreased compared with perfusion with VWF alone (Figure 6A right panel and 6B). Thus, at a moderately high shear rate of 1250 s–1, the presence of VWF or fibronectin in the perfusate enhances thrombus formation by a similar degree and there is no additive effect. At a high shear rate of 5000 s–1, VWF enhances thrombogenesis much more than fibronectin and indeed fibronectin blunts the effect of VWF.
When localization of perfused VWF and fibronectin was studied by confocal microscopy, VWF poorly colocalized with deposited fibronectin on platelet thrombi at a shear rate of 1250 s–1 (Figure 6C).
Discussion
In ex vivo studies in which platelets and red blood cells were reconstituted with fibronectin-depleted plasma, the absence of plasma fibronectin caused a decrease in platelet adhesion.13,14 Consistent with these observations, mice lacking plasma fibronectin form less cohesive thrombi that shed platelets.8 The mechanisms of the enhancing effect of plasma fibronectin on platelet thrombus formation have been obscure. We recently demonstrated ex vivo that 2 types of insolubilized fibronectin—fibronectin cross-linked to fibrin matrices and assembled in developing platelet thrombi—enhance platelet thrombus formation on fibrin synergistically.9 In the present paper, we describe investigations of whether these 2 types of insolubilized fibronectin enhance platelet thrombus formation on substrates of fibrillar type I collagen. We found that coperfusion of plasma fibronectin during platelet adhesion and build-up on collagen enhances thrombogenesis at a shear rate of 1250 s–1 but not 5000 s–1 due to deposition of fibronectin in developing thrombi. Cross-linking of fibronectin to collagen, however, did not cause the collagen substrate to be more thrombogenic. The fact that fibronectin–cross-linked collagen, unlike fibronectin–cross-linked fibrin, did not influence platelet thrombus formation suggests that the interaction of platelets with collagen causes activation that is so strong that any further activation due to interaction with cross-linked fibronectin is trivial. This strong activation may explain why, during platelet build-up, the impact of fibronectin assembly on platelet thrombus formation on a collagen substrate is 2- to 3-fold at maximum compared with 3- to 6-fold on a fibrin substrate.9
Effect of cross-linking of fibronectin to collagen on platelet thrombus formation under shear conditions. (A) A 100-μL mixture of 20 μg/mL collagen, 50 μg/mL plasma fibronectin, 2 mM CaCl2, and 2 U/mL thrombin in the absence or presence of 5 μg/mL FXIII was incubated at 4°C overnight, and 40 μL was subjected to electrophoresis in SDS under reduced conditions. Lane 1: 2 μg fibronectin (FN); lane 2: 1 μg collagen (CL); lane 3: the protein mixture without FXIII; and lane 4: the protein mixture with FXIII. (B) The mixtures were coated on coverslips or Petri dishes. A cell suspension was mixed with 50 μg/mL soluble fibronectin, and perfused over surfaces coated with collagen, fibronectin-bound collagen (FN-bound CL), or fibronectin–cross-linked collagen (FN–cross-linked CL) at a wall shear rate of 1250 s–1 for 5 minutes. Microscopy was performed as described in Figure 3. Bar = 100 μm. Thrombus volume and number of adherent platelets were calculated as described in Figure 3. Values represent the mean ± SD (n = 3).
Effect of cross-linking of fibronectin to collagen on platelet thrombus formation under shear conditions. (A) A 100-μL mixture of 20 μg/mL collagen, 50 μg/mL plasma fibronectin, 2 mM CaCl2, and 2 U/mL thrombin in the absence or presence of 5 μg/mL FXIII was incubated at 4°C overnight, and 40 μL was subjected to electrophoresis in SDS under reduced conditions. Lane 1: 2 μg fibronectin (FN); lane 2: 1 μg collagen (CL); lane 3: the protein mixture without FXIII; and lane 4: the protein mixture with FXIII. (B) The mixtures were coated on coverslips or Petri dishes. A cell suspension was mixed with 50 μg/mL soluble fibronectin, and perfused over surfaces coated with collagen, fibronectin-bound collagen (FN-bound CL), or fibronectin–cross-linked collagen (FN–cross-linked CL) at a wall shear rate of 1250 s–1 for 5 minutes. Microscopy was performed as described in Figure 3. Bar = 100 μm. Thrombus volume and number of adherent platelets were calculated as described in Figure 3. Values represent the mean ± SD (n = 3).
Effect of perfused VWF and fibronectin on thrombogenesis on collagen under shear conditions and localization of VWF and fibronectin. A suspension of platelets and red blood cells with or without 50 μg/mL FITC-fibronectin (FITC-FN) and 200 μg/mL unlabeled fibronectin and/or 10 μg/mL VWF was perfused over surfaces coated with 20 μg/mL collagen at a wall shear rate of 1250 s–1 or 5000 s–1 for 5 minutes. (A) Coverslips were taken out of the chamber, stained with rhodamine-phalloidin, and processed for epifluorescence microscopy. The small and large pictures were taken with 10 × (bar = 100 μm) and 100 × (bar = 10 μm) objectives, respectively. (B) Thrombus volume and number of adherent platelets were calculated as described in Figure 3. Values represent the mean ± SD (n = 3-4). *P < .05 compared with when the perfusate contained just VWF. (C) After perfusion, nonpermeabilized platelets were incubated with mouse anti-VWF followed by rhodamine-conjugated anti–mouse IgG antibody. Confocal microscopy was performed as described in “Materials and methods.” The images represent a 1-μm slice taken from 4-μm height of the coverslip. The bottom right panel shows a perpendicular section through the line. Bar = 10 μm.
Effect of perfused VWF and fibronectin on thrombogenesis on collagen under shear conditions and localization of VWF and fibronectin. A suspension of platelets and red blood cells with or without 50 μg/mL FITC-fibronectin (FITC-FN) and 200 μg/mL unlabeled fibronectin and/or 10 μg/mL VWF was perfused over surfaces coated with 20 μg/mL collagen at a wall shear rate of 1250 s–1 or 5000 s–1 for 5 minutes. (A) Coverslips were taken out of the chamber, stained with rhodamine-phalloidin, and processed for epifluorescence microscopy. The small and large pictures were taken with 10 × (bar = 100 μm) and 100 × (bar = 10 μm) objectives, respectively. (B) Thrombus volume and number of adherent platelets were calculated as described in Figure 3. Values represent the mean ± SD (n = 3-4). *P < .05 compared with when the perfusate contained just VWF. (C) After perfusion, nonpermeabilized platelets were incubated with mouse anti-VWF followed by rhodamine-conjugated anti–mouse IgG antibody. Confocal microscopy was performed as described in “Materials and methods.” The images represent a 1-μm slice taken from 4-μm height of the coverslip. The bottom right panel shows a perpendicular section through the line. Bar = 10 μm.
Collagen-mediated platelet adhesion results in inside-out signaling to activate platelet αIIbβ3 integrin25 and release or generation of activators such as ADP and thromboxane A2.26 α2β1 and glycoprotein VI are the major receptors for platelet adhesion to collagen and activation of platelets.25,27,28 Glycoprotein VI is associated with the Fc receptor γ-chain, which accomplishes inside-out signaling through Syk, SLP-76, phosphatidylinositol 3-kinase, and phospholipase Cγ2.25,27 In static experiments, platelets adherent to collagen are aggregated and assemble fibronectin in the absence of an exogenous activator.5 Under shear conditions, however, LPA enhanced platelet thrombus formation on collagen in the absence of perfused fibronectin and even more in the presence of perfused fibronectin. LPA binds to platelet receptors, LPA1, LPA2, or LPA3, to initiate signaling pathways through Gi/o,Gq,G12/13,or Gs.29 The platelet thrombi stained positively for fibrinogen secreted from activated platelets and, if present in the perfusate, fibronectin. The staining patterns overlapped only partially. The results suggest that interaction of perfused LPA with the receptors on collagen-activated platelets causes additional inside-out signaling that results in stronger cohesion due to both fibrinogen-αIIbβ3 interactions and interaction of platelets with multimerizing fibronectin.
We found that platelets adherent to VWF alone or a mixture of collagen and VWF, surprisingly, did not assemble plasma fibronectin under static conditions, whereas platelets adherent to collagen alone did. Examination of the surfaces by enzyme-linked immunosorbent assay (ELISA) with monoclonal antibody to type I collagen or VWF demonstrated that both proteins were present on the cocoated surface at densities comparable with those on surfaces in which only one protein was coated (Jielin Xu and D.F.M., unpublished data, March 2006). The result of the cocoating experiment suggests that platelet adhesion to VWF, like to fibrinogen and vitronectin,7 suppresses subsequent fibronectin assembly when a surface with an adhesive ligand that supports fibronectin assembly by adherent platelets is present. The mechanism of the suppression is not known. Of interest, when collagen alone was coated followed later by VWF, bound VWF, which could be demonstrated by ELISA (Jielin Xu and D.F.M., unpublished data, March 2006), no longer suppressed the supportive effect of collagen on fibronectin assembly by adherent platelets. This finding suggests that VWF adsorbs to tissue culture plastic in a conformation different from the conformation assumed upon binding to collagen-coated surfaces. Platelet glycoprotein Ib/IX/V complex interacts with the A1 domain of VWF via glycoprotein Ibα, which is important for initiation of platelet adhesion under high shear conditions,10,22 and αIIbβ3 integrin binds to the RGD sequence in the C domain region of VWF.30 One possibility is that suppression under static conditions is triggered by the αIIbβ3-RGD–containing region interaction, just as the αIIbβ3-QAGDV–containing γ-chain tail interaction accounts for suppression when platelets bind to fibrinogen.7
Inclusion of fibronectin and VWF in the perfusate at the moderately high shear of 1250 s–1 caused effects on platelet thrombus formation on a collagen-coated substrate that seemed to be independent of one another. Either protein perfused alone enhanced thrombus formation, and the effects were not additive when the 2 proteins were perfused together. Both proteins were deposited in thrombi, but there was no codistribution. At the high shear rate of 5000 s–1 however, the effect of VWF dominated. The formation of thrombi depended upon perfusion of VWF, and when VWF and fibronectin were perfused together, little fibronectin was deposited in the platelet thrombi. Fibronectin, however, was detected on the collagen substrate, and thrombus formation was attenuated compared with perfusions done only with VWF. The chains of type I collagen bind to both VWF and fibronectin.31,32 Binding of VWF to collagen is inhibited by fibronectin,33 suggesting that fibronectin and VWF may interact with the same binding sites on collagen. It was recently reported that C1qTNF-related protein-1 inhibits platelet thrombus formation on collagen surfaces by perturbing VWF binding to collagen under high shear conditions.34 By also competing with VWF for binding sites on collagen, fibronectin may blunt the enhancement of VWF-mediated formation of platelet thrombi on collagen at the high shear rate.
Matrix and cell surface molecules exposed at sites of vessel injury are dependent on the type of injury.35 Despite the alterations seen in vessel injury models in plasma fibronectin conditional knock-out mice,8 Sakai et al found that the knock-out mice have normal bleeding time and in vitro clotting time.36 This discrepancy suggests that plasma fibronectin is not important at very high shear conditions in which VWF is crucial for hemostasis and thrombosis37 or in a stagnant condition in which red blood cells are entrapped within fibrin. Our findings that deposition of fibronectin enhances the number of adherent platelets and thrombus size on collagen surfaces, along with previous studies showing an even more marked effect on fibrin,9 define conditions (moderately high shear at sites of vascular injury severe enough to expose collagen and/or tissue factor) in which fibronectin may be a key determinant of hemostatic and thrombotic events.
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2006-02-002063.
Supported by National Institutes of Health grant HL21644.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jielin Xu for helping with ELISAs.