Abstract
Mitochondrial ferritin (MtFt) is a mitochondrial iron-storage protein whose function and regulation is largely unknown. Our previous results have shown that MtFt overexpression markedly affects intracellular iron homeostasis in mammalian cells. Using tumor xenografts, we examined the effects of MtFt overexpression on tumor iron metabolism and growth. The expression of MtFt dramatically reduced implanted tumor growth in nude mice. Mitochondrial iron deposition in MtFt-expressing tumors was directly observed by transmission electron microscopy. A cytosolic iron starvation phenotype in MtFt-expressing tumors was revealed by increased RNA-binding activity of iron regulatory proteins, and concomitantly both an increase in transferrin receptor levels and a decrease in cytosolic ferritin. MtFt overexpression also led to decreases in total cellular heme content and heme oxygenase-1 levels. In addition, elevated MtFt in tumors was also associated with a decrease in total aconitase activity and lower frataxin protein level. In conclusion, our study shows that high MtFt levels can significantly affect tumor iron homeostasis by shunting iron into mitochondria; iron scarcity resulted in partially deficient heme and iron-sulfur cluster synthesis. It is likely that deprivation of iron in the cytosol is the cause for the significant inhibition of xenograft tumor growth.
Introduction
Iron is indispensable for nearly all forms of life. Because iron is virtually insoluble and can be highly toxic when present in excess in its free form, cells possess specialized molecules for the acquisition, transport, and storage of iron in safe and accessible forms. Ferritins are ubiquitous iron-storage proteins made of 24 subunits that form a spherical shell accommodating up to 4500 iron atoms.1,2 Nearly all of the cellular ferritins are found in the cytoplasm where their expression is controlled post-transcriptionally by iron. The size of the labile iron pool (LIP) modulates the binding of iron regulatory proteins (IRPs) to iron responsive elements (IREs) present in the 5′-untranslated regions (UTRs) of ferritin mRNAs. When the level of iron in the LIP is low, translation of ferritin mRNA is suppressed by the binding of IRPs to IREs, whereas iron supplementation up-regulates ferritin synthesis through the dissociation of IRPs from IREs.3-6 Cytosolic ferritin is composed of 2 subunits, H and L, which have approximately 50% sequence identity; the former subunit has ferroxidase activity that is essential for the storage of iron in ferritin as ferricoxohydrate phosphate, whereas the latter subunit has a nucleation site that is involved in iron-core formation.1,2
Mitochondrial ferritin (MtFt) is a recently identified ferritin heavy chain like protein.7 In humans, MtFt is encoded by an intronless gene on chromosome 5q23.1 and is expressed as a 30-kDa precursor protein, which possesses a mitochondrial leader sequence and is targeted exclusively to mitochondria.7-9 Mice have a similar MtFt gene localized on chromosome 18D1 (gene ID: 67 634). Both human and mouse MtFt precursors are imported into mitochondria and processed to an approximately 22 kDa mature protein in the mitochondrial matrix,8,10 where they assemble into homopolymeric ferritin shells with ferroxidase activity.11 Normally, MtFt is expressed at extremely low levels in most cells and tissues except the testis9 where it shows substantial levels of expression. However, it is highly expressed in erythroblast mitochondria (“ringed sideroblasts”) of patients with X-linked and acquired sideroblastic anemia12 as well as those with refractory anemia with ringed sideroblasts13 (RARS, a subgroup of myelodys-plastic syndrome patients). It is well-known that ringed sideroblasts are pathologic erythroid precursors containing excessive mitochondrial deposits of non-heme iron with a characteristic perinuclear distribution accounting for their ring appearance.14-16 Importantly, it has been shown that the iron in ringed sideroblasts is primarily stored within MtFt.12
Mitochondria play a key role in iron metabolism, as both heme synthesis and iron-sulfur cluster (ISC) assembly occur mainly, if not entirely, within the organelles.17-20 Mitochondrial iron content must be tightly regulated, as both mitochondrial iron deficiency and excess impair the metabolic and respiratory activities of the organelle.17,19,20 So far, several human disorders characterized by mitochondrial iron overload have been linked with defects in mitochondrial iron transport and utilization.20 Besides the erythroblasts of patients with X-linked and acquired sideroblastic anemia,14-18 mitochondrial iron accumulation has also been well documented in cardiomyocytes of patients with Friedreich ataxia21,22 and in erythroblasts of patients with sideroblastic anemia with ataxia.23 In addition, yeast studies have demonstrated that a defect in the ISC assembly pathway also leads to mitochondrial iron homeostasis disruption.24
Iron also plays an important role in the regulation of cell growth via oxygen transport and utilization and DNA synthesis.25,26 In order to maintain high proliferative rates, cancer cells require large amounts of iron for related proteins, including ribonucleotide reductase (RNR), whose activity relies on a labile iron center in its subunit B (R2).27-29 This characteristic renders tumor cells sensitive to iron deprivation. Many in vitro and in vivo studies and clinical trials have demonstrated that iron chelators are effective antineo-plastic agents.30-35
Recent results from our laboratory10 and others8 show that MtFt, overexpressed in human H1299 cells and HeLa cells, is a functional protein that can modulate iron metabolism. Overexpression of MtFt leads to cellular iron redistribution whereby large amounts of iron are translocated to mitochondria and stored in MtFt. This results in cytosolic iron deprivation and decreases both cytosolic and mitochondrial aconitase activities.10 Therefore, we speculate that iron scarcity by MtFt expression may suppress tumor growth. In the current study, we took advantage of tumor xenografts as an in vivo system to further study the function of MtFt overexpression on cellular iron metabolism and explore the possibility that MtFt iron sequestration inhibits tumor growth.
Materials and methods
Materials
Paraformaldehyde, glutaraldehyde solution (25%, Grade I), anti–β-actin antibody, horseradish peroxidase–conjugated anti–rabbit, anti–mouse, and anti–horse immunoglobulin G (IgG) were purchased from Sigma (Oakville, ON, Canada). Antibodies against hemagglutinin (HA) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antifrataxin antibody was generously provided by Dr Grazia Isaya (Mayo Clinic College of Medicine, Rochester, MN). Antitransferrin receptor (TfR) antibody was purchased from Zymed (South San Francisco, CA). Antiferritin antibody was obtained from Dako (Carpinteria, CA) and anti–poly-ADP-ribose-polymerase (PARP) antibodies were purchased from R&D Systems (Minneapolis, MN) and Roche Diagnostics (Indianapolis, IN).
Plasmid constructs and generation of a stable MtFt-expressing cell line
The mouse mitochondrial ferritin cDNA encoding the full open reading frame was polymerase chain reaction (PCR) amplified and cloned into the EcoRI/BamHI site of pUHD10-3 to yield pUHD-MtFt with a C-terminal hemagglutinin (HA) epitope.10 The pUHD-MtFt plasmid was cotransfected with the puromycin-resistant pBabe plasmid into tTA-H1299 cells36 by Lipofectamine Plus (Invitrogen, Burlington, ON, Canada). Stable clones were positively selected and one of these stable transfectants, the B9 clone, which was characterized in our previous study,10 was used in the current study.
Cell cultures
H1299 parental cells (non–small cell lung carcinoma) and B9 clone (overexpressing MtFt) were maintained in Dulbecco modified Eagle medium supplemented with 10% (vol/vol) tetracycline-free fetal bovine serum (Clontech, Mississauga, ON, Canada), 2 mM glutamine, 100 U/mL penicillin, and 0.1 ng/mL streptomycin. MtFt expression was induced by removal of tetracycline from the culture medium (tet-off system).
Nude mouse xenograft system
Female athymic Balb/c nu/nu mice, 5 weeks of age and specific pathogen-free, were obtained from Charles River Laboratories (Wilmington, DE). Mice were housed in microisolator cages with autoclaved bedding in a specific pathogen-free facility with 12-hour light-dark cycles. Animals received water and food ad libitum, and were observed for signs of tumor growth, activity, feeding, and pain, in accordance with the guidelines of the McGill University Animal Care Committee. Two groups of mice (8 mice in the H1299 group and 14 mice in the B9 group, which included 6 mice with doxycycline administration in the drinking water) were inoculated with 2 × 106 H1299 and B9 cells in 0.2 mL phosphate-buffered saline (PBS; 1 × 107cells/mL) subcutaneously. Tumor dimensions were measured weekly using micrometer calipers after the tumors became visible. Tumor volumes were calculated using the following formula: volume = 0.5a × b2, where a and b represent the larger and smaller tumor diameters, respectively. After 4, 6, 9, and 10 weeks of injection, mice were humanely killed with Avertin (0.4 mL of 2.5% Avertin) and the primary tumors and organs (livers and spleens) were excised, immediately weighed, and stored in –80°C.
Administration of doxycycline to mice that underwent transplantation, to inhibit MtFt expression
Doxycycline (Dox; 0.2 mg/mL) was given to mice bearing B9 tumors in drinking water. The water bottles were protected from light and the Dox solution was changed twice a week. One group of mice was given Dox at the time when tumors became visible (about 4 week after injection) up to 10 weeks after injection.
Western blotting
Tumors were homogenized and lysed with Munro buffer (10 mM HEPES, pH 7.5, 3 mM MgCl2, 40 mM NaCl, 5% glycerol, 1 mM dithiothreitol, and 0.2% Nonidet P-40) and protein content was determined by the Bradford assay (Bio-Rad, Hercules, CA). The cell lysates were immediately boiled in Laemmli loading buffer for 10 minutes. Equal amounts of protein were loaded, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose blotting membrane (Pall, Pensacola, FL). The blots were blocked by incubating them for 1 hour with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBST) and hybridized with primary antibodies. After washing 3 times for 15 minutes each with PBST, the blots were incubated for 1 hour with horseradish peroxidase–linked secondary antibody. Peroxidase-coupled secondary antibodies were detected with the ECL plus (Amersham, Buckinghamshire, United Kingdom).
IRP binding ability
A gel retardation assay, as described previously,10 was used to measure the interaction between IRPs and IREs.
Transmission electron microscopy (TEM) study
The tumors were excised and minced into 1-mm3 pieces and fixed with fresh 4% paraformaldehyde solution and 0.5% glutaraldehyde solution in PBS or 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, for 2 hours at 4°C, then washed with PBS or 0.1 M cacodylate buffer 3 times (10 minutes each) and kept in PBS or cacodylate at 4°C. The next day, the samples were postfixed with osmium tetroxide (0.1%) and potassium ferrocyanide 1.5% in H2O, dehydrated in graded alcohols, and embedded in Epon resin. Ultrathin sections, cut with a Reichert Ultracut ultramicrotome, were stained with uranyl acetate and lead citrate and examined with a Philips 410LS transmission electron microscope (Philips, Eindhoven, The Netherlands) equipped with a charge-coupled device (CCD) camera at a voltage of 80 Kv.
Heme content measurement
Heme content in tumors and livers was measured according to an established method.37 Briefly, tissues were weighed and homogenized in a glass-Teflon homogenizer containing RIPA buffer while keeping the samples on ice. Heme content in the tissue lysates was determined following its conversion to protoporphyrin IX by boiling in concentrated oxalic acid, by spectrofluorometry (LS55; PerkinElmer, Wellesley, MA).37 Protein concentration was determined by the Bradford assay (Bio-Rad).
Iron concentration measurement
Total nonheme iron was measured using the ferrozine method described by Fish.38
Aconitase activity measurement
Total aconitase activities (cytosolic and mitochondrial) were measured by isocitric dehydrogenase–coupled nicotinamide adenine dinucleotide phosphate (NADPH) formation as previously described.10
In vitro culture of xenograft-derived tumor cells
Six weeks after injection of B9 cells into 2 groups of nude mice (Dox-on group [200 μg/mL in drinking water] and Dox-off group), tumor xenografts were dissociated mechanically, minced into small pieces, and treated with trypsin (0.25%)–EDTA (0.38 g/L) (Invitrogen) for 30 minutes at 37°C. After removing tissue clumps, B9 Dox-on and B9 Dox-off xenograft-derived cells were cultured separately in vitro, and treated with and without Dox, respectively. Four days later, both Dox-on and Dox-off cells were seeded in 24-well plates (5000 cells/well) for the cell proliferation assay.
Measurement of cell proliferation
Cell proliferation was measured by MTS assay (Non-radioactive Cell Proliferation Assay; Promega, Madison, WI) according to the manufacturer's instructions with minor modifications.
Statistical analysis
All values are expressed as mean plus or minus the standard error of the mean (SEM) of 4 or more samples. Statistical analysis was performed using the Student t test. Results were considered statistically significant when P values were lower than .05 (except in Figure 6C, where P < .1 was considered a statistically significant difference).
Results
Engraftment of H1299 cells and B9 cells in athymic nude mice
To examine the tumorgenicity of parental H1299 cells and MtFt-expressing H1299 cells (B9 clone),10 2 × 106 cultured cells were injected subcutaneously into the flanks of nude (nu/nu) Balb/c mice. The mice consistently developed visible solid tumors within 3 to 4 weeks. The tumors grown in inoculated mice were localized at the site of injection with no obvious signs of invasion and metastasis during the 9 weeks. The tumors of mice injected with parental H1299 cells exhibited exponential growth characterized by shorter doubling time relative to B9 cell–derived tumors. Mice bearing H1299 cell–derived tumors developed abnormalities such as loss of body weight (40%-50%) and severe fatigue 7 to 9 weeks after subcutaneous injection and subsequently died within a week. In contrast, mice injected with B9 cells showed reduced tumor growth rates in comparison to H1299 cell–derived tumors, negligible weight loss, and did not die during our experimental period of 10 weeks.
MtFt expression in tumor xenografts
MtFt protein expression in tumor xenografts 4, 6, and 9 weeks after initial injection was analyzed by Western blotting using an anti-HA tag antibody (Figure 1). While no MtFt expression was detected in parental cells, strong expression of the protein was observed in all the B9 cell–derived tumors at different time points (Figure 1A, upper panel: 4 and 6 weeks; Figure 1B, first panel: 9 weeks). In agreement with our previous report,10 only mature MtFt can be observed in the solid tumors; the protein migrated as a single band with an apparent molecular mass of approximately 22 kDa on SDS-PAGE.
Expression of MtFt in tumor xenografts and effects of MtFt expression on TfR, cytosolic ferritin levels, and RNA binding activity of IRPs. (A) MtFt expression in parental H1299 cell (H)–derived and MtFt-expressing B9 cell (B9)–derived tumor xenografts at 4 and 6 weeks after tumor injection. (B) Enhanced expression of TfR and decreased expression of cytosolic ferritin were observed in MtFt-expressing tumor xenografts. The tumor lysates were analyzed for protein expression of MtFt, TfR, cytosolic ferritin, and actin by Western blotting. (C) Increased RNA binding activity of IRPs in B9 cell–derived tumor lysates. Nine weeks after tumor cell injection, tumors were dissected and homogenized in Munro buffer. Equal amounts of tumor extracts were assayed for their ability to retard the migration of a 32P-labeled IRE probe in the absence (i) or presence of 2% β-ME (ii).
Expression of MtFt in tumor xenografts and effects of MtFt expression on TfR, cytosolic ferritin levels, and RNA binding activity of IRPs. (A) MtFt expression in parental H1299 cell (H)–derived and MtFt-expressing B9 cell (B9)–derived tumor xenografts at 4 and 6 weeks after tumor injection. (B) Enhanced expression of TfR and decreased expression of cytosolic ferritin were observed in MtFt-expressing tumor xenografts. The tumor lysates were analyzed for protein expression of MtFt, TfR, cytosolic ferritin, and actin by Western blotting. (C) Increased RNA binding activity of IRPs in B9 cell–derived tumor lysates. Nine weeks after tumor cell injection, tumors were dissected and homogenized in Munro buffer. Equal amounts of tumor extracts were assayed for their ability to retard the migration of a 32P-labeled IRE probe in the absence (i) or presence of 2% β-ME (ii).
MtFt expression induced tumor cellular iron scarcity in tumor xenografts
To scrutinize the function of MtFt in tumor iron metabolism, we examined the effect of MtFt expression on the levels of TfR and cytosolic ferritins as well as IRP binding activity. Both TfR and ferritin proteins were examined by Western blotting. In all B9-derived tumors, dramatically increased expression of TfR protein was consistently observed as compared with the H1299 parental cell–derived tumors (Figure 1B, second panel). This increase in levels of TfR is consistent with a cellular response to iron deficiency. The ferritin levels from the same tumor lysates were measured and a significant decrease in both H-ferritin and L-ferritin steady-state levels was found in B9-derived tumors as compared with H1299 cell–derived tumors (Figure 1B, third panel), whereas the levels of β-actin did not change (Figure 1B, bottom panel). Since IRPs sense intracellular iron levels by binding to IREs, present within the 5′- and 3′-UTRs of cytosolic ferritin and TfR mRNA, respectively, IRP binding activity was analyzed by gel retardation assays using an IRE probe. Expression of MtFt in B9 cell–derived tumor xenografts led to a significant increase in IRP binding activity (more than 3 times on average; quantified by densitometry) as compared with H1299 parental tumors (Figure 1Ci). There was no significant change in total IRPs activity that was revealed when the cell lysates were pretreated with β-mercaptoethanol (β-ME, which is commonly employed to convert cytosolic aconitase into the IRE-binding conformation in vitro39 ; Figure 1Cii). Since human tumor xenografts generated in mice (including xenograft tumors from cancerous human cells and surrounding mouse host tissue and vascular tissue) were estimated to contain between 10% and 20% host tissue, demonstrated by histologic analysis,40 both human and mouse IRPs were observed in our system. We therefore assume that the prominent upper band in our gel shift experiments corresponds to the human IRPs, while the less abundant, faster migrating band represents “contaminating” murine IRPs. In summary, the expression of MtFt in tumor xenografts promoted an apparent iron-deficient phenotype in B9 cell–derived tumors, as we previously observed in cell cultures.10
MtFt expression changed the intracellular iron content and decreased heme levels
We next examined the effects of MtFt expression on iron content and distribution in tumor xenografts. Strikingly, the total cellular heme amount was approximately 17.5% higher in the H1299 tumors than in the B9 tumors (Figure 2A), whereas there was no significant difference of heme content in the livers of mice bearing either H1299 cell– or B9 cell–derived tumors (Figure 2B). Moreover, the non–heme iron amount was much higher (75%) in B9 tumors than in H1299 tumors (7.70±1.99 ng Fe/mg protein in B9 tumors versus 4.41±1.54 ng Fe/mg protein in H1299 tumors; P < .01). Following the observation that total heme levels decreased in B9-derived tumor xenografts, we measured the level of heme oxygenase-1 (HO-1), a 32-kDa protein inducible by heme, which catalyzes the degradation of heme to produce equimolar quantities of biliverdin, CO, and free iron. In our experimental system, higher HO-1 protein levels were consistently observed in all H1299 cell–derived tumors as compared with B9 cell–derived tumors (Figure 2C).
MtFt expression significantly affected ISC metabolic pathways
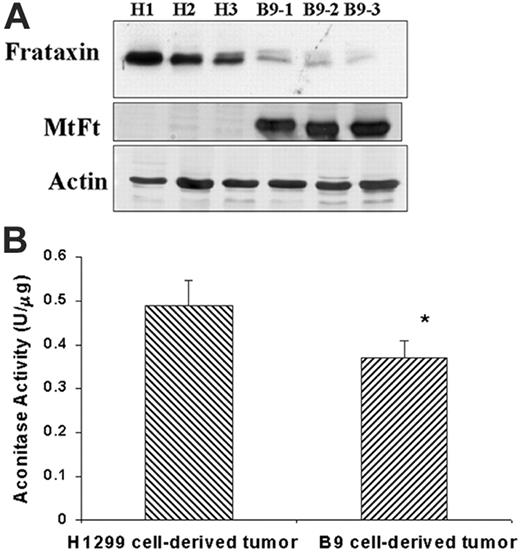
Both heme biosynthesis and iron sulfur cluster assembly pathways rely on the availability of intracellular iron.17-20,24 Moreover, a very recent study indicates that cellular heme biosynthesis is significantly affected by ISC assembly,41 and therefore we evaluated the effects of MtFt expression on ISC biosynthesis. Frataxin, a mitochondrial matrix protein, plays an important role in ISC synthesis and mitochondrial iron metabolism, and its defect causes Friedreich ataxia.21,42-45 We measured frataxin protein levels in H1299 cell– and B9 cell–derived tumors by Western blotting. Figure 3A shows that frataxin protein markedly decreased in MtFt-expressing tumors as compared with H1299 parental tumors, whereas actin levels remained constant. Next, we measured the activity of aconitase, an ISC-containing enzyme which converts citric acid to isocitric acid.46 The total aconitase activity (both cytosolic and mitochondrial), was measured in tumor extracts, with isocitric dehydrogenase–coupled NADPH formation. As expected, the total aconitase activity significantly decreased (by 33%) in B9 tumors as compared with H1299 tumors (Figure 3B). Together, these results indicate that ISC synthesis is sensitive to mitochondrial iron sequestration, leading us to propose that MtFt expression alters ISC cluster synthesis.
MtFt expression affects heme metabolism of tumor xenografts. Total heme content in the tumors (A) and in the livers (B) of tumor-bearing mice were measured by fluorescent spectrophotometry.37 Tumor and liver lysates were incubated with 2 M oxalic acid for 30 minutes in a boiling water bath, following which the fluorescence of heme-derived protoporphyrin IX was measured using 662 nm emission and 400 nm excitation (*P < .05). (C) Effect of MtFt expression on HO-1 protein expression. Tumor lysates identical to those in panel A were used to measure HO-1 protein levels by Western blotting.
MtFt expression affects heme metabolism of tumor xenografts. Total heme content in the tumors (A) and in the livers (B) of tumor-bearing mice were measured by fluorescent spectrophotometry.37 Tumor and liver lysates were incubated with 2 M oxalic acid for 30 minutes in a boiling water bath, following which the fluorescence of heme-derived protoporphyrin IX was measured using 662 nm emission and 400 nm excitation (*P < .05). (C) Effect of MtFt expression on HO-1 protein expression. Tumor lysates identical to those in panel A were used to measure HO-1 protein levels by Western blotting.
Effects of MtFt expression on frataxin expression and aconitase activity in tumors. (A) Frataxin and MtFt expression (Western blotting) in H1299-derived tumors (H) and MtFt-expressing tumors (B9). (B) Total aconitase activities of fresh whole tumor lysates (*P < .01 compared with H1299 cell–derived tumors).
Effects of MtFt expression on frataxin expression and aconitase activity in tumors. (A) Frataxin and MtFt expression (Western blotting) in H1299-derived tumors (H) and MtFt-expressing tumors (B9). (B) Total aconitase activities of fresh whole tumor lysates (*P < .01 compared with H1299 cell–derived tumors).
Morphologic observation of iron deposition in mitochondria of MtFt-expressing tumor xenografts
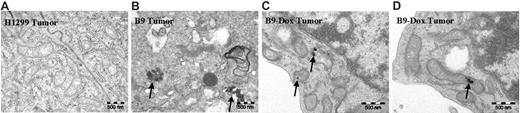
TEM has revealed (Figure 4B) that non–heme iron accumulated in B9 cell–derived tumors, and that this iron was indeed deposited in mitochondria in a form that was similar to that observed in ringed sideroblasts,47,48 whereas there was no detectable iron in the mitochondria of parental H1299 cell–derived tumors (Figure 4A). The accumulation of non–heme iron in mitochondria elicited some unusual mitochondrial morphology (Figure 4B). Importantly, oral administration of doxycycline (which blocks MtFt expression in the B9 tumors; Figure 5B) significantly decreased iron accumulation in the mitochondria (Figure 4C-D). These observations confirm that, in MtFt-expressing tumors, iron shunted to and accumulated in the mitochondria is stored in a form similar to the aberrant iron storage in mitochondria observed in pathologic conditions, such as sideroblastic anemia.47,48
MtFt expression significantly inhibited tumor growth
One of the intriguing findings of the current study is that significant inhibition of tumor growth was observed upon MtFt expression in tumor xenografts. In B9 tumors, as much as 75% reduction of tumor weight was found 9 weeks after tumor implantation as compared with the H1299 cell–derived tumors (Figure 5Ai). Measurement of tumor growth over time also indicated that MtFt expression significantly inhibited tumor burden size from the point at which the tumor became visible (4 weeks after injection) to 9 weeks after tumor inoculation (Figure 5Aii). The expression of MtFt in B9 cells can be repressed by tetracycline via specific binding to tetracycline-responsive promoter.10 In order to achieve the repression of MtFt and confirm that inhibition of tumor growth is directly linked with the expression of MtFt, doxycycline (Dox, a more active form of tetracycline) in drinking water was given to mice that underwent transplantation with B9 tumor. The result of Western blotting (Figure 5B) showed that the expression of MtFt protein was undetectable after the 6 weeks of Dox administration (Figure 5B, first panel). TfR protein levels also went down to control levels (Figure 5B, second panel). Tumor weight analysis (Figure 5C) demonstrated that Dox administration significantly (P < .1) stimulated B9 tumor burden as compared with the B9 tumor growth without Dox administration. Taken together, these results directly link the expression of MtFt with the tumor growth inhibition.
Transmission electron micrographs of H1299 and B9 (MtFt-expressing) tumors. No mitochondrial iron accumulation was observed in H1299 cell–derived tumors (A), whereas conspicuous iron deposits were detected in mitochondria of B9 cell–derived tumors (B). Oral administration of doxycycline to mice bearing B9 cell–derived tumors virtually abrogated mitochondrial iron accumulation (C-D). Representative transmission electron micrographs are shown. For all images, total original magnification is × 44/400.
Transmission electron micrographs of H1299 and B9 (MtFt-expressing) tumors. No mitochondrial iron accumulation was observed in H1299 cell–derived tumors (A), whereas conspicuous iron deposits were detected in mitochondria of B9 cell–derived tumors (B). Oral administration of doxycycline to mice bearing B9 cell–derived tumors virtually abrogated mitochondrial iron accumulation (C-D). Representative transmission electron micrographs are shown. For all images, total original magnification is × 44/400.
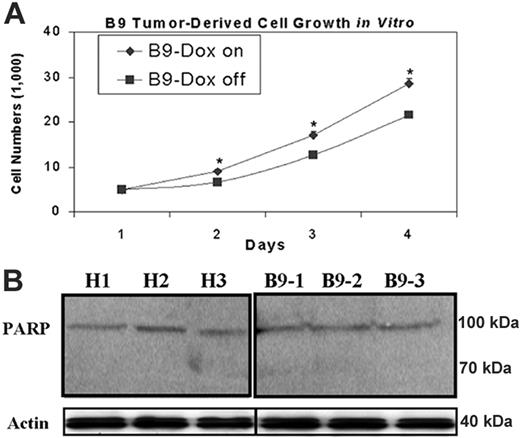
As iron chelators can both suppress cell proliferation33,35,49,50 and induce cell apoptosis,51 it is important to elucidate the mechanism responsible for abrogated tumor xenograft growth. Therefore, we measured both proliferation of in vitro–cultured B9 xenograft-derived tumor cells, treated with or without doxycycline, and an early apoptotic event in tumor xenografts, respectively. Using an MTS-based cell proliferation assay, we observed a significant decrease in cell proliferation in MtFt-overexpressing tumor-derived cells (B9 Dox-off cells) compared with B9 Dox-on cells, in which MtFt expression was suppressed by Dox (Figure 6A). In contrast, we did not observe apoptosis in any of the tumor tissues, as indicated by the lack of cleavage of the intact PARP to an 89-kDa fragment (an early indicator of apoptosis; Figure 6B). Taken together, our data indicate that in our system, MtFt overexpression inhibits tumor xenograft growth mainly, if not entirely, via suppression of cell proliferation.
MtFt expression inhibited tumor growth. (Ai) Weights of H1299 cell– and B9 cell–derived tumors (9 weeks after injection) and (ii) measurement of tumor burden growth over time. (B) MtFt and TfR expression (Western blotting) was repressed by oral administration of Dox to the mice (B9-Dox) (“Materials and methods”). (C) Weights of tumors (10 weeks after transplantation) obtained from mice that had (B9-Dox) or did not have (B9) doxycycline in their drinking water (*P < .1).
MtFt expression inhibited tumor growth. (Ai) Weights of H1299 cell– and B9 cell–derived tumors (9 weeks after injection) and (ii) measurement of tumor burden growth over time. (B) MtFt and TfR expression (Western blotting) was repressed by oral administration of Dox to the mice (B9-Dox) (“Materials and methods”). (C) Weights of tumors (10 weeks after transplantation) obtained from mice that had (B9-Dox) or did not have (B9) doxycycline in their drinking water (*P < .1).
Discussion
MtFt is an H-ferritin-like protein expressed exclusively in mitochondria sites of heme biosynthesis where most, if not all, Fe-S clusters are assembled. Under normal circumstances, MtFt is expressed in only a small number of tissues, mainly the testis.9 While MtFt is virtually undetectable in normal erythroid tissues, the ringed sideroblasts of patients with sideroblastic anemia have been shown to contain conspicuous levels of MtFt.7,12,13
Although the physiologic function of MtFt is still elusive, recent results from our laboratory10 and those of others8 show that overexpression of MtFt leads to a rapid and efficient redistribution of iron from various sources in the cytosol to mitochondria, where it is deposited in a form not available for metabolic use. Since MtFt expression prevented, at least in part, oxidative stress–induced damage of frataxin-defective yeast cells,52 it is tempting to speculate that MtFt ameliorates iron-mediated toxicity that would, without the expression of this protein, seriously damage erythroblast mitochondria of patients with sideroblastic anemia.
When overexpressed in cultured cells, MtFt causes a cytosolic iron starvation phenotype, exemplified by increased RNA-binding activities of IRPs, with consequent increases in TfR and decreases in cytosolic ferritin levels as well as decreases in aconitase (both mitochondrial and cytoplasmic) activities.10 In the present study, we observed similar changes in the tumor tissues; however, the notable exception was that MtFt expression in tumors, as compared with cells cultured in vitro, abrogated neoplastic cells to proliferate in vivo. MtFt expression significantly changed both non–heme iron and heme levels in the tumor xenografts. MtFt-expressing tumors exhibit a significant increase in non–heme iron levels, although they display an iron deficiency phenotype in the cytosol (Figure 1B-C). Meanwhile, heme iron content decreased significantly in MtFt-expressing tumors (Figure 2A). Interestingly, TEM analysis (Figure 4) of tumors, in which we overexpressed MtFt, revealed iron overloaded mitochondria that exhibited very similar morphology to those seen in the erythroblasts of patients with sideroblastic anemia.47,48 Strikingly, MtFt overexpression dramatically reduced (by more than 75%) tumor burden, as compared with the control tumors (Figure 5A). Oral administration of Dox to mice, inoculated with MtFt-expressing tumor cells, abrogated MtFt expression and dramatically decreased TfR expression (Figure 5B); these results provided a clear link between MtFt expression and tumor growth inhibition. Additionally, these results are the first to demonstrate that MtFt overexpression results in an accumulation of non–heme iron in mitochondria, as well as a marked decrease in cellular heme content in vivo. Since heme is a physiologic inducer of HO-1, it is highly likely that decreased levels of heme are responsible for a dramatic reduction of HO-1 levels in MtFt-expressing tumors (Figure 2C). Blockage of in vivo tumor xenograft growth by MtFt expression most likely results from suppression of cell proliferation (Figure 6).
Effects of MtFt overexpression on in vitro–cultured xenograft-derived tumor cell growth and apoptosis of tumor xenografts. (A) MtFt expression decreased in vitro growth of xenograft-derived tumor cells (“Materials and methods”). (B) MtFt expression did not lead to cleavage of PARP in H1299 cell– and B9 cell–derived tumor xenografts. Tumor lysates (prepared as in Figure 3A; see “Materials and methods”) were used to measure PARP protein levels by Western blotting. Representative of 3 independent experiments that produced similar results (mean of triplicate ± SD); *P < .01.
Effects of MtFt overexpression on in vitro–cultured xenograft-derived tumor cell growth and apoptosis of tumor xenografts. (A) MtFt expression decreased in vitro growth of xenograft-derived tumor cells (“Materials and methods”). (B) MtFt expression did not lead to cleavage of PARP in H1299 cell– and B9 cell–derived tumor xenografts. Tumor lysates (prepared as in Figure 3A; see “Materials and methods”) were used to measure PARP protein levels by Western blotting. Representative of 3 independent experiments that produced similar results (mean of triplicate ± SD); *P < .01.
Previous studies on cytosolic ferritin overexpression have revealed that H-ferritin could regulate cell growth based on its potential of modulating the cellular labile iron pool iron levels.52-54 Marked overexpression of H-ferritin in HeLa cells attenuated cell growth in a manner that is dependent on its ferroxidase activity to incorporate iron,53 whereas moderate overexpression of H-ferritin, as well as partial repression of H- and L-ferritin, produced no significant effect on cell growth.52,54 Therefore, in cell culture, it appears that the effects of elevated ferritin on cell growth are dependent on the extent to which cellular iron homeostasis is disrupted by the expression of ferritins. Our current study is consistent with this notion, since MtFt expression caused profound changes in iron homeostasis in the tumors.
A striking observation of the current study is that expression of mitochondrial ferritin leads to dramatically inhibited tumor xenograft growth in vivo. We took advantage of the highly efficient incorporation of iron into the newly identified mitochondrial ferritin in mitochondria to test the concept of “cellular iron chelation therapy for inhibition of tumor growth.”55,56 Our data not only confirm our previous observation that a cytosolic iron-starvation phenotype develops in MtFt-overexpressing cells, but also, more importantly, demonstrate that in vivo tumor iron limitation is a viable strategy for reducing tumor burden. Numerous studies have shown that tumor cells are more sensitive to iron deprivation than normal cells since they seem to require more iron for their metabolism26,49-51 ; such sensitivity is probably due to higher iron requirements of cancer cells for rapid cellular proliferation. The inhibition of tumor growth that we observed can likely be attributed to a general decrease in levels or activities of proteins, especially heme and ISC-containing proteins. Our study confirms and extends the notion26,35,49,55,56 that iron chelation therapy can be an effective way to inhibit tumor growth, at least in some circumstances.
Prepublished online as Blood First Edition Paper, June 6, 2006; DOI 10.1182/blood-2006-04-018341.
Supported by grants from the Canadian Institutes of Health Research, Ottawa (P.P.) and by a fellowship from the Fonds de la Recherche en Santé du Québec, Montreal, Quebec, Canada (G.N.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Lukas Kuhn for pST18-fer plasmid containing the ferritin IRE, and Drs Heather A. O'Neill and Grazia Isaya for frataxin antibody. We also thank Ms Johanne Ouellette for her help in TEM experiments.