Abstract
L-selectin directs neutrophils to sites of inflammation, and upon their activation, surface expression of the receptor is rapidly down-regulated by ectodomain shedding. Tumor necrosis factor–α–converting enzyme (TACE, or ADAM17) is a sheddase of L-selectin; however, Adam17 gene targeting (ADAM17ΔZn/ΔZn) in mice is perinatal lethal and its role in L-selectin shedding by mature neutrophils has not been determined. This was addressed here by using radiation-chimeric mice reconstituted with ADAM17ΔZn/ΔZn fetal liver cells. ADAM17-deficient neutrophils, monocytes, and lymphocytes failed to shed L-selectin in response to PMA, as did neutrophils infiltrating the inflamed peritoneum. In addition, the absence of functional ADAM17 resulted in significantly increased levels of L-selectin surface expression by peripheral-blood leukocytes, indicating the sheddase also plays a role in the constitutive cleavage of L-selectin. Interestingly, not all manners of L-selectin turnover required ADAM17. Plasma L-selectin levels were similar between ADAM17ΔZn/ΔZn-chimeric and control mice, as was the shedding of L-selectin by neutrophils undergoing spontaneous apoptosis. The latter process, however, was diminished by a metalloprotease inhibitor, indicating the role of a sheddase other than ADAM17. Together, our data reveal that L-selectin's surface density on neutrophils is regulated by ADAM17, but homeostatic L-selectin cleavage is not.
Introduction
L-selectin (CD62L) is a type-1 transmembrane protein that is constitutively and uniformly expressed by neutrophils. It plays a critical role in directing these cells to sites of inflammation. L-selectin's adhesive role is diverse and includes facilitating direct and indirect neutrophil tethering along the vascular wall.1 The latter process both accelerates and prolongs their accumulation.2-5
Consistent with L-selectin's broad role in directing leukocyte accumulation, it is tightly regulated. This includes a rapid and efficient down-regulation from the surface of neutrophils upon their activation by various stimuli, with virtually all of the L-selectin molecules being released by a proteolytic process.6 In addition to neutrophil activation, L-selectin shedding also occurs by these cells upon induced and spontaneous apoptosis.7 The cleavage of L-selectin takes place proximal to the cell membrane, releasing an intact ectodomain fragment.8,9 The soluble ectodomain portion of L-selectin is detected in the blood plasma, demonstrating that L-selectin shedding is a physiologic process. The functional relevance of L-selectin shedding is not yet fully understood. Studies involving gene-targeted mice expressing noncleavable L-selectin indicate that ectodomain shedding regulates surface L-selectin density, and this affects the efficiency of leukocyte adhesion and migration.20 Homeostatic L-selectin cleavage accounts for high levels of the receptor in the plasma of healthy individuals and mice relative to other leukocyte membrane proteins,10,11 and perhaps this process maintains an adhesion buffer.
L-selectin cleavage primarily occurs by a zinc-dependent metalloprotease activity in a cis manner.12-14 Tumor necrosis factor (TNF)–α–converting enzyme (TACE), also referred to as a disintegrin and metalloprotease (ADAM17), has been proposed to be a primary sheddase of L-selectin. Adam17 gene targeting (ADAM17 lacking a Zn-binding domain; ADAM17ΔZn/ΔZn) results in perinatal lethality in mice. An examination of their fetal thymocytes revealed that L-selectin surface expression did not down-regulate upon their activation with a phorbol ester.15 In addition, reconstitution experiments using recombinant ADAM17 increased L-selectin shedding in an ADAM17-deficient cell line; however, L-selectin ectodomain shedding also occurred by an ADAM17-independent process.14 At this time, there is no direct evidence that ADAM17 is the primary sheddase of L-selectin by mature leukocytes. We addressed this using radiation-chimeric mice reconstituted with ADAM17ΔZn/ΔZn leukocytes.
Materials and methods
Antibodies and other reagents
The monoclonal antibodies (mAbs) PE-conjugated anti–L-selectin (MEL-14, rat IgG2a), allophycocyanin-conjugated anti-CD11b (M1/70, rat IgG2b), allophycocyanin-conjugated anti-CD3e (145-2C11, hamster IgG), and FITC-conjugated Ly-6G (Gr-1) (RB6-8C5, rat IgG2b) were purchased from eBioscience (San Diego, CA). Biotin-conjugated anti–L-selectin (LAM1-116) mAb was purchased from Ancell (Bayport, MN). Conjugated and unconjugated rat and hamster isotype-matched negative control antibodies were purchased from Caltag Laboratories (Burlingame, CA). Horseradish peroxidase conjugated to streptavidin was purchased from Pierce (Rock-ford, IL). Annexin V–FITC was purchased from BD Pharmingen (San Diego, CA). RPMI, PBS, HEPES, and molecular-grade water were purchased from Mediatech (Herndon, VA). PMA was purchased from Sigma (St Louis, MO). TAPI-0 (Peptides International, Louisville, KY), a hydroxamate metalloprotease inhibitor, has previously been described.7,13,16 Cyanogen bromide–activated Sepharose 4B was purchased from Amer-sham Biosciences (Uppsala, Sweden). Erythrolyse was purchased from Serotech (Oxford, United Kingdom).
Animals and manipulations
Experimental procedures were approved by the Animal Care and Use Committee of the University of Minnesota. C57BL/6 wild-type mice were purchased from the Jackson Laboratory (Bar Harbor, ME). ADAM17+/ΔZn mice (C57BL/6J/129Sv) were generously provided by Amgen (Seattle, WA) via Dr David Lee (University of North Carolina). ADAM17 deficiency in mice is lethal between embryonic day 17.5 and soon after birth.15 Thus, fetal liver cells were isolated from ADAM17+/+ and ADAM17ΔZn/ΔZn embryos at gestation day 16.5 and injected into irradiated C57BL/6J recipient mice (10 Gy [1000 rad]; 1/3 of a homogenized liver per mouse). The genotype of the embryos was determined by polymerase chain reaction. Peritonitis was induced in the chimeric mice and wild-type C57BL/6 mice by thioglycollate injection [1 mL of 3% wt/vol; Sigma].
Neutrophil culture
Tissue-culture dishes (24-well) were blocked with PBS containing 20% normal mouse serum. After extensively washing coated wells with PBS, freshly isolated bone marrow neutrophils were then cultured for approximately 24 hours at a concentration of 2 × 106 to 3 × 106/mL in RPMI plus 5 mM HEPES. All reagents used for neutrophil isolation and incubations were sterile and were tested for endotoxin. For neutrophil treatment with TAPI-0 (75 μg/mL), the agent was added to the medium and incubations were performed for the indicated time points.
Cell labeling and flow cytometry
Leukocytes from peripheral blood, bone marrow (erythrocytes were lysed using Erythrolyse, as per the manufacturer's instructions), and the peritoneal cavity were stained with particular mAbs, as previously described.17 Cells were fixed in 1% paraformaldehyde and the 1- to 3-color stains were analyzed on a FACSCalibur instrument (Becton Dickinson, San Jose, CA). In some cases, cells were incubated with PMA (25 ng/mL) at 37°C for 20 minutes before staining the cells with mAbs. For cell staining that involved annexin V–FITC, nonfixed cells were used, and following their staining with antibodies, the cells were treated with annexin V–FITC (per the manufacturer's instructions) and immediately examined by flow cytometry, as previously described.7
L-selectin ELISA
Sandwich enzyme-linked immunosorbent assay (ELISA) for mouse L-selectin was performed using a kit from R&D Systems (Minneapolis, MN) per the manufacturer's instructions. All plasma samples were diluted 1/100 in PBS and then passed through a syringe filter to remove membrane fragments larger than 0.22 μm.
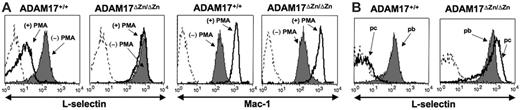
Induced L-selectin shedding by neutrophils from ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice. (A) Peripheral-blood leukocytes from the chimeric mice were treated with or without PMA for 20 minutes, as indicated, and then double-stained for surface expression of L-selectin (left row) or Mac-1 (right row) and neutrophil marker Ly-6G. (B) Peripheral-blood (pb) and peritoneal-cavity (pc) neutrophils from the chimeric mice following 2 hours of thioglycollate-induced peritonitis were double-stained for surface expression of L-selectin and neutrophil marker Ly-6G. Relative staining levels were determined by flow cytometry. For all histogram plots, the dashed line indicates staining by an isotype-matched negative control mAb. The y-axis indicates cell number and the x-axis indicates log-10 fluorescence. Results are representative of 3 or more independent experiments.
Induced L-selectin shedding by neutrophils from ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice. (A) Peripheral-blood leukocytes from the chimeric mice were treated with or without PMA for 20 minutes, as indicated, and then double-stained for surface expression of L-selectin (left row) or Mac-1 (right row) and neutrophil marker Ly-6G. (B) Peripheral-blood (pb) and peritoneal-cavity (pc) neutrophils from the chimeric mice following 2 hours of thioglycollate-induced peritonitis were double-stained for surface expression of L-selectin and neutrophil marker Ly-6G. Relative staining levels were determined by flow cytometry. For all histogram plots, the dashed line indicates staining by an isotype-matched negative control mAb. The y-axis indicates cell number and the x-axis indicates log-10 fluorescence. Results are representative of 3 or more independent experiments.
Immunoprecipitation and immunoblotting
Mouse serum were precleared and immunoprecipitated with Sepharose-bound, isotype-matched negative control mAb and Sepharose-bound MEL-14 mAb, respectively. The MEL-14–Sepharose was washed extensively and specifically bound material was eluted with SDS sample buffer and boiling. Samples were resolved on a 12.5% ProPure SDS-PAGE gel (Amresco, Solon, OH) under nonreducing conditions. The separated proteins were electrotransferred to a PVDF membrane, which was blocked with Tris-buffered saline (TBS)–T containing 20% normal goat serum. The membrane was probed with biotin-conjugated LAM1-116 mAb, directed against the ectodomain of human and mouse L-selectin,18 followed by horseradish peroxidase conjugated to streptavidin. The membrane was washed and visualized by addition of SuperSignal chemiluminescent substrate (Pierce) and exposure to film, as per the manufacturer's instructions.
Results
ADAM17 is critical for induced L-selectin shedding in vitro and in vivo
Lethally irradiated wild-type C57BL/6 mice were injected with fetal liver cells isolated from ADAM17+/+ or ADAM17ΔZn/ΔZn embryos. Following hematopoietic-cell reconstitution for at least 10 weeks, leukocyte counts and differentials were assessed in the chimeric mice and the wild-type C57BL/6 mice. Total leukocyte counts were similar for both groups of chimeric mice, although total leukocyte and absolute lymphocyte counts tended to be higher for both groups of chimeric mice compared with age-matched C57BL/6 mice (data not shown).
The effects of ADAM17 deficiency on L-selectin shedding was initially assessed by treating peripheral-blood leukocytes from the ADAM17ΔZn/ΔZn-chimeric and control mice with PMA. Surface L-selectin levels greatly decreased on neutrophils from ADAM17+/+-chimeric mice (Figure 1A) and wild-type C57BL/6 mice (data not shown) upon their activation, whereas there was no apparent decrease in the surface expression of L-selectin on neutrophils from the ADAM17ΔZn/ΔZn-chimeric mice upon their activation (Figure 1A), as determined by flow cytometry. Unlike control mice, peripheral-blood T cells (CD3+) and monocytes (Mac-1+/Ly-6G–) from ADAM17ΔZn/ΔZn-chimeric mice also failed to down-regulate their surface L-selectin (data not shown). These findings indicate that induced L-selectin shedding is mediated by ADAM17, and that this proteolytic process is not functionally redundant. ADAM17 deficiency did not prevent leukocyte activation, as neutrophils from ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice up-regulated their surface expression of Mac-1 (CD11b/CD18) following PMA treatment (Figure 1A), and they underwent characteristic shifts in their forward and side-light scatter profiles (data not shown).
Transendothelial migration of neutrophils into sites of inflammation leads to their activation in a manner that is much more complex than can be mirrored in vitro. Neutrophils that have migrated into the inflamed peritoneal cavity of mice have greatly reduced levels of L-selectin expression compared with neutrophils in the peripheral blood.19 This does not, however, occur by neutrophils expressing a cleavage-resistant L-selectin transgene,20 indicating the involvement of ectodomain shedding. We examined the role of ADAM17 in L-selectin cleavage upon neutrophil transendothelial migration by injecting thioglycollate into the peritoneal cavity of the ADAM17ΔZn/ΔZn-chimeric and control mice. Following 2 hours of induced peritonitis, neutrophils that infiltrated the inflamed peritoneal cavity of ADAM17+/+-chimeric mice (Figure 1B) and wild-type C57BL/6 mice (data not shown) expressed considerably lower levels of L-selectin compared with neutrophils in their peripheral blood. Neutrophil infiltration into the inflamed peritoneal cavity of ADAM17ΔZn/ΔZn-chimeric mice was not diminished, and these cells were found to express equivalent or higher levels of surface L-selectin compared with neutrophils in the peripheral blood (Figure 1B). Activated neutrophils are reported to express L-selectin mRNA and actively synthesize L-selectin,7,8,21 which may increase the levels of surface L-selectin by activated neutrophils deficient in ADAM17.
In the absence of ADAM17, basal expression of surface L-selectin increases
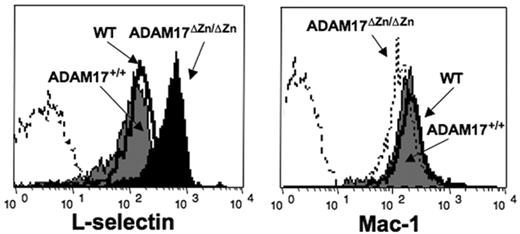
Surface L-selectin levels were observed to be more than 2-fold higher on peripheral-blood neutrophils from ADAM17ΔZn/ΔZn-chimeric mice compared with ADAM17+/+-chimeric mice and wild-type C57BL/6 mice (Figure 2). This was also the case for bone marrow–derived neutrophils (data not shown) as well as peripheral-blood lymphocytes and monocytes (Table 1). Noncleavable cell-surface determinants such as Mac-1 were expressed at equivalent levels on peripheral-blood neutrophils from ADAM17ΔZn/ΔZn-chimeric mice and control mice (Figure 2), demonstrating that ADAM17 deficiency did not cause a global up-regulation in expression of cell-surface molecules.
ADAM17 deficiency does not alter L-selectin shedding upon spontaneous neutrophil apoptosis or the production of plasma L-selectin
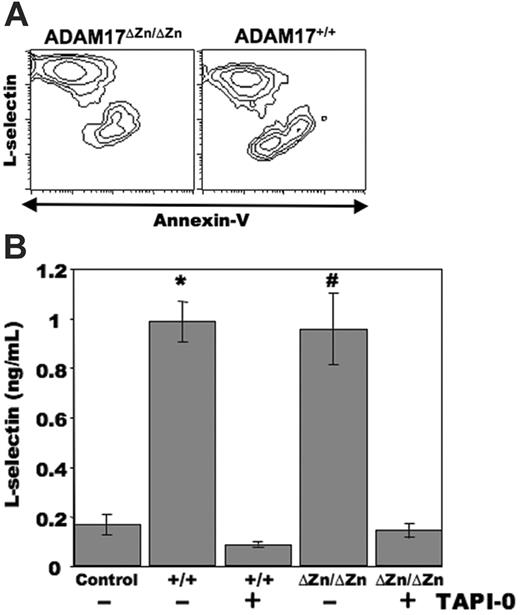
Neutrophils have a short lifespan and undergo spontaneous apoptosis in culture.22 We have recently reported that in addition to leukocyte activation, L-selectin shedding occurs by human neutrophils undergoing apoptosis.7 Mouse neutrophils were cultured for approximately 24 hours, and apoptotic cells were distinguished by annexin V–FITC reactivity in combination with flow cytometry. As with human neutrophils, we observed a considerable down-regulation in L-selectin surface expression by neutrophils undergoing apoptosis (annexin V–bright) when compared with the nonapoptotic neutrophils (annexin V–dull) from wild-type C57BL/6 mice (data not shown) and ADAM17+/+-chimeric mice (Figure 3A). Of interest was that a similar proportion of neutrophils from the ADAM17ΔZn/ΔZn-chimeric mice underwent spontaneous apoptosis as well, and these cells efficiently down-regulated their surface L-selectin (Figure 3A). The cultured neutrophils from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice were observed to produce equivalent amounts of soluble L-selectin, as determined by ELISA, which was greatly diminished by the hydroxamate metalloprotease inhibitor TAPI-0 (Figure 3B). We have shown that TAPI-0 specifically abrogates L-selectin proteolysis, but not other cellular events,12,13,16 and, as observed with human neutrophils,7 it did not have a significant effect on the level of spontaneous apoptosis by mouse neutrophils (data not shown). These findings thus suggest that a sheddase mechanism other than ADAM17 participates in the down-regulation of L-selectin by neutrophils undergoing spontaneous apoptosis.
Basal L-selectin expression levels by neutrophils from ADAM17ΔZn/ΔZn and control mice. Resting peripheral-blood leukocytes from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice, and wild-type C57BL /6 mice (WT) were double-stained for surface expression of L-selectin (left panel) or Mac-1 (right panel) and neutrophil marker Ly-6G. Relative staining levels were determined by flow cytometry. The dashed line indicates staining by an isotype-matched negative control mAb. The y-axis indicates cell number and the x-axis indicates log-10 fluorescence. Results are representative of 3 or more independent experiments.
Basal L-selectin expression levels by neutrophils from ADAM17ΔZn/ΔZn and control mice. Resting peripheral-blood leukocytes from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice, and wild-type C57BL /6 mice (WT) were double-stained for surface expression of L-selectin (left panel) or Mac-1 (right panel) and neutrophil marker Ly-6G. Relative staining levels were determined by flow cytometry. The dashed line indicates staining by an isotype-matched negative control mAb. The y-axis indicates cell number and the x-axis indicates log-10 fluorescence. Results are representative of 3 or more independent experiments.
L-selectin down-regulation by neutrophils from ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice following spontaneous apoptosis. (A) Bone marrow neutrophils from the indicated mice were cultured for approximately 24 hours, and then triple-stained for surface expression of L-selectin, neutrophil marker Ly-6G, and annexin V–FITC. Relative staining levels were determined by flow cytometry. The contour plots represent gated neutrophils, and the x- and y-axes indicate log-10 fluorescence. (B) Bone marrow neutrophils from the ADAM17ΔZn/ΔZn (ΔZn/ΔZn)– and ADAM17+/+ (+/+)–chimeric mice were cultured for approximately 24 hours in the presence or absence of the metalloprotease inhibitor TAPI-0, as indicated. Media supernatant was then collected and passed through a syringe filter to remove membrane fragments larger than 0.22 μm. The presence of soluble L-selectin in the media was determined by ELISA analysis, as described in “Materials and Methods.” Neutrophils from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice were plated in triplicate wells, and the supernatant from each well was analyzed in duplicate. Each bar represents the mean ± SD. *P < .01 versus ADAM17+/+ plus TAPI-0; #P < .01 versus ADAM17ΔZn/ΔZn plus TAPI-0. Data are representative of 3 independent experiments.
L-selectin down-regulation by neutrophils from ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice following spontaneous apoptosis. (A) Bone marrow neutrophils from the indicated mice were cultured for approximately 24 hours, and then triple-stained for surface expression of L-selectin, neutrophil marker Ly-6G, and annexin V–FITC. Relative staining levels were determined by flow cytometry. The contour plots represent gated neutrophils, and the x- and y-axes indicate log-10 fluorescence. (B) Bone marrow neutrophils from the ADAM17ΔZn/ΔZn (ΔZn/ΔZn)– and ADAM17+/+ (+/+)–chimeric mice were cultured for approximately 24 hours in the presence or absence of the metalloprotease inhibitor TAPI-0, as indicated. Media supernatant was then collected and passed through a syringe filter to remove membrane fragments larger than 0.22 μm. The presence of soluble L-selectin in the media was determined by ELISA analysis, as described in “Materials and Methods.” Neutrophils from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice were plated in triplicate wells, and the supernatant from each well was analyzed in duplicate. Each bar represents the mean ± SD. *P < .01 versus ADAM17+/+ plus TAPI-0; #P < .01 versus ADAM17ΔZn/ΔZn plus TAPI-0. Data are representative of 3 independent experiments.
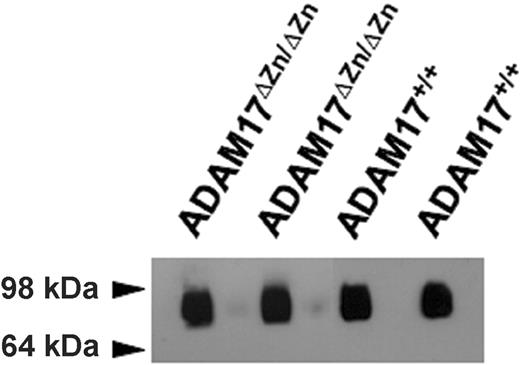
Continuous L-selectin shedding maintains high levels of soluble receptor in the blood of healthy individuals and mice.10,11 The role of ADAM17 in homeostatic L-selectin shedding was assessed by comparing the levels of plasma L-selectin from ADAM17ΔZn/ΔZn-chimeric mice and control mice. Remarkably, plasma L-selectin levels for the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice were found not to be significantly different (0.47 ± 0.12 μg/mL and 0.51 ± 0.17 μg/mL, respectively [SD; n = 4 for each group of chimeric mice]). In addition, equivalent levels of L-selectin were detected in the plasma of ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice by immunoblot analysis (Figure 4).
Discussion
L-selectin plays a broad role in directing neutrophils to diverse inflammatory settings. Efficient and rapid ectodomain shedding tightly regulates its surface expression. An important deficiency in our current understanding of this process is the sheddase mechanism(s) involved. We demonstrate that ADAM17 is the primary sheddase of L-selectin upon the overt activation of mature neutrophils both in vitro and in vivo. In addition, ADAM17 deficiency resulted in an overall increase in L-selectin expression by resting leukocytes, indicating a basal activity by the sheddase. Considering that a similar level of augmented L-selectin surface expression occurs by peripheral-blood leukocytes from gene-targeted mice expressing a noncleavable L-selectin construct,20 ADAM17 would thus appear to be the primary means of constitutive L-selectin shedding as well.
Despite the critical role of ADAM17 in induced and constitutive L-selectin shedding, equivalent levels of plasma L-selectin were detected in the ADAM17ΔZn/ΔZn-chimeric mice and control mice. L-selectin enters the plasma as a cleaved and intact receptor.20 We did not have an antibody to the cytoplasmic region of mouse L-selectin to assess the proportion of plasma L-selectin from the ADAM17ΔZn/ΔZn-chimeric mice that was intact receptor. However, various evidence suggest that intact L-selectin is unlikely to account for the normal plasma levels of L-selectin occurring in the ADAM17ΔZn/ΔZn-chimeric mice. For instance, Venturi et al have established that plasma L-selectin is derived primarily from receptor cleavage, as gene-targeted mice expressing noncleavable L-selectin have considerably lower levels of plasma L-selectin.20 Hence, their study did not reveal a compensatory event by intact L-selectin. In addition, the plasma samples examined by ELISA in our study were subjected to 0.22-μm filtration, which would remove much of the membrane fragments that are a source of intact L-selectin. Our findings thus indicate that ADAM17 is not critical for homeostatic L-selectin shedding. Certain inflammatory or disease conditions in humans can increase the levels of plasma L-selectin,23-26 which may reflect ADAM17 activity. However, this effect appears not to be as apparent in mice,11 and changes in plasma L-selectin levels in control and ADAM17ΔZn/ΔZn-chimeric mice were also not evident during the inflammatory process investigated here.
Immunoprecipitation and immunoblot blot analysis of plasma L-selectin levels from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice. Plasma (approximately 40 μL) from 2 ADAM17ΔZn/ΔZn- and 2 ADAM17+/+-chimeric mice were subjected to immunoprecipitation using the L-selectin mAb MEL-14 and then to nonreducing SDS-PAGE and immunoblotting, as described in “Materials and Methods.”
Immunoprecipitation and immunoblot blot analysis of plasma L-selectin levels from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice. Plasma (approximately 40 μL) from 2 ADAM17ΔZn/ΔZn- and 2 ADAM17+/+-chimeric mice were subjected to immunoprecipitation using the L-selectin mAb MEL-14 and then to nonreducing SDS-PAGE and immunoblotting, as described in “Materials and Methods.”
The underlying events that contribute to the continuous production of cleaved L-selectin in the blood of healthy individuals are not well understood at this time. Homeostatic L-selectin shedding appears to be an important process, since reduced levels of plasma L-selectin correlate with susceptibility to inflammatory disease.27,28 Of interest is that similar levels of L-selectin shedding occurred by neutrophils from the ADAM17ΔZn/ΔZn- and ADAM17+/+-chimeric mice upon their spontaneous apoptosis. Considering that in humans approximately 1.6 × 109 neutrophils/kg body weight are turned over every day,29 senescent neutrophils undergoing apoptosis may be a key ADAM17-independent source of plasma L-selectin. A metalloprotease inhibitor, however, was found to reduce the down-regulation of L-selectin surface expression by apoptotic neutrophils and their production of soluble L-selectin (Figure 3B; Walcheck et al7 ). Incidentally, we have also shown that an ADAM17-deficient cell line can spontaneously cleave L-selectin within its membrane-proximal stalk region by a metalloprotease process.14 An additional sheddase of L-selectin has yet to be described. Various zinc-dependent metalloproteases are reported to participate in ectodomain shedding, including particular matrix metalloproteases and ADAMs.30 ADAM10 is an appealing candidate in that of the ADAM family members, ADAM17 and ADAM10 are the most similar in terms of structure (29% identical and 50% similar at the amino-acid level), domain composition, and function.31-34 Indeed, other substrates can be cleaved by both ADAMs, including amyloid precursor protein, interleukin-6 receptor (IL-6R), and pro–TNF-α.31,35-37 Condon et al have reported that ADAM10 is not a sheddase of L-selectin upon cell activation.38 Ongoing studies are examining its role, as well as other metalloproteases, in ectodomain shedding during neutrophil apoptosis.
L-selectin shedding may have distinct purposes and therefore is regulated by different sheddases. For instance, induced and constitutive L-selectin cleavage appears to control the receptor's cell-surface density, and this regulates neutrophil migration into sites of inflammation,20 whereas homeostatic L-selectin shedding may maintain soluble L-selectin levels in the blood, perhaps to diminish leukocyte-endothelium interactions occurring below a critical level of inflammation. Considering that homeostatic L-selectin turnover is a constant process, most L-selectin shedding may occur by a protease other than ADAM17.
It is clear from various in vitro and in vivo studies that L-selectin cleavage is not required for neutrophils to undergo transendothelial migration.20,39,40 Our findings are consistent with these studies. However, assessing the effects of the absence of functional ADAM17 on neutrophil migration into sites of inflammation were beyond the scope of this study, as the contributions and counterbalances of various putative ADAM17 substrates need to be considered, including intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and TNF-α.32,34,41,42
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2006-02-005827.
Supported by grant HL61613 (B.W.) from the National Institutes of Health.
Y.L. performed research; J.B. performed research; A.H. contributed analytic tools; and B.W. designed research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Stephen Jameson and Xiao-Jie Ding for their technical assistance in generating the radiation chimeric mice; Drs David Lee, Susan Wohler-Sunnarborg, Roy Black, and Jacques Peschon for providing a breeding pair of ADAM17+/ΔZn mice; and Elaine Raines for thoughtful discussions.