Abstract
HLA-G is a major histocompatibility complex class Ib molecule whose constitutive tissue distribution is restricted mainly to trophoblast cells at the maternal-fetal interface during pregnancy. In this study, we demonstrated the ability of the soluble HLA-G1 (sHLA-G1) isoform to inhibit fibroblast growth factor-2 (FGF2)-induced capillary-like tubule formation. Using a rabbit corneal neovascularization model, we further showed that sHLA-G1 inhibits FGF2-induced angiogenesis in vivo. We also demonstrated that sHLA-G1 induces endothelial cell apoptosis through binding to BY55/CD160, a glycosylphosphatidylinositolanchored receptor expressed by endothelial cells. Furthermore, we showed that the specific CL1-R2 anti-CD160 monoclonal antibody mimics sHLA-G1-mediated inhibition of endothelial cell tube formation and induction of apoptosis. Thus, the engagement of CD160 in endothelial cells may be essential for the inhibition of angiogenesis. sHLA-G1/CD160-mediated antiangiogenic property may participate in the vascular remodeling of maternal spiral arteries during pregnancy, and, given that we found that CD160 is strongly expressed in the vasculature of a murine tumor, it offers an attractive therapeutic target for preventing pathologic neovascularization.
Introduction
HLA-G is a human major histocompatibility complex (MHC) class Ib gene characterized by a unique promoter region, limited polymorphism, restricted constitutive tissue distribution, and several spliced transcripts encoding either membrane-bound or soluble proteins.1 The soluble HLA-G1 (sHLA-G1) isoform derives from mRNA retaining intron 4,2 which contains a stop codon that precludes translation of the transmembrane domain. Such intron 4 retention is unique among all HLA class I molecules described to date. This 37-kDa, intron 4-retaining sHLA-G1 isoform associates noncovalently with β2-microglobulin (β2m).2 Soluble HLA-G can also be generated by metalloproteinase-mediated release of surface HLA-G containing only extracellular domains.3 The predominant expression of sHLA-G1 in the placenta, at a time when polymorphic HLA-A and HLA-B class Ia molecules are repressed, is consistent with important immunologic functions during pregnancy.4 sHLA-G1 induces apoptosis of activated CD8+ T and natural killer (NK) cells5,6 and down-regulates the CD4+ T-cell alloproliferation response.7 The observation that some anti-HLA-G monoclonal antibodies bound to HLA-G-negative placental endothelial cells8,9 led to our hypothesis that sHLA-G1 might bind to these cells and be involved in the modulation of placental angiogenesis or uterine vessel remodeling.8 Several further observations are in line with such a novel function of HLA-G. Among them is that a defect of HLA-G expression in extravillous cytotrophoblast is associated with preeclampsia,10,11 a common complication of pregnancy in which HLA-G+ endovascular trophoblast invasion of maternal spiral arteries is abrogated, compromising blood flow to the maternal interface.12 In addition, it has been shown that HLA-G inhibits the transendothelial migration of NK cells13 and the rolling adhesion of activated NK cells on endothelial cells.14 To date, the potential role of sHLA-G1 in the modulation of angiogenesis has not been addressed.
Angiogenesis, the formation of new capillaries from preexisting blood vessels, is a crucial component of embryonic vascular development and differentiation, wound healing, and organ regeneration.15 It also contributes to the progression of pathologic conditions that depend on neovascularization, including tumor growth, ischemic ocular disease, and rheumatoid arthritis. Although the most important mediators of angiogenesis, the vascular endothelial growth factor (VEGF) and the fibroblast growth factor (FGF) families, are well defined,16 angiogenesis is a complex process involving multiple gene products expressed by different cell types, all contributing to an integrated sequence of events.17,18
To test our hypothesis that sHLA-G1 is a regulator of endothelialcell activity, we first investigated its in vitro effect. This study demonstrated that sHLA-G1 inhibits FGF2- or VEGF-induced angiogenesis and triggers apoptosis of endothelial cells by interaction with the glycosylphosphatidylinositol (GPI)-anchored CD160 receptor19,20 expressed on endothelial cells. Moreover, in vivo, sHLA-G1 abolishes angiogenesis. Interestingly, we show by immunohistochemistry performed ex vivo that CD160 is expressed solely at the vascular level in a mouse tumor model.
Materials and methods
Cells and reagents
Human umbilical vein endothelial cells (HUVECs; Tebu, Le Perray-en-Yvelines, France) and human microvascular endothelial cells (HMVECs; Cambrex, San Diego, CA) were maintained in endothelial basal medium (EBM; Cambrex) supplemented with 5% fetal calf serum (FCS) and 1 ng/mL FGF2 or VEGF-A 165 (R&D Systems, Minneapolis, MN) every other day. SGHEC-7 cells, a HUVEC-derived cell line, and the SGHPL-4 extravillous trophoblast cell line were cultured as previously described.21,22 Human aortic smooth muscle cells (HAOSMCs; Cambrex) were maintained in RPMI supplemented with 10% FCS. Primary human fibroblasts were obtained from skin biopsy specimens of healthy subjects and were grown in Dulbecco modified Eagle medium (DMEM) containing 10% FCS. Human Jurkat T cells and Jurkat transfected with CD160 (Jurkat-CD160) cells have been previously described.23 NK92 is a human NK-cell line expressing CD160.23 CD4+ T cells were purified from human peripheral-blood mononuclear cells using the MACS separation system (Miltenyi Biotec, Auburn, CA). The B-lymphoblastoid cell line 721.221-sHLA-G1, a gift from Dr D. Geraghty (Fred Hutchinson Cancer Research Center, Seattle, WA), was obtained by transfection of intron 4 containing sHLA-G1 cDNA, as described.2 The resultant transfected protein corresponded to the sHLA-G1 heavy chain noncovalently associated with endogenous β2m. The sHLA-G1-β2m fusion monochain (sHLA-G1mono) construct was engineered by connecting the last residue of the α3 domain of HLA-G to the first codon of the human β2m sequence through a 15-residue spacer.24 sHLA-G1mono was transfected in the HLA class I-negative trophoblast-derived cell line JAR.24 Recombinant sHLA-G1 and sHLA-G1mono molecules were purified from the corresponding transfectant cell-culture supernatants by W6/32 monoclonal antibody (mAb) immunoaffinity column and were eluted at basic pH, as described.24 Positive fractions evaluated by HLA-G-specific ELISA were pooled, concentrated, and analyzed either by SDS-PAGE stained with Coomassie blue or by Western blot using HLA-G-specific mAb24 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Monoclonal antibodies used included CL1-R2 (IgG1) anti-CD160,23 produced in one of our laboratories, anti-CD8 (OKT8; Coulter Immunotech, Fullerton, CA), anti-ILT4/CD85d (a gift from Dr M. Colonna, Washington University, St Louis, MO), anti-ILT2/CD85j (BD Biosciences, San Jose, CA), and dialyzed mouse IgG1 or IgG2a isotype controls (DAKO, Trappes, France). HLA-G1 tetramers were produced as previously described25 with synthetic self-peptide RIIPRHLQL26 after the addition of streptavidinphycoerythrin (PE; BD Biosciences). Labeling of HUVECs, Jurkat, and Jurkat-CD160 by PE-conjugated HLA-G tetramers was performed at 37°C for 1 hour. For Jurkat-CD160 and Jurkat, tetramers were cross-linked with anti-HLA class I W6/32 mAb, as previously described.27
In vitro capillary tube formation assay
Growth factor-reduced Matrigel (BD Biosciences) was diluted in collagen (1:6 vol/vol) and kept on ice. One hundred sixty microliters of this solution was added to each well of 8-well culture slides precoated with type 1 rat tail collagen and left at 37°C for 1 hour. A HUVEC suspension, untreated or treated with FGF2 (10 ng/mL), sHLA-G1 (1 μg/mL), sHLA-G1mono (1 μg/mL), mAb CD160 (1 or 10 μg/mL), or IgG1 isotype control (10 μg/mL) was seeded into the Matrigel/collagen gel for 24 hours at 37°C. Microtubules were quantified by microscopy, as previously described.28 Briefly, the culture medium was removed, and cells were rinsed twice with PBS and fixed for 30 minutes at room temperature in a 4% paraformaldehyde solution. Then the cells were washed twice with PBS and stained with Masson trichrome. The extent of the microcapillary network was measured with an automated computer-assisted image analysis software (Imagenia 2000; Biocom, Les Ulis, France), and the total length of the capillaries in each well was determined. The mean microcapillary network length (in micrometers) was calculated for each experimental condition. Experiments were performed in triplicate and repeated 3 times.
Apoptosis assays
Apoptosis was evaluated by 2 methods: time-lapse microscopy29 and flow cytometry analysis using the FITC-conjugated annexin V/PI assay.30 For the time-lapse microscopy assay, HUVEC, SGHEC-7 endothelial cell, or SGHPL-4 trophoblast cell lines were seeded into 6-well plates at 2.5 × 105 cells/well in normal culture medium, as described earlier.21,22 After 15 hours, sHLA-G1, sHLA-G1mono (0.1 or 1 μg/mL), CL1-R2 anti-CD160 mAb (1-10 μg/mL), IgG1 isotype control (10 μg/mL), or zVAD-fmk (50 μM; Calbiochem) was added to the wells. The plate was transferred to an inverted fluorescence microscope (IX70; Olympus, Tokyo, Japan) with motorized stage and cooled CCD camera and enclosed in a heated, humidified chamber at 37°C in 5% CO2. Images were taken every 15 minutes for 0 to 50 hours, and time-lapse sequences were analyzed using ImagePro Plus version 4 (Media Cybernetics, Silver Spring, MD). In each field of view, 40 cells were randomly chosen. The experiments were repeated at least 4 times. Apoptotic cells were scored according to the time at which clear apoptotic morphology was first observed.29 For the annexin V/PI assay, 2 × 105 cells were seeded into a 6-well plate in RPMI 1% FCS for 24 hours and then incubated for an additional 12 hours in RPMI 0.5% FCS. Cells were left untreated or were treated with sHLA-G1 (1 μg/mL), mAb CD160 (10 μg/mL), or control IgG1 (10 μg/mL) for 50 hours in the presence of VEGF (50 ng/mL). At the end of the treatment, the floating cells were collected by centrifugation, whereas adherent cells were harvested by trypsin-EDTA solution to produce a single-cell suspension. The cells were then pelleted by centrifugation and washed twice with PBS. Apoptotic cell death was identified by double staining with FITC-conjugated annexin-V and PI using the annexin V-FITC Apoptosis Detection kit (DAKO, Carpinteria, CA) according to the manufacturer's instructions. Cells were analyzed by flow cytometry on a FACScan (Becton Dickinson) using the fluorescence 1 (FL1-540 nm) signal detector for FITC conjugates and the fluorescence 2 (FL2-600 nm) signal detector for PI. Ten thousand events were recorded for each sample. Data were analyzed using CellQuest software (Becton Dickinson).
Western blot analysis of cleaved PARP expression
SGHEC-7 endothelial cells were seeded in culture plates. After 16 hours, the cells were incubated with sHLA-G1 (0.1 μg/mL) for 60 hours. Cells were lysed in RIPA buffer with 0.1 mg/mL PMSF, 30 μL/mL aprotinin (Sigma A6279 solution at 5-10 trypsin-inhibitor U/mL; Sigma, Gillingham, United Kingdom), and 1 mM sodium orthovanadate at 4°C for 30 minutes. Equal amounts of proteins determined by Bradford assay were loaded and separated by SDS-PAGE and transferred to a nitrocellulose membrane. After incubation in blocking buffer for 1 hour at room temperature, the membrane was incubated with rabbit polyclonal anti-human cleaved PARP (Promega, Southampton, United Kingdom) for 1 hour. Anti-rabbit IgG peroxidase (A6154; Sigma) was added for 1 hour. Detection of membrane-bound mAb was carried out by chemiluminescence (ECLPlus; Amersham Biosciences, Balfont, United Kingdom). Actin expression after stripping and reprobing the blot confirmed equal loading.
Rabbit corneal angiogenesis assay
The corneal pocket assay used in this study has been previously described.31 Male New Zealand albino rabbits were anesthetized. A corneal pocket was created by incision on the superior side of the corneal stroma 2 mm from the limbus. Slow-releasing implants of hydrogel hydrated with PBS containing FGF2 (500 ng) were inserted into this pocket. Twenty-four and 72 hours after pellet implantation, 5 μg sHLA-G1 (1 mg/mL in PBS) or an equal volume of PBS (control) was injected into the subconjunctiva on the superior side near the limbus. Neovascularization of the cornea was measured on day 8 after implantation by microscopic examination with a slit lamp and was scored according to a 5-grade scale (grade 0, normal avascular cornea; grade 1, less than 1-mm-long neovessels; grade 2, 1-mm-long neovessels; grade 3, 1- to 2-mm-long neovessels; grade 4, neovessels extending to the implant). The average mean score obtained after implantation of a pellet containing no angiogenic factor was less than 0.5.31 Mean ± SD was determined on 8 implants per group.
VEGF and sHLA-G1 cell-binding competition experiments
VEGF and sHLA-G1 were radiolabeled with Na125I to a specific activity of 2.4 × 104 and 1.1 × 105 cpm/ng, respectively.32 Wells containing 2 × 105 serum-starved HUVECs were untreated or were pretreated with VEGF (0.1
μg/mL) or FGF2 (0.1 μg/mL) or sHLA-G1 (0.1, 1.0, or 10 μg/mL) at 37°C for 24 hours or were processed immediately for binding assays. Briefly, dishes were rinsed in cold DMEM supplemented with 0.2% gelatin and 20 mM HEPES (pH 7.3) and were incubated at 4°C for 2 hours with 2 ng/mL 125I-VEGF or 125I-sHLA-G1 in the absence or presence of indicated concentrations of cold competitors. Cells were then rinsed in the same medium and lysed in 0.02 M Tris, 10 mM EDTA, 0.3 M NaCl, 0.1% SDS, 1% Triton X-100, and 0.05% Tween 20 RIPA buffer, and radioactivity was counted in a γ counter.
Cell phenotyping
Subconfluent HUVECs, HVMECs, HAOSMCs, or fibroblasts were scraped in PBS-EDTA and incubated in the presence or absence of 0.1 μg/mL HLA-G1 at 4°C. After 2 hours, cells were incubated with anti-CD8, anti-CD85d, anti-CD85j, or CL1-R2 anti-CD160-specific mAbs or immunoglobulin-isotype control (20 μg/mL) followed by F(ab′)2-FITC or F(ab′)2-PE-conjugated anti-mouse IgG. Nonviable cells were excluded by the use of propidium iodide (PI). Samples were analyzed on a Coulter-Epics ELITE flow cytometer (Beckman Coulter, Roissy, France).
Reverse transcription-polymerase chain reaction (RT-PCR) and cDNA sequencing
CD160 transcripts were detected by RT-PCR using the following primer sequences: 5′-TGCAGGATGCTGTTGGAACCC-3′ and 3′-TCAGCCTGAACTGAGAGTGCCTTC-5′. cDNA quality was confirmed by amplification of β-actin using the appropriate primers. Amplification conditions for CD160 and β-actin were 95°C for 45 seconds, 60°C for 30 seconds, and 72°C for 1 minute (35 cycles) using an MJ Research PTC-200 Peltier Thermal Cycler (Bio-Rad, Marnes-la-Coquette, France). For CD160 sequencing, a Taq High Fidelity was used (Invitrogen, Carlsbad, CA). PCR product was purified (qiaex II; Qiagen, Valencia, CA) and analyzed with the following primer sequences: BY01, 5′-TGCAGGATGCTGTTGGAACCC-3′); BY03, 3′-TCAGCCTGAACTGAGAGTGCCTTC-5′); BY02, 5′CAGCTGAGACTTAAAAGGGATC-3′); and BY04, 3′-CACCAACACCATCTATCCCAG-5′).
Immunohistochemistry
Subconfluent Lewis lung carcinoma cells were trypsinized, washed twice, and suspended in PBS. Cells (2 × 105) were injected subcutaneously into the dorsal midback region of C57BL/6 female mice (Iffa-Credo, L'Arbresle, France). Tumors were taken on day 21, fixed with 10% formalin (Sigma) overnight at 4°C, and embedded in paraffin (embedder; Leica, Wetzlar, Germany). Five-micrometer sections were placed in a DAKO Autostainer and incubated with TNB blocking buffer (TSA kit; New England Nuclear, Beverly, MA), peroxidase-blocking reagent (DAKO), and mouse immunoglobulin-blocking reagent (Vector Laboratories, Paris, France). Sections were incubated with CL1-R2 anti-CD160 mAb (10 μg/mL), followed by biotin-labeled goat anti-mouse IgG and avidin-biotin complex (Vector Laboratories). They were stained with DAB (Vector Laboratories), counter-stained with hematoxylin, viewed under a microscope (E-800; Nikon, Tokyo, Japan), and digitized with a DMX 1200 camera (Nikon) equipped with a Nikon Plan Fluor 40×/0.75 numeric aperture (NA) objective. Images were acquired using Nikon ACT-1 software version 2.63 and were processed using Explora Nova Macrowriter T1.20 software (Explora Nova, La Rochelle, France).
Statistical analysis
Where applicable, results were presented as mean ± SEM or SD of “n” independent experiments and were assessed with the Mann-Whitney U test, analysis of variance, or Student t test, as appropriate, and Everstat (SAS Institute, Cary, NC) or Prism (GraphPad, San Diego, CA) software; P values of less than .05 were considered significant.
Results
sHLA-G1 inhibits FGF2-mediated capillary tube formation
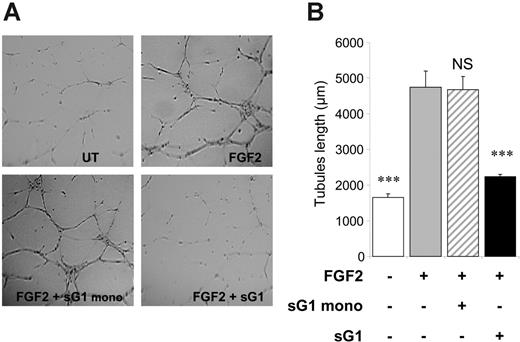
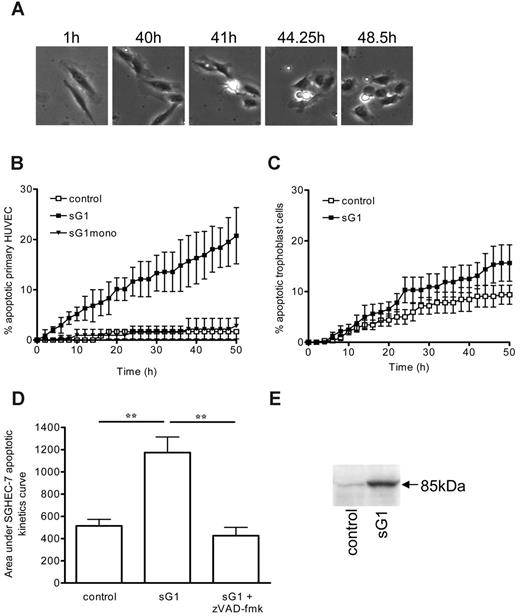
We investigated whether sHLA-G1 heavy chain noncovalently associated with β2m could interfere with proangiogenic factor functions in vitro. We evaluated the capacity of sHLA-G1 to inhibit capillary tube formation by endothelial cells cultured on Matrigel. Purified recombinant sHLA-G1, when added exogenously to HUVECs, significantly inhibited FGF2-induced tubule-like formation (morphology [Figure 1A] and quantification [Figure 1B]). In contrast, the sHLA-G1mono-negative control had no effect. These findings indicated that sHLA-G1 inhibits in vitro proangiogenic factor-mediated endothelial-cell capillary tube formation.
sHLA-G1 induces apoptosis of endothelial cells
Having shown the inhibitory effect of sHLA-G1 on a function relevant to angiogenesis (tubule formation), we then used apoptosis assays to interrogate the possible mechanisms by which sHLA-G1 altered the end point of angiogenesis. Apoptosis is indeed an important regulator of angiogenesis.33 We found that incubation of HUVECs with recombinant sHLA-G1 clearly induced apoptosis, as determined by time-lapse digital image microscopy (Figure 2). Phase-contrast images of the experiment (Figure 2A) and supplemental video data (Video S1: sHLA-G1; Video S2: control) show that sHLA-G1-treated cell morphology was characterized by cytoplasmic and nuclear shrinkage, by a change to a phase-bright appearance, and by the formation of membrane blebs/blisters (Figure 2A). Such an effect of sHLA-G1, but not of sHLA-G1mono, was time dependent (Figure 2B). Comparable kinetics curve was obtained using SGHEC-7 cells (data not shown). The endothelial-cell type specificity of this sHLA-G1-induced apoptosis was demonstrated by the absence of any significant effect of this molecule on human trophoblast cells (Figure 2C). Use of the broad-spectrum caspase inhibitor zVAD-fmk prevented recombinant sHLA-G1-mediated apoptosis (Figure 2D), implicating the caspase pathway in the sHLA-G1-mediated apoptosis of endothelial cells. This was further demonstrated by the detection of cleaved poly (ADP-ribose) polymerase (PARP) by Western blot analysis after sHLA-G1 treatment of endothelial cells (Figure 2E). By contrast, no p85-cleaved PARP was detected after a similar sHLA-G1 treatment of trophoblast cells (Figure S2). These findings together demonstrated that sHLA-G1 induced caspase-dependent apoptosis of endothelial cells.
sHLA-G1 inhibits FGF2-mediated HUVEC capillary tubule-like formation. (A) In vitro tubule-like capacity of HUVECs. HUVECs were seeded on Matrigel in the absence (UT, untreated) or presence of FGF2 (10 ng/mL) and after the addition of sG1 or sG1mono (1 μg/mL). Photographs of each well were taken after 24 hours, and angiogenesis was quantified as described in “Materials and methods.” Data are representative of 5 separate experiments, each performed in triplicate. After sG1 treatment, the branches and tubules formed were less developed than in sG1mono-treated or untreated HUVECs. (B) Branches from each cell were counted from 1 representative field per well. Data indicate the mean ± SEM of 3 wells and are representative of 5 independent experiments. Images were visualized using a Nikon Eclipse E-800 microscope equipped with a Plan Apo 4×/0.2 NA objective. Images were acquired using a Nikon DXM-1200F camera with Nikon ACT-1 software, and were processed using Morphoexpert software version 2.5 (Explora Nova). ***P < .001, ANOVA.
sHLA-G1 inhibits FGF2-mediated HUVEC capillary tubule-like formation. (A) In vitro tubule-like capacity of HUVECs. HUVECs were seeded on Matrigel in the absence (UT, untreated) or presence of FGF2 (10 ng/mL) and after the addition of sG1 or sG1mono (1 μg/mL). Photographs of each well were taken after 24 hours, and angiogenesis was quantified as described in “Materials and methods.” Data are representative of 5 separate experiments, each performed in triplicate. After sG1 treatment, the branches and tubules formed were less developed than in sG1mono-treated or untreated HUVECs. (B) Branches from each cell were counted from 1 representative field per well. Data indicate the mean ± SEM of 3 wells and are representative of 5 independent experiments. Images were visualized using a Nikon Eclipse E-800 microscope equipped with a Plan Apo 4×/0.2 NA objective. Images were acquired using a Nikon DXM-1200F camera with Nikon ACT-1 software, and were processed using Morphoexpert software version 2.5 (Explora Nova). ***P < .001, ANOVA.
sHLA-G1 inhibits corneal angiogenesis in vivo
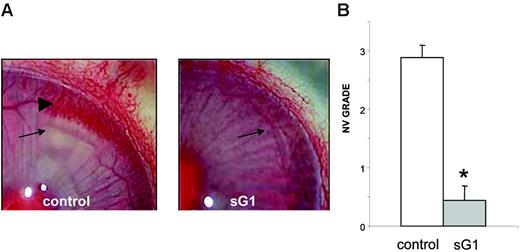
A rabbit corneal pocket assay was used to determine whether sHLA-G1 could also inhibit angiogenesis in vivo. Neovascularization arose from the limbus toward the FGF2-containing pellet and was easily detected after 8 days in the control corneas (Figure 3A, left). In contrast, the injection of sHLA-G1 (Figure 3A, right) almost totally abrogated corneal neovascularization. These results, reproduced on 8 implants per group (Figure 3B), clearly demonstrated that sHLA-G1 had an antiangiogenic effect on FGF2-induced angiogenesis in vivo.
sHLA-G1 binds directly to the CD160 receptor
It was then important to identify the receptor involved in the antiangiogenic effects of sHLA-G1. We first tested whether sHLA-G1 interfered with VEGF receptors by performing radioreceptor assay-binding experiments at 4°C on HUVECs incubated for 2 hours (equilibrium time) with 125I-VEGF or 125I-sHLA-G1 in the presence of various concentrations of cold competitors. We analyzed the binding of 125I-sHLA-G1 to HUVECs and found that cold VEGF or FGF2 competitors had no effect, whereas unlabeled sHLA-G1 inhibited this binding as a function of concentration, with IC50 values in the nanomolar range (Figure 4A). In competition experiments using 125I-VEGF as ligand, we found that cold VEGF displaced its binding to HUVECs with IC50 values in the nanomolar range, whereas sHLA-G1 had no effect (Figure 4B).
Furthermore, we found that cold sHLA-G1 competitor did not inhibit the binding of 125I-VEGF to porcine aortic endothelial cells (PAECs)-VEGF-R2 or PAEC-NPL1 transfectants (data not shown). These results demonstrate that sHLA-G1 bound specifically to endothelial cells without interfering with the VEGF receptors.
sHLA-G1 induces apoptosis of endothelial cells. (A) Time-lapse digital image microscopy of HUVECs after treatment with sHLA-G1. Apoptotic morphology could be detected over time, as evidenced by cytoplasmic retraction and a phase-bright appearance and membrane blebbing and blistering. These apoptotic changes are also illustrated in Videos S1 (sHLA-G1) and S2 (control). Images were visualized using an Olympus 1X70 microscope equipped with a UPlanF1 4×/0.13 NA objective. Images were captured using a Hamamatsu C4742-95-12NR camera and Image Pro Plus software version 4.5.1.29 (Media Cybernetics). (B) Kinetics curve of apoptosis induction. HUVECs were un-treated (control) or were incubated with sHLA-G1 (sG1; 1 μg/mL) or sHLA-G1mono (sG1mono; 1 μg/mL). Time-lapse microscopy was carried out to assess the appearance of apoptotic morphology. Although data were obtained every 15 minutes, data points are shown at 2-hour intervals for clarity. Mean ± SEM of pooled data from 4 experiments are shown. P < .001 between sHLA-G1 and sHLA-G1mono or control at the 50-hour time point, as determined by repeated-measures ANOVA with Tukey posttest. (C) Kinetics curve of apoptosis induction of trophoblast cells after incubation with sHLA-G1 compared with untreated (control) cells. Mean ± SEM of pooled data from 4 experiments is shown. Nonsignificance between sHLA-G1 and control at the 50-hour time point, as determined by the Mann-Whitney U test (P = .114) or paired t test (P = .127). (D) SGHEC-7 endothelial-cell apoptosis induction by sG1 (0.1 μg/mL), compared with untreated (control) cells, in the presence or absence of the caspase inhibitor zVAD-fmk assessed by time-lapse microscopy. Mean ± SEM of pooled data from 4 experiments is shown. Area under the curve was calculated from the kinetics curves. **P < .001, ANOVA. (E) Western blot analysis of p85 cleaved PARP expression. SGHEC-7 endothelial cells were incubated in the absence (control) or presence of sG1 (0.1 μg/mL) for 60 hours (confluent monolayer).
sHLA-G1 induces apoptosis of endothelial cells. (A) Time-lapse digital image microscopy of HUVECs after treatment with sHLA-G1. Apoptotic morphology could be detected over time, as evidenced by cytoplasmic retraction and a phase-bright appearance and membrane blebbing and blistering. These apoptotic changes are also illustrated in Videos S1 (sHLA-G1) and S2 (control). Images were visualized using an Olympus 1X70 microscope equipped with a UPlanF1 4×/0.13 NA objective. Images were captured using a Hamamatsu C4742-95-12NR camera and Image Pro Plus software version 4.5.1.29 (Media Cybernetics). (B) Kinetics curve of apoptosis induction. HUVECs were un-treated (control) or were incubated with sHLA-G1 (sG1; 1 μg/mL) or sHLA-G1mono (sG1mono; 1 μg/mL). Time-lapse microscopy was carried out to assess the appearance of apoptotic morphology. Although data were obtained every 15 minutes, data points are shown at 2-hour intervals for clarity. Mean ± SEM of pooled data from 4 experiments are shown. P < .001 between sHLA-G1 and sHLA-G1mono or control at the 50-hour time point, as determined by repeated-measures ANOVA with Tukey posttest. (C) Kinetics curve of apoptosis induction of trophoblast cells after incubation with sHLA-G1 compared with untreated (control) cells. Mean ± SEM of pooled data from 4 experiments is shown. Nonsignificance between sHLA-G1 and control at the 50-hour time point, as determined by the Mann-Whitney U test (P = .114) or paired t test (P = .127). (D) SGHEC-7 endothelial-cell apoptosis induction by sG1 (0.1 μg/mL), compared with untreated (control) cells, in the presence or absence of the caspase inhibitor zVAD-fmk assessed by time-lapse microscopy. Mean ± SEM of pooled data from 4 experiments is shown. Area under the curve was calculated from the kinetics curves. **P < .001, ANOVA. (E) Western blot analysis of p85 cleaved PARP expression. SGHEC-7 endothelial cells were incubated in the absence (control) or presence of sG1 (0.1 μg/mL) for 60 hours (confluent monolayer).
sHLA-G1 inhibits FGF2-induced corneal angiogenesis in vivo. Neovascularization was assessed 8 days after the insertion of implants containing FGF2 in rabbit corneal pockets, in the presence or absence of sHLA-G1 (5 μg/injection). (A) Representative image of each group (control, PBS subconjunctival injections; sG1, sHLA-G1 subconjunctival injections) at day 8 after FGF2 pellet implantation. (Arrows) Pellet implants. (Arrowhead) Newly formed vessels. The rabbit's eyes were examined under a OPMI-1 FC slit lamp biomicroscope (Carl Zeiss, Berlin, Germany) using a 2.4×/1.6 NA objective. (B) Neovascularization (NV) scores (mean ± SEM) for 8 implant groups. *P < .001, Student t test.
sHLA-G1 inhibits FGF2-induced corneal angiogenesis in vivo. Neovascularization was assessed 8 days after the insertion of implants containing FGF2 in rabbit corneal pockets, in the presence or absence of sHLA-G1 (5 μg/injection). (A) Representative image of each group (control, PBS subconjunctival injections; sG1, sHLA-G1 subconjunctival injections) at day 8 after FGF2 pellet implantation. (Arrows) Pellet implants. (Arrowhead) Newly formed vessels. The rabbit's eyes were examined under a OPMI-1 FC slit lamp biomicroscope (Carl Zeiss, Berlin, Germany) using a 2.4×/1.6 NA objective. (B) Neovascularization (NV) scores (mean ± SEM) for 8 implant groups. *P < .001, Student t test.
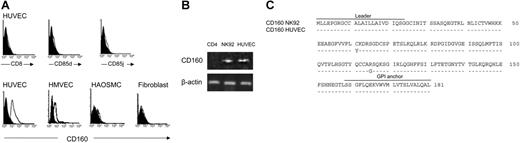
With the use of flow cytometry and specific mAbs, we investigated whether HUVECs expressed some of the HLA-G receptors described to date, including CD8,6 ILT4/CD85d,34 ILT2/CD85j,34 and CD160.27 We found that HUVECs expressed CD160, though not at constant levels, but not CD8, CD85d, or CD85j (Figure 5A). Similarly, HMVECs bound anti-CD160 mAb (Figure 5A), as did bovine endothelial cells (data not shown), suggesting that the CD160 epitope recognized by this mAb was conserved among species. In contrast, smooth muscle cells and fibroblasts in primary culture did not express CD160 (Figure 5A). To confirm that CD160 is expressed by HUVECs, we performed reverse transcription-polymerase chain reaction (RT-PCR) analysis with CD160-specific primers. We demonstrated that the CD160 mRNA was present in HUVECs, as in the NK92-cell line, whereas CD4+ T cells were negative (Figure 5B). It should be noted that HUVECs did not express the potential HLA-G receptor KIR2DL4 transcripts (data not shown). Then HUVECs and NK92 cDNA were isolated and sequenced. Predicted amino acid sequence alignment of HUVECs and NK92 CD160 proteins showed that they were both similar to the CD160 sequence already published,20 with the exception of 2 substituted residues (Figure 5C), indicating a possible allelic form.
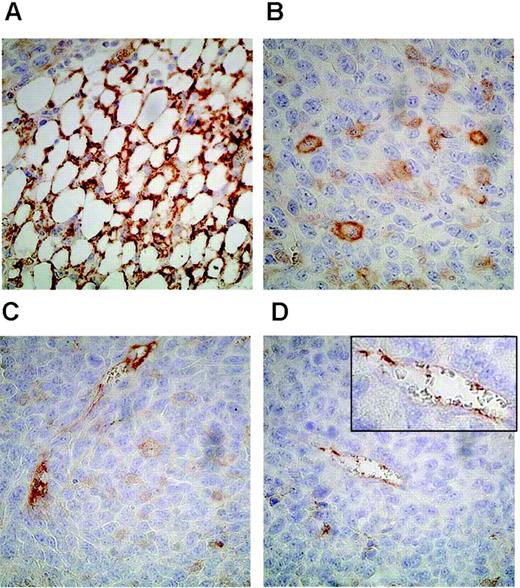
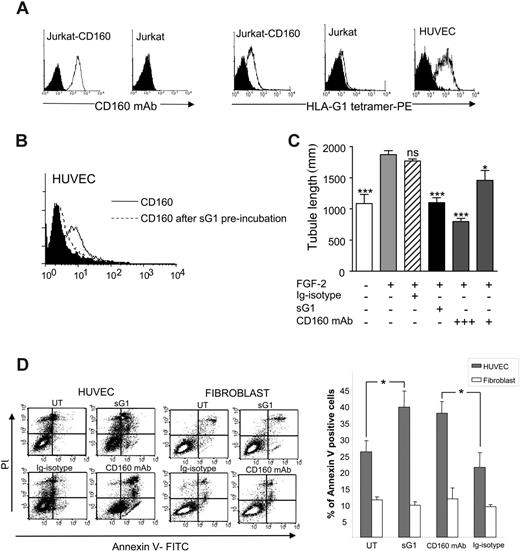
We then investigated whether CD160 was also expressed on endothelial cells in vivo and did not result from culture conditions. For this purpose, Lewis lung carcinoma cells were injected subcutaneously in C57/BL6 mice. Immunohistochemical analysis of the tumor was performed on day 21 with the CL1-R2 anti-CD160 mAb. We found that this mAb strongly stained endothelial cells of microvessels at the periphery of the tumors (Figure 6A-B) and inside them (Figure 6C-D), whereas no staining was detected with IgG isotype control (data not shown). In contrast, tumor cells remained unstained. Such reactivity of this anti-human CD160 mAb was not surprising because the previous identification and sequencing of human and mouse CD160 encoding cDNA revealed a strong homology between the 2 species.20,35 We then analyzed whether sHLA-G1 could effectively bind to the CD160 receptor expressed by endothelial cells. We first found that an HLA-G1 tetramer specifically bound to HUVECs as it did on CD160-transfected Jurkat, but not on parental Jurkat, cells (Figure 7A). sHLA-G1-CD160 direct interaction on HUVECs was further demonstrated by showing that preincubation of HUVECs with recombinant sHLA-G1 specifically blocked the binding of anti-CD160 mAb (Figure 7B), whereas preincubation with VEGF did not (data not shown).
sHLA-G1 does not interfere with VEGF receptors. (A) HUVECs were incubated with 125I-sHLA-G1 (2 ng/mL [50 pM]) in the absence (-) or presence of cold VEGF (1 μg/mL), FGF2 (1 μg/mL), or sHLA-G1 (sG1, 0.1, 1.0, or 10 μg/mL). Unlabeled sG1, but not VEGF or FGF2, prevented 125I-sHLA-G1 binding. (B) HUVECs were incubated with 125I-VEGF (2 ng/mL) in the presence of cold sG1, FGF2, or VEGF. Unlike cold VEGF, cold sG1 did not abrogate iodinated VEGF binding. In these conditions, the IC50 of cold ligands was 200 pM for VEGF and 2500 pM for sHLA-G1. Results are mean ± SEM of triplicate wells and are representative of 3 independent experiments.
sHLA-G1 does not interfere with VEGF receptors. (A) HUVECs were incubated with 125I-sHLA-G1 (2 ng/mL [50 pM]) in the absence (-) or presence of cold VEGF (1 μg/mL), FGF2 (1 μg/mL), or sHLA-G1 (sG1, 0.1, 1.0, or 10 μg/mL). Unlabeled sG1, but not VEGF or FGF2, prevented 125I-sHLA-G1 binding. (B) HUVECs were incubated with 125I-VEGF (2 ng/mL) in the presence of cold sG1, FGF2, or VEGF. Unlike cold VEGF, cold sG1 did not abrogate iodinated VEGF binding. In these conditions, the IC50 of cold ligands was 200 pM for VEGF and 2500 pM for sHLA-G1. Results are mean ± SEM of triplicate wells and are representative of 3 independent experiments.
To further demonstrate that sHLA-G1 antiangiogenic function was mediated through interaction with CD160, we tested whether soluble anti-CD160 mAb could antagonize or mimic sHLA-G1 activity in the in vitro Matrigel tube and apoptosis assays. The results clearly showed that CL1-R2 mAb mimicked sHLA-G1 because it inhibited FGF2-mediated tubule vessel growth (Figure 7C) and induced HUVEC apoptosis, as demonstrated by annexin V/PI assay (Figure 7D) and time-lapse microscopy (data not shown and Figure 2). CD160 mAb-mediated apoptosis of HUVECs was similar to sHLA-G1-mediated apoptosis, whereas none of these apoptosis inducers were active on control fibroblast cells in primary culture (Figure 7D) or on trophoblast cells (Figure 2C). Altogether, these data further demonstrated that CD160 expressed by endothelial cells is a functional receptor able to trigger an antiangiogenic cell response.
Discussion
In this study, we demonstrated that the sHLA-G1 molecule, known to exert immune regulatory function,1 also displays antiangiogenic properties in vitro and in vivo. This included the inhibition of vessel formation, the induction of endothelial cell apoptosis, and the in vivo inhibition of FGF2-induced rabbit corneal neoangiogenesis. Spatial and temporal regulation of the vasculature at the maternal-fetal interface plays an important role in ensuring adequate blood supply to nourish the developing embryo, suggesting that locally acting factors regulate vascular cells.36 The ability of sHLA-G1 to induce apoptosis of confluent and proliferating endothelial cells might be relevant in the utero-placental environment because effacement of preexisting maternal endothelial cells rather than inhibition of angiogenesis would be required. Soluble HLA-G1 is indeed secreted by endovascular trophoblasts that replace vascular cells of the maternal spiral arteries, thereby increasing the diameter of these vessels several fold and transforming them into high-conductance vessels.12 We hypothesize that during early pregnancy, sHLA-G1 apoptotic effects on these maternal endothelial cells might contribute to such replacement and vascular remodeling. Defects of HLA-G expression, including diminishment of soluble HLA-G in preeclamptic placentas characterized by a shallow cytotrophoblast invasion and a reduced flow of maternal blood to the feto-placental unit,37 favor such a hypothesis. A recent report using an ex vivo model of maternal spiral artery perfused with extravillous cytotrophoblast has shown that adding these cells induced the apoptosis of endothelial cells.38 Knowing that extravillous cytotrophoblasts do produce sHLA-G4 might lead to the conclusion that such apoptosis is mediated by sHLA-G.
HUVECs and HMVECs express the CD160 receptor. (A) HUVECs, HMVECs, HAOSMCs, and fibroblasts in primary culture were analyzed by flow cytometry after incubation with anti-CD8-, anti-CD85d-, anti-CD85j-, or CL1-R2 (anti-CD160)-specific mAbs (open profiles) or IgG isotype controls (filled profiles), followed by PE-labeled conjugates. Results are representative of 6 independent experiments. (B) CD160 mRNA was expressed by HUVECs and NK92 cells (positive control) but notbyCD4+ T cells (negative control). RT-PCR analysis, using CD160-specific primers, compared with β-actin control primers. (C) Predicted amino acid sequence alignment of CD160 expressed in HUVECs and NK92. Dotted lines indicate identity.
HUVECs and HMVECs express the CD160 receptor. (A) HUVECs, HMVECs, HAOSMCs, and fibroblasts in primary culture were analyzed by flow cytometry after incubation with anti-CD8-, anti-CD85d-, anti-CD85j-, or CL1-R2 (anti-CD160)-specific mAbs (open profiles) or IgG isotype controls (filled profiles), followed by PE-labeled conjugates. Results are representative of 6 independent experiments. (B) CD160 mRNA was expressed by HUVECs and NK92 cells (positive control) but notbyCD4+ T cells (negative control). RT-PCR analysis, using CD160-specific primers, compared with β-actin control primers. (C) Predicted amino acid sequence alignment of CD160 expressed in HUVECs and NK92. Dotted lines indicate identity.
Immunohistochemical staining of Lewis lung carcinoma tumor sections with anti-CD160 mAb demonstrating CD160+ vessels. (A) Vessel network staining in brown was localized at the periphery of the tumor. Blood vessels in the periphery (B) and the center of the tumor (C-D) were also stained with CD160 mAb, whereas tumor cells remained unstained. Original magnification, × 400. (Inset magnification, × 4000)
Immunohistochemical staining of Lewis lung carcinoma tumor sections with anti-CD160 mAb demonstrating CD160+ vessels. (A) Vessel network staining in brown was localized at the periphery of the tumor. Blood vessels in the periphery (B) and the center of the tumor (C-D) were also stained with CD160 mAb, whereas tumor cells remained unstained. Original magnification, × 400. (Inset magnification, × 4000)
Different mechanisms have been reported to explain the activity of angiogenesis inhibitors, including inhibition of endothelial-cell remodeling,39 induction of endothelial-cell apoptosis,40 or chemorepulsion of endothelial cells.41 In this report, we demonstrate novel inhibitory actions of sHLA-G1 directed to endothelial cells. In addition, we report that sHLA-G1-treated endothelial cells progressively showed apoptotic morphology. The mechanism of this induced apoptosis remains incompletely characterized despite clear implication of caspases (Figure 2D-E). The Fas/FasL pathway might also be involved, as it has been shown for the sHLA-G1-induced apoptosis of activated CD8+ T cells.6 It is interesting that a role for apoptosis and Fas/FasL interactions in the remodeling of uterine arteries during pregnancy has recently been demonstrated.38
In this study, we also used the sHLA-G1-β2m fusion monochain (sHLA-G1mono)23 as a negative control molecule prepared and purified exactly like the conformational sHLA-G1. This control molecule did not exhibit antiangiogenic activities in the assays described in this report. It is unlikely that the conformation of this construct was grossly altered because the W6/32 conformational mAb still recognized it (see “Materials and methods”). However, it is possible that the presence of a β2m-linked 15-residue spacer to sHLA-G1 heavy chain impairs the formation of sHLA-G1mono multimers. The oligomerization of sHLA-G1 is essential for its activity and efficient recognition by CD85j/ILT2 receptors,42 which might explain the unresponsiveness of sHLA-G1mono, which might not bind to CD160 for the same reason. This hypothesis is supported by the recently published crystal structure of the HLA-G protein presenting a model of HLA-G oligomers and the relative position of the CD85j/ILT2 receptor near the complex.43 It has also been shown that HLA-G can be found as a free heavy chain because of the dissociation of β2m from the conformed protein.44 Moreover, β2m-free HLA-G and conformed HLA-G can form complexes able to modulate HLA-G affinity to some NK receptors.44 In the sHLA-G1mono construct, the β2m is covalently linked to the HLA-G1 heavy chain. Such a mechanism of dissociation of the β2m from the heavy chain would thus be impossible, and this might contribute to the inefficiency of sHLA-G1mono to activate CD160 present on endothelial cells.
sHLA-G1 binds to the CD160 receptor expressed by endothelial cells. (A, left) anti-CD160 mAb (open profiles) stains Jurkat-CD160 but not untransfected Jurkat (filled profiles, isotype control). Flow cytometry analysis. (right) sHLA-G1 tetramer binds to HUVECs and Jurkat-CD160 control transfectant (open profiles) but not to untransfected Jurkat cells (filled profiles, control staining with streptavidin-PE). Flow cytometry analysis. (B) Recombinant sHLA-G1 (sG1) blocks CD160 mAb binding to HUVECs (filled profile, isotype control). Flow cytometry analysis. Results are representative of 3 independent experiments. (C) Soluble CL1-R2 anti-CD160 mAb triggers inhibition of in vitro angiogenesis. HUVECs were seeded on Matrigel in the presence or absence of FGF2 (10 ng/mL) and sHLA-G1 (sG1, 1 μg/mL) or CD160 mAb (+++, 10 μg/mL; +, 1 μg/mL) or IgG1-isotype control (10 μg/mL). Photographs of each well were taken after 24 hours and angiogenesis quantified. Results are mean ± SD of triplicate wells and are representative of 5 independent experiments. ***P < .001. *P < .005. ns indicates not significant (ANOVA) compared with FGF2-treated cells. (D) Soluble CL1-R2 anti-CD160 mAb induces HUVEC but not fibroblast apoptosis. HUVECs were treated with sG1 (1 μg/mL), CD160 mAb (10 μg/mL), or control IgG1 (10 μg/mL) or were untreated (UT) for 50 hours in the presence of VEGF (50 ng/mL). Apoptotic cells were detected by flow cytometry using annexin V/PI double staining. (Left) Results of representative experiment. (Right) Histograms. Data are shown as mean ± SEM percentage of annexin V-positive cells. *P ≤ .02, Student t test.
sHLA-G1 binds to the CD160 receptor expressed by endothelial cells. (A, left) anti-CD160 mAb (open profiles) stains Jurkat-CD160 but not untransfected Jurkat (filled profiles, isotype control). Flow cytometry analysis. (right) sHLA-G1 tetramer binds to HUVECs and Jurkat-CD160 control transfectant (open profiles) but not to untransfected Jurkat cells (filled profiles, control staining with streptavidin-PE). Flow cytometry analysis. (B) Recombinant sHLA-G1 (sG1) blocks CD160 mAb binding to HUVECs (filled profile, isotype control). Flow cytometry analysis. Results are representative of 3 independent experiments. (C) Soluble CL1-R2 anti-CD160 mAb triggers inhibition of in vitro angiogenesis. HUVECs were seeded on Matrigel in the presence or absence of FGF2 (10 ng/mL) and sHLA-G1 (sG1, 1 μg/mL) or CD160 mAb (+++, 10 μg/mL; +, 1 μg/mL) or IgG1-isotype control (10 μg/mL). Photographs of each well were taken after 24 hours and angiogenesis quantified. Results are mean ± SD of triplicate wells and are representative of 5 independent experiments. ***P < .001. *P < .005. ns indicates not significant (ANOVA) compared with FGF2-treated cells. (D) Soluble CL1-R2 anti-CD160 mAb induces HUVEC but not fibroblast apoptosis. HUVECs were treated with sG1 (1 μg/mL), CD160 mAb (10 μg/mL), or control IgG1 (10 μg/mL) or were untreated (UT) for 50 hours in the presence of VEGF (50 ng/mL). Apoptotic cells were detected by flow cytometry using annexin V/PI double staining. (Left) Results of representative experiment. (Right) Histograms. Data are shown as mean ± SEM percentage of annexin V-positive cells. *P ≤ .02, Student t test.
The direct inhibitory effect of sHLA-G1 on vessel formation is most likely mediated through the functional CD160 receptor because the CL1-R2 anti-CD160 mAb mimics the inhibition of FGF2-induced capillary tubule formation by endothelial cells cultured in Matrigel and the induction of endothelial cell apoptosis. sHLA-G1 acts directly on CD160 receptor. Given that various HLA class I molecules may bind to CD160,27 it cannot be excluded that other soluble MHC class I molecules could also trigger this receptor to exert antiangiogenic functions. Collectively, these findings provide important mechanistic insight into the antiangiogenic action of sHLA-G1. Further investigation is needed to determine and compare the signaling pathways used by endothelial cells and NK cells after CD160 engagement and leading to antiangiogenic activities and endothelial cell apoptosis for the former and cytokine production45 and cytotoxicity23 for the latter.
In addition to the clear importance in the placental/uterine environment, the identification of CD160 as an inhibitory signaling receptor for angiogenesis could be useful for experimental antiangiogenic therapy to prevent tumor cell growth. Our immunohistochemical analysis of a mouse-grafted tumor showed that CD160, encoded by a gene conserved in this species,35,46 was present in endothelial cells of the tumor vasculature but was not expressed by tumor cells. Future goals are to examine the potential CD160/sHLA-G1-mediated antiangiogenic effect in different tumors and to explore the possible therapeutic use of CD160 mAb in the regulation of pathologic neovascularization.
Prepublished online as Blood First Edition Paper, June 29, 2006; DOI 10.1182/blood-2005-12-019919.
Supported by a Convention Industrielle de Formation par la Recherche-AbTECH Company (CIFRE-AbTECH) fellowship (P.F.), Institut National de la Santé et de la Recherche Médicale (P.L.B., A.B., J.P.), Université Paul Sabatier de Toulouse (P.L.B.), Ligue Nationale contre le Cancer (J.P.), Association pour la Recherche sur le Cancer (J.P., A.B.), British Heart Foundation (J.E.C.), Etablissement Français des Greffes (P.L.B.), Ligue Régionale contre le Cancer, Comités Départements Ariège and Haute Garonne (P.L.B.), Comité Paris (A.B., J.P.), and European Union Network of Excellence EMBIC (Control of Embryo Implantation) (LSHM-CT-2004-512040) (P.L.B.).
P.L.B., J.P., and A.B. contributed equally to this study.
S.C. and J.E.C. contributed equally to this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Marco Colonna for the gift of CD85d mAb, Dr Dan Geraghty for the gift of 721.221-sHLA-G1 cells, and Prof Alain Hovnanian for the gift of primary fibroblasts. We also thank Isabelle Senegas, Josette Desjobert, Carine Berge, and Marie-Claude Laplace for their contributions to this work.

![Figure 4. sHLA-G1 does not interfere with VEGF receptors. (A) HUVECs were incubated with 125I-sHLA-G1 (2 ng/mL [50 pM]) in the absence (-) or presence of cold VEGF (1 μg/mL), FGF2 (1 μg/mL), or sHLA-G1 (sG1, 0.1, 1.0, or 10 μg/mL). Unlabeled sG1, but not VEGF or FGF2, prevented 125I-sHLA-G1 binding. (B) HUVECs were incubated with 125I-VEGF (2 ng/mL) in the presence of cold sG1, FGF2, or VEGF. Unlike cold VEGF, cold sG1 did not abrogate iodinated VEGF binding. In these conditions, the IC50 of cold ligands was 200 pM for VEGF and 2500 pM for sHLA-G1. Results are mean ± SEM of triplicate wells and are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/8/10.1182_blood-2005-12-019919/4/m_zh80200602400004.jpeg?Expires=1767875825&Signature=NfrFvqIvTZvq7LNZQlfPWV9MiM6O1Rsm49FjKwSJ4k122nwrA5P6QdLZ5e9q9bCTlje70CsVt4j5qprOBQjS7J1sLqfOqd6vUQ42~gMZhDcOIxTiYn8bQDb7rlL2uxNUbY69XZFXt~WZlilLaf7zirTCrPcLl8O6wjuuyIurBNEKIMoNsjRbBjvNq5Y-917rShu1PVcSznJ5qr-hZ-3PHtTEQ3imSIUzZCWkA4j37qJH2bC8tdx9C2LYsIm7kvamTpi8R5MzHZJHkFSZx9SgRg2HAjHSF4hLtYYHFCsP20i1HApl7faVicV8GQMtcBfx9nck3Ctmdn78ASwbVp1NpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)