Abstract
B-lymphocyte stimulator (BLyS) is a member of the tumor necrosis factor (TNF) ligand superfamily. Although BLyS costimulates adaptive immune cells, the ability of BLyS to stimulate innate immune cells has not been described. Here, we show that BLyS strongly induces human monocyte survival, and activation as measured by proinflammatory cytokine secretion and up-regulation of costimulatory molecule expression. In addition, monocytes cultured with BLyS differentiated into macrophage-like cells. Regarding BLyS receptor(s) expression, freshly isolated monocytes bound low levels of exogenous BLyS and expressed primarily intracellular TACI, and cell surface TACI levels increased following monocyte activation. Of interest, bone marrow monocytes from some multiple myeloma patients expressed significant levels of cell surface TACI at isolation. Our findings indicate that BLyS plays a role in activating innate immune cells. Moreover, this study may explain more clearly why high BLyS production is often correlated with certain inflammatory autoimmune diseases and B-lymphocyte malignancies.
Introduction
Monocytes are produced in the bone marrow; they circulate in blood for 1 to 3 days before entering tissues or undergo apoptosis if they do not encounter specific survival signals.1-3 The mechanisms regulating monocyte apoptosis are not fully understood. It is known, however, that lipopolysaccharide (LPS), granulocyte-macrophage colony stimulating factor (GM-CSF), macrophage CSF (M-CSF), TNF-α, and interleukin-1β (IL-1β) all inhibit monocyte apoptosis and induce prolonged monocyte survival.3-5 Monocytes play an important role in initiating activation of the innate immune system, for example, phagocytosis further activates monocytes to secrete proinflammatory cytokines and chemokines in inflamed tissues.6 Monocytes can differentiate into either macrophages,7 which display enhanced ability to phagocytose, or dendritic cells (DCs),8 which function as antigen-presenting cells that play a central role in activating the adaptive immune system. These cells provide a first-line host defense against viral and bacterial infection.
B-lymphocyte stimulator (BLyS) is a member of the TNF family (also named BAFF, zTNF4, THANK, and Tall-1), which is expressed as a full-length 285-amino acid transmembrane molecule, and cleaved from cells as a 152-amino acid soluble ligand following processing by a furinlike protease.9,10 Soluble BLyS exists as a trimer or oligomer and is thought to be the primary effector of in vivo function. However, cell-associated BLyS also induced proliferation of anti-IgM-stimulated B lymphocytes,10 and more recently, T cells were shown to respond only to immobilized BLyS.11,12
BLyS is produced by myeloid lineage cells, malignant B cells, activated T cells, and bone marrow stromal cells.13-17 Monocyte stimulation with IFN-γ, TNF-α, or IL-10 increases BLyS production.14 Bacterial components such as LPS and peptidoglycan can also up-regulate BLyS secretion by macrophages, dendritic cells, and monocytes.14,18
BLyS has 3 receptors: BCMA (B-cell maturation antigen), TACI (transmembrane activator and CAML interactor), and BAFF-R (BAFF receptor).19-22 These receptors belong to the TNF receptor superfamily; all receptors possess an extracellular domain containing multiple cysteine-rich domains (CRDs) and intracellular sequences containing TNF receptor-associated protein (TRAF) binding sites. These receptors are primarily expressed in B-lineage cells. BCMA is exclusively expressed in B cells. However, a subset of T cells has also been shown to express TACI and BAFF-R.11,12,23 Because of the expression pattern of BLyS receptors, most studies to date have focused on the effects of BLyS on adaptive immune cells, and these studies show that BLyS costimulates B-cell proliferation and induces cell survival18,24 and can also function as a T-cell costimulatory molecule.11,12,23 Whether BLyS has functional effects on other cell lineages has not been reported.
BLyS transgenic mice developed a syndrome with similarities to systemic lupus erythematosus (SLE) in humans.24,25 Furthermore, BLyS levels are higher in human autoimmune disease, such as SLE and Sjogren syndrome,26-28 rheumatoid arthritis (RA),29,30 as well as in multiple myeloma (MM).31,32 Monocytes/macrophages are recruited into inflamed tissues and they play a key role in initiating immune responses. However, monocytes are currently viewed as BLyS-producing cells, and the possibility that BLyS may also play a signaling role in monocytes has not been previously demonstrated. Of interest, a recent study showed that the incidence of apoptosis among BLyS-expressing cells, mostly CD68+ monocytes/macrophages, in salivary gland tissue was significantly lower in patients with Sjogren syndrome compared with healthy controls.26
Here, we show that BLyS strongly induces monocyte survival, activation, and differentiation into macrophage-like cells. Thus, our data suggest that BLyS regulates the function of innate immune cells as well as adaptive immune cells.
Materials and methods
Cell isolation and culture
Mononuclear cells (MNCs) were isolated from buffy coats of healthy donors and bone marrow cells from healthy donors or patients with MM, monoclonal gammopathy of undetermined significance (MGUS), and primary amyloidosis (AL) by Ficoll-Paque density gradient centrifugation. All patients provided written informed consent in accordance with the Declaration of Helsinki. The Mayo Clinic Rochester institutional review board approved the protocol to obtain blood from volunteers, as well as obtaining bone marrow from individuals with various conditions of monoclonal gammopathy. Monocytes were purified by positive selection using the StemSep Ab enrichment cocktail (StemCell Technologies, Vancouver, BC) in combination with StemSep magnetic colloid. The purity of isolated CD14+ cells routinely exceeded 98%. We cultured CD14+ cells (0.5 × 106/1 mL) in 48-well plates (Becton Dickinson, San Jose, CA) or 2 × 106/2 mL in 24-well plates (Corning, Corning, NY) in RPMI 1640 (Biosource, Camarillo, CA) supplemented with 5% or 10% FBS with or without stimuli.
Cytokines, antibodies, and reagents
Recombinant human GM-CSF and M-CSF were purchased from R&D Systems (Minneapolis, MN). Recombinant human IL-4 was purchased from Peprotech (Rocky Hill, NJ). Recombinant human BLyS (aa 83-286, long form of flag-tagged BLyS) purchased from Calbiochem (La Jolla, CA) was used in all experiments unless otherwise indicated. Recombinant human BLyS was also purchased from Alexis Biochemicals (San Diego, CA), Chemicon International (Temecula, CA), and Research Diagnostics (Flanders, NJ). LPS and polymyxin B were purchased from Sigma-Aldrich (St Louis, MO).
Biotinylated anti-TACI, anti-BCMA, goat isotype control, and phycoerythrin (PE)-conjugated anti-TACI antibodies were purchased from R&D Systems. PE-conjugated anti-BAFF-R and isotype control antibodies were purchased from eBioscience (San Diego, CA). PE-conjugated streptavidin was purchased from Caltag (Burlingame, CA). Biotinylated anti-flag M2 antibody was purchased from Sigma-Aldrich. PE-conjugated CD14, CD80, and CD86; fluorescein isothiocyanate (FITC)-conjugated CD3 and CD14; and PE- or FITC-conjugated mouse IgG1 isotype control were purchased from Becton Dickinson. PE-conjugated mouse IgG2b isotype control antibody, FITC-CD19, CD40, and CD1a were purchased from BD Biosciences Pharmingen (San Diego, CA). FITC-conjugated annexin V was purchased from Caltag. For Western blotting, rabbit anti-IκBα antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-p52 antibody was obtained from Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase-conjugated anti-IgG was purchased from Amersham (Piscataway, NJ).
FACS analysis
Cells to be analyzed by flow cytometry were routinely washed with fluorescence-activated cell sorting (FACS) buffer (PBS supplemented with 2% FBS containing 2 mM EDTA and 0.05% sodium azide). To detect BLyS binding, cells were incubated with or without 2 μg/mL human flag-BLyS for 1 hour at 4°C. After washing 2 times with 4 mL FACS buffer, biotinylated anti-flag M2 antibody was added for 20 minutes at 4°C. Cells were washed and then incubated with PE-conjugated streptavidin for 15 minutes at 4°C. For detecting BLyS receptors, cells were incubated with biotinylated anti-TACI and anti-BCMA, or PE-anti-BAFF-R for 30 minutes to 1 hour at 4°C. After washing, PE-conjugated streptavidin was added and cells were incubated for an additional 15 minutes at 4°C. To detect intracellular BLyS binding receptors, cells were first fixed and permeabilized using a fixation/permeabilization kit purchased from Caltag. For all flow cytometric analyses, we used the FcγR blocking reagent (Miltenyi Biotech, Auburn, CA) before, or with, primary antibody staining in monocytes.
For analysis of apoptosis by flow cytometry, 0.5 × 106/1 mL monocytes were cultured with 5% FBS/RPMI for 3 days, washed with PBS, and stained with FITC-conjugated annexin V for 20 minutes at 4°C in annexin V binding buffer (140 mM NaCl, 5 mM CaCl2, 10 mM HEPES [pH 7.4]). After washing with annexin V binding buffer, cells were resuspended with 0.5 ng/mL propidium iodide (PI) in annexin V binding buffer, and immediately analyzed using a FACSCalibur (Becton Dickinson). Data were analyzed with FlowJo software (Tree Star, Ashland, OR) and WinMDI software (The Scripps Research Institute, La Jolla, CA).
Immunoblotting
Freshly isolated or stimulated CD14+ monocytes (2 × 106) were lysed using radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% NP-40, 0.1% SDS, 1 mM EDTA, 15 mM sodium molybdate, 1 mM NaF) supplemented with protease inhibitors (10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL pepstatin, and 1 mM PMSF). Lysates were cleared of insoluble material by centrifugation for 15 minutes at 13 226g and subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P membranes (Millipore, Bedford, MA). Membranes were blocked in TBS-T (25 mM Tris-HCl [pH 7.2], 150 mM NaCl, 0.2% Tween) supplemented with 5% nonfat skim milk for 1 hour at room temperature. IκBα, TACI, and p52 were detected using specific antibodies for overnight incubation at 4°C. After washing 3 times with TBS-T, the membranes were incubated with horseradish peroxidase-conjugated anti-IgG for 1 hour at room temperature. After washing 4 times with TBS-T, proteins in the membranes were then visualized using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL).
Quantification of cytokine secretion
IL-6, TNF-α, and IL-1β levels were determined in cell-free culture supernatants using cytokine-specific enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems. Results are shown in nanograms per milliliter or picograms per milliliter (as mean ± SD, n = 3).
Statistical analysis
Statistical analysis was performed using the Student t test. Values of P less than .05 were considered significant.
Results
BLyS prevents monocytes from undergoing spontaneous apoptosis
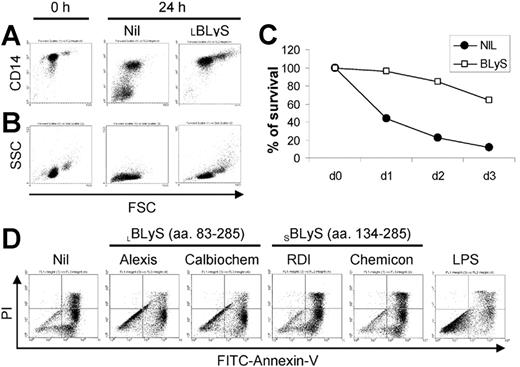
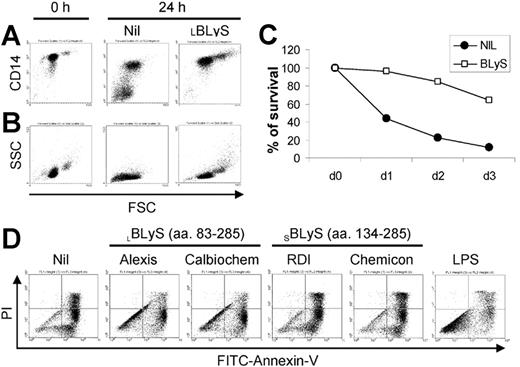
During our studies of BLyS on the survival of malignant plasma cells obtained from MM patients, we observed that BLyS appeared to augment monocyte survival in cultures of MM patient bone marrow MNCs (data not shown). To pursue this observation, we began this study by assessing the effects of exogenous BLyS on peripheral blood monocytes obtained from healthy adults. As shown in Figure 1A, monocytes cultured in media alone expressed lower levels of CD14, and a significant subset completely lost CD14 expression in a manner that correlated with the appearance of a subset of nonviable cells as revealed by decreased forward light scatter (Figure 1B). In striking contrast, monocytes cultured with BLyS maintained high levels of CD14 expression and the cells increased in size. It has been reported by others that CD14 expression is maintained only on viable monocytes.3 The apparent ability of BLyS to augment in vitro monocyte survival was also consistent with cell morphology as analyzed by light microscopy (data not shown). In addition, cell aggregation was readily apparent in monocytes cultured with BLyS (data not shown).
BLyS induces monocyte survival. CD14+ monocytes were cultured ± 100 ng/mL BLyS for 24 hours before assessing CD14 expression levels by flow cytometry (A) or cell morphology (B) as revealed by forward (FSC) versus side scatter (SSC). (C) To assess the effects of BLyS on monocyte survival over time, cells were cultured ± 100 ng/mL LBLyS for 1 to 3 days and the percentage of viable cells was determined by FITC-annexin V and PI staining. (D) Four commercial preparations of BLyS (100 ng/mL) were compared with 20 ng/mL LPS for their ability to promote monocyte survival after 3 days of culture.
BLyS induces monocyte survival. CD14+ monocytes were cultured ± 100 ng/mL BLyS for 24 hours before assessing CD14 expression levels by flow cytometry (A) or cell morphology (B) as revealed by forward (FSC) versus side scatter (SSC). (C) To assess the effects of BLyS on monocyte survival over time, cells were cultured ± 100 ng/mL LBLyS for 1 to 3 days and the percentage of viable cells was determined by FITC-annexin V and PI staining. (D) Four commercial preparations of BLyS (100 ng/mL) were compared with 20 ng/mL LPS for their ability to promote monocyte survival after 3 days of culture.
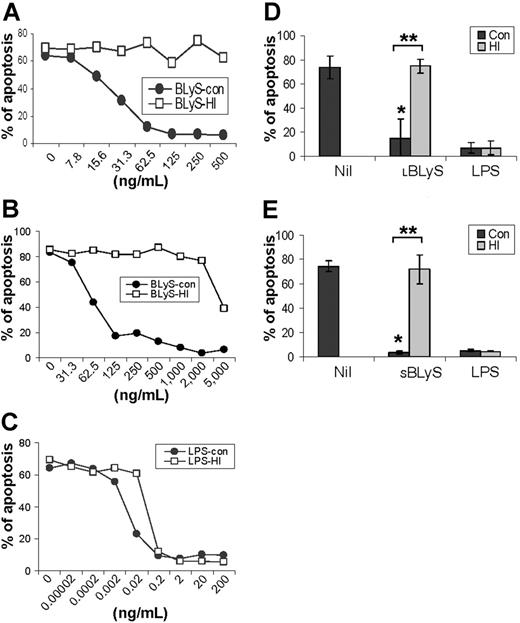
To more rigorously determine whether BLyS attenuated monocyte apoptosis in vitro, we next analyzed monocyte apoptosis using annexin V and PI staining. Figure 1C shows that BLyS promoted monocyte survival compared with cells cultured in media alone. Monocyte survival is known to be enhanced when cells are cultured in the presence of human serum.33,34 However, in results not shown, BLyS augmented monocyte survival even when cells were cultured with human serum for 3 days (80% viability in the absence of BLyS versus > 90% viability in the presence of BLyS, P < .01, n = 3). Because of a previous report that BLyS binds to B cells, but not T cells, monocytes/macrophages, granulocytes, or natural killer (NK) cells,9 our observations that BLyS augmented monocyte survival were unexpected and prompted us to test several additional preparations of recombinant BLyS. Of note, recombinant human BLyS is available in 2 different forms. One form contains most of the extracellular domain as well as the stalk region (amino acids 83-285) and is hereafter referred to as the long form of BLyS (LBLyS). The other form includes amino acids 134 to 285 and lacks the stalk region and is hereafter referred to as the short form of BLyS (SBLyS). Our initial screen of these 2 forms of BLyS demonstrated differences in their effects on monocyte survival when used at a uniform concentration of 100 ng/mL (Figure 1D). Both preparations of LBLyS clearly enhanced monocyte survival, whereas 2 preparations of SBLyS appeared to be ineffective. The differential effect of LBLyS versus SBLyS on monocytes was surprising given that we were able to demonstrate that all 4 of these BLyS preparations were able to enhance anti-Ig-stimulated human B-cell proliferation to a similar degree (data not shown). Because it was formally possible that the BLyS-promoting effects on monocyte survival may be dependent upon absolute levels, particularly given that the long form of BLyS may be more likely to exist as a multimer, we next carried out a more comprehensive titration of the effects of both SBLyS and LBLyS on in vitro monocyte survival. LBLyS promoted monocyte survival at concentrations as low as 15.6 ng/mL and achieved maximum effectiveness when used at 62.5 ng/mL (Figure 2A), whereas 2 to 3 times more SBLyS was needed to see a comparable level of LBLyS activity (Figure 2B).
BLyS-mediated enhancement of monocyte survival does not result from endotoxin contamination
Our results thus far suggest the intriguing possibility that monocytes may be capable of both producing BLyS14 and responding to BLyS. However, because endotoxin is a potent activator of monocytes,3,35 it was formally possible that endotoxin contamination accounted for the ability of BLyS to stimulate monocyte survival. We therefore subjected both BLyS and LPS, as a control, to heat inactivation by boiling for 30 minutes, and then assessed the ability of the heat-inactivated BLyS and LPS to augment in vitro monocyte survival compared with control, non-heat-inactivated reagents. LBLyS induced monocyte survival in a dose-dependent manner, and its ability to augment monocyte survival was completely abrogated by heat inactivation (Figure 2A). The ability of SBLyS to promote monocyte survival was also destroyed by heat inactivation, although very high levels of SBLyS may introduce some levels of endotoxin (Figure 2B), as suggested by the resistance of the survival-promoting activity to heat inactivation. In contrast, LPS strongly enhanced monocyte survival, and, as expected, this activity was resistant to heat inactivation (Figure 2C). To assess the reproducibility of the effects of SBLyS and LBLyS on promotion of monocyte survival, we next chose 100 ng/mL LBLyS, 500 ng/mL SBLyS, and 20 ng/mL LPS, and then repeated the experiments on monocytes obtained from 3 to 5 different donors. As seen in Figure 2D, LBLyS strongly and reproducibly induced monocyte survival, and those effects were completely abrogated by heat inactivation (P < .001) (Figure 2D). Statistically significant results were also observed when SBLyS at a concentration of 500 ng/mL was used (P < .001) (Figure 2E). Consistent with the results shown in Figure 2A-C, heat inactivation had no effect on the ability of LPS to augment monocyte survival (Figure 2D-E). In addition, when the LPS inhibitor, polymyxin B, was assessed for its effects on BLyS-induced survival, whereas it completely inhibited LPS-induced survival, it was largely without effect on BLyS-induced survival (Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article). Collectively, these results indicate that BLyS highly enhances monocyte survival, and this effect does not result from trace levels of endotoxin contamination.
Titration of BLyS-mediated enhancement of monocyte survival and effects of heat inactivation on biologic activity. Control (con; non-heat inactivated) and heat-inactivated (HI) (A) LBLyS (Calbiochem), (B) SBLyS (RDI), and (C) LPS were titrated over the indicated range of concentrations, and monocyte survival was determined on day 3. Data are representative of 3 independent experiments. (D) Apoptosis analysis of cells stimulated with control (con) or heat-inactivated 100 ng/mL LBLyS (HI) or 20 ng/mL LPS (HI) (data shown represent mean ± SD of 5 independent experiments); and (E) cells stimulated with control (con) or heat-inactivated 500 ng/mL SBLyS (HI) or 20 ng/mL LPS (HI) (data shown represent mean ± SD of 3 independent experiments). *When compared with Nil, P < .001. **When control LBLyS or SBLyS compared with heat-inactivated LBLyS or SBLyS, P < .001.
Titration of BLyS-mediated enhancement of monocyte survival and effects of heat inactivation on biologic activity. Control (con; non-heat inactivated) and heat-inactivated (HI) (A) LBLyS (Calbiochem), (B) SBLyS (RDI), and (C) LPS were titrated over the indicated range of concentrations, and monocyte survival was determined on day 3. Data are representative of 3 independent experiments. (D) Apoptosis analysis of cells stimulated with control (con) or heat-inactivated 100 ng/mL LBLyS (HI) or 20 ng/mL LPS (HI) (data shown represent mean ± SD of 5 independent experiments); and (E) cells stimulated with control (con) or heat-inactivated 500 ng/mL SBLyS (HI) or 20 ng/mL LPS (HI) (data shown represent mean ± SD of 3 independent experiments). *When compared with Nil, P < .001. **When control LBLyS or SBLyS compared with heat-inactivated LBLyS or SBLyS, P < .001.
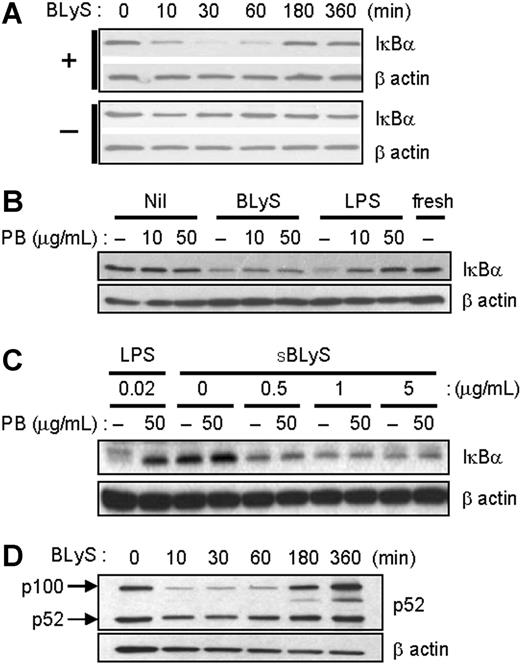
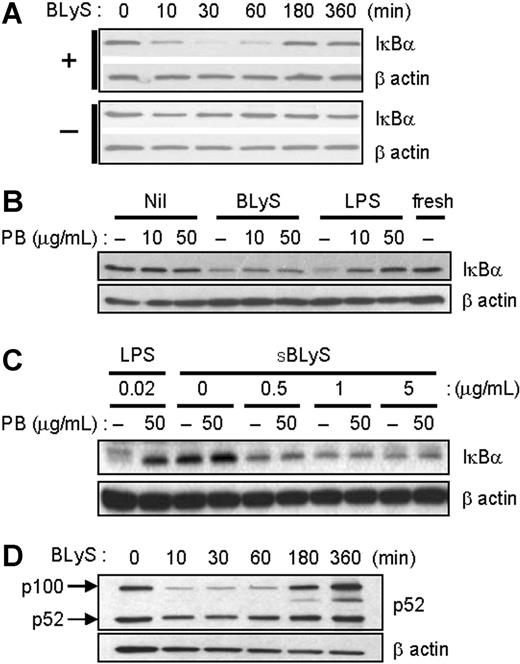
BLyS stimulates NF-κB activation. (A) After cell stimulation with 200 ng/mL LBLyS for each indicated time point at 37°C, cell extracts were analyzed for IκBα degradation. Unstimulated cells were used as a control (bottom panel). (B) Cells were stimulated with 200 ng/mL LBLyS or 20 ng/mL LPS for 30 minutes ± 10 or 50 μg/mL polymyxin B (PB), and cell lysates were immunoblotted for IκBα. (C) Cells were stimulated with the indicated concentrations of SBLyS or LPS for 30 minutes ± 50 μg/mL polymyxin B before analysis of IκBα levels by immunoblotting. (D) Cells were stimulated as described in panel A, and cell lysates were analyzed for p52 expression levels. β-Actin was used as a loading control in all experiments.
BLyS stimulates NF-κB activation. (A) After cell stimulation with 200 ng/mL LBLyS for each indicated time point at 37°C, cell extracts were analyzed for IκBα degradation. Unstimulated cells were used as a control (bottom panel). (B) Cells were stimulated with 200 ng/mL LBLyS or 20 ng/mL LPS for 30 minutes ± 10 or 50 μg/mL polymyxin B (PB), and cell lysates were immunoblotted for IκBα. (C) Cells were stimulated with the indicated concentrations of SBLyS or LPS for 30 minutes ± 50 μg/mL polymyxin B before analysis of IκBα levels by immunoblotting. (D) Cells were stimulated as described in panel A, and cell lysates were analyzed for p52 expression levels. β-Actin was used as a loading control in all experiments.
BLyS stimulates NF-κB activity
Several previous studies have demonstrated BLyS-mediated activation of the classical and nonclassical NF-κB pathways in B cells.36-38 We therefore asked if BLyS stimulation enhances NF-κB activation in monocytes. To measure classical NF-κB activation, monocytes were stimulated with LBLyS for varying lengths of time, and NF-κB activation was measured by IκBα degradation using immunoblotting. Activated NF-κB components are released from IκBα, a specific inhibitor that retains NF-κB components in the cytoplasm.39 As shown in Figure 3A, BLyS promotes IκBα degradation as early as 10 minutes, and cellular levels of IκBα returned to baseline levels approximately 3 hours later. These results are similar to what has been reported in B-lineage cells.36,40 To exclude endotoxin contamination again, we stimulated monocytes with BLyS or LPS with or without 10 or 50 μg/mL of the LPS inhibitor, polymyxin B, and then analyzed IκBα degradation. In LPS-stimulated monocytes, IκBα degradation was inhibited by polymyxin B in a dose-dependent manner. However, BLyS still induced IκBα degradation even in the presence of polymyxin B (Figure 3B). SBLyS was also able to activate the classical NF-κB pathway in the presence of polymyxin B (Figure 3C).
Next, we determined whether BLyS also induces nonclassical NF-κB activation in monocytes. LBLyS stimulation triggered generation of p52 from p100 molecules with delayed kinetics, and, of interest, it also increased the relative levels of p100 over time (Figure 3D). In summary, these results indicate that BLyS induces processing of both IκBα and p100 in monocytes, and that this did not result from endotoxin contamination.
BLyS promotes monocyte activation and differentiation
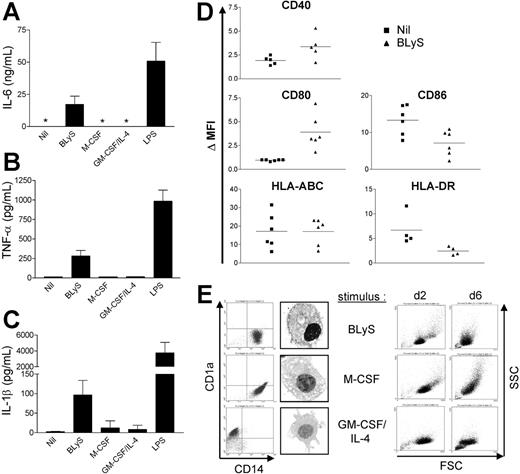
Activated monocytes express proinflammatory cytokines such as IL-6, IL-1β, TNF-α, and IL-8.6 Therefore, we next determined whether BLyS promotes cytokine production by monocytes using ELISA. We were unable to detect IL-6 in supernatants harvested from monocytes cultured in media alone for 24 hours; however, BLyS-treated cells produced IL-6 (Figure 4A). We also confirmed that BLyS induced IL-6 expression at the mRNA level using reverse-transcription-polymerase chain reaction (RT-PCR) (data not shown). Moreover, BLyS also induced TNF-α and IL-1β secretion by monocytes (Figure 4B and 4C, respectively). It is important to note that M-CSF or GM-CSF plus IL-4 did not stimulate monocyte IL-6 or TNF-α secretion, however, addition of these cytokines did promote monocyte survival as effectively as did BLyS as revealed by cell morphology (Figure 4E right column). Thus, BLyS-stimulated monocyte secretion of IL-6, TNF-α, and IL-1β did not simply reflect higher monocyte viability but instead suggested that BLyS triggers monocytes to secrete those cytokines. As a further measure of BLyS-induced activation/maturation of monocytes, we analyzed the effects of BLyS on expression levels of several cell surface markers. Compared with control cells, BLyS significantly up-regulated cell surface CD80 and CD40 expression (Figure 4D; P = .009 and P = .023, respectively) and significantly decreased CD86 expression (P = .018). MHC class I was not changed, and HLA-DR was slightly decreased by BLyS stimulation, albeit not significantly (P = .073). Collectively, these data suggest that BLyS enhances monocyte activation and secretion of proinflammatory cytokines. In addition, BLyS induced monocyte aggregation (data not shown), further adding support to the conclusion that BLyS induces monocyte activation.
BLyS promotes monocyte activation and differentiation. Cells were stimulated with the indicated stimuli for 24 hours before isolation of cell-free supernatants and assessment of secreted (A) IL-6, (B) TNF-α, and (C) IL-1β levels by ELISA. *Values that were below the limits of detection. (A-C) Data shown represent mean ± SD of 3 different experiments. (D) Isolated monocytes were cultured ± 200 ng/mL LBLyS for 2 days before assessment of expression of the indicated surface costimulatory molecules on viable cells by flow cytometry. Each symbol represents the ΔMFI, which is a reflection of fold induction of each molecule relative to the isotype control. (E) Monocyte differentiation was determined by measuring surface expression of CD14 and CD1a at day 6. Cell morphology was analyzed by flow cytometry and by light microscopy using an Olympus AX70 microscope (Olympus America, Center Valley, PA) equipped with a UPlan FL objective at a magnification of 60×(1.25 numeric aperture) under oil. Images were collected using a Diagnostic Instruments camera (model 1.4.0; Sterling Heights, MI) and SPOT image acquisition software version 3.0.4 (Diagnostic Instruments).
BLyS promotes monocyte activation and differentiation. Cells were stimulated with the indicated stimuli for 24 hours before isolation of cell-free supernatants and assessment of secreted (A) IL-6, (B) TNF-α, and (C) IL-1β levels by ELISA. *Values that were below the limits of detection. (A-C) Data shown represent mean ± SD of 3 different experiments. (D) Isolated monocytes were cultured ± 200 ng/mL LBLyS for 2 days before assessment of expression of the indicated surface costimulatory molecules on viable cells by flow cytometry. Each symbol represents the ΔMFI, which is a reflection of fold induction of each molecule relative to the isotype control. (E) Monocyte differentiation was determined by measuring surface expression of CD14 and CD1a at day 6. Cell morphology was analyzed by flow cytometry and by light microscopy using an Olympus AX70 microscope (Olympus America, Center Valley, PA) equipped with a UPlan FL objective at a magnification of 60×(1.25 numeric aperture) under oil. Images were collected using a Diagnostic Instruments camera (model 1.4.0; Sterling Heights, MI) and SPOT image acquisition software version 3.0.4 (Diagnostic Instruments).
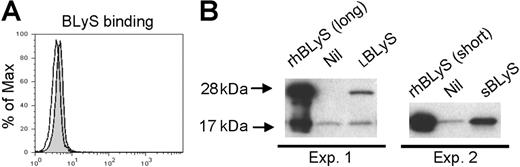
BLyS binding in monocytes. (A) Freshly isolated monocytes were incubated without (open curve) or with (gray shaded curve) flag-tagged BLyS, and binding was visualized by flow cytometry using a biotinylated anti-flag antibody and PE-streptavidin. (B) Freshly isolated monocytes were incubated with LBLyS (Exp 1) or SBLyS (Exp 2), or without BLyS (Nil) before washing, cell lysis, and analysis of BLyS binding by Western blotting
BLyS binding in monocytes. (A) Freshly isolated monocytes were incubated without (open curve) or with (gray shaded curve) flag-tagged BLyS, and binding was visualized by flow cytometry using a biotinylated anti-flag antibody and PE-streptavidin. (B) Freshly isolated monocytes were incubated with LBLyS (Exp 1) or SBLyS (Exp 2), or without BLyS (Nil) before washing, cell lysis, and analysis of BLyS binding by Western blotting
Monocytes can either differentiate into macrophages in vitro in response to M-CSF,7 or immature DCs in response to GM-CSF and IL-4.8 To determine whether BLyS is involved in monocyte differentiation, monocytes were cultured with BLyS for 6 days. For controls, monocytes were cultured with GM-CSF and IL-4, or M-CSF. We found that monocytes cultured with BLyS maintained high levels of CD14 expression, but did not express CD1a, a specific DC marker (Figure 4E, left column). Cells cultured with BLyS displayed macrophage morphology as evidenced by the presence of many vacuoles in the cytoplasm (Figure 4E, left panel). BLyS, M-CSF, and GM-CSF plus IL-4 had a similar effect on cell viability on the basis of FSC versus SSC at day 2. The overall cell size of BLyS-cultured cells exhibited similarity with M-CSF-cultured cells, although they were smaller compared with cells stimulated by M-CSF at day 6 (Figure 4E, right column). These results suggest that BLyS promotes differentiation of monocytes into macrophage-like cells.
TACI is expressed in monocytes
Our results thus far clearly demonstrate that exogenous BLyS stimulates monocyte survival and activation. To determine by what mechanism this occurs, we next used flow cytometry to assess BLyS binding and expression of the known BLyS binding receptors (BAFF-R, TACI, and BCMA) on monocytes. The level of BLyS binding on freshly isolated monocytes was very low when assessed by flow cytometry (Figure 5A) and somewhat variable between experiments (data not shown). In an alternative strategy to detect BLyS binding to freshly isolated monocytes, we next used Western blotting. As shown in Figure 5B, there were trace levels of a 17-kDa band in both preparations of monocytes, however, the intensity of this band was clearly increased when cells were incubated with SBLyS (Expt 2) and a 28-kDa band appeared when cells were incubated with LBLyS.
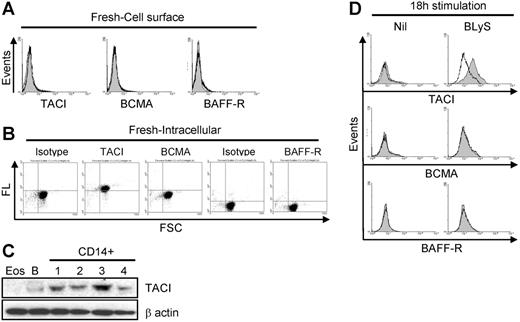
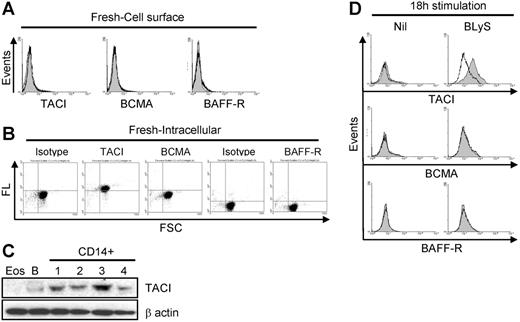
Of interest, despite our ability to demonstrate binding of exogenous BLyS (Figure 5A-B), expression of TACI, BCMA, and/or BAFF-R was either absent or below the levels of detection by flow cytometry (Figure 6A). In contrast, intracellular analysis of BLyS binding receptor expression using permeabilized cells demonstrated that TACI was highly expressed, whereas BCMA and BAFF-R were not (Figure 6B). Moreover, TACI expression was confirmed at the protein level by Western blotting (Figure 6C) and mRNA level by RT-PCR (data not shown). Because other members of the TNF family have been shown to pre-exist in the cytoplasm and translocate to the surface following activation,41-43 we next examined TACI expression on monocytes that had been cultured overnight with or without BLyS. As seen in Figure 6D, TACI expression was barely detectable in control cells, however, TACI was clearly present on cells cultured with BLyS, albeit the expression levels were variable among donors. BCMA and BAFF-R remained undetectable on the cell surface after culturing (Figure 6D). We also found that IL-10 (Figure S2) was able to increase surface TACI expression. Therefore, we suggest that TACI is the responsible receptor for the BLyS-mediated effects in monocytes.
TACI is expressed in bone marrow monocytes from MM patients
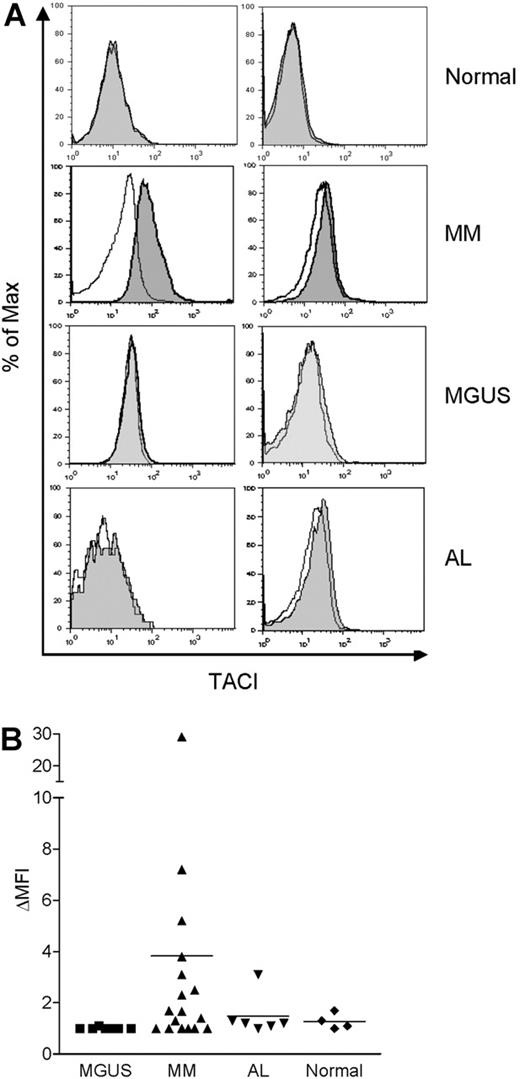
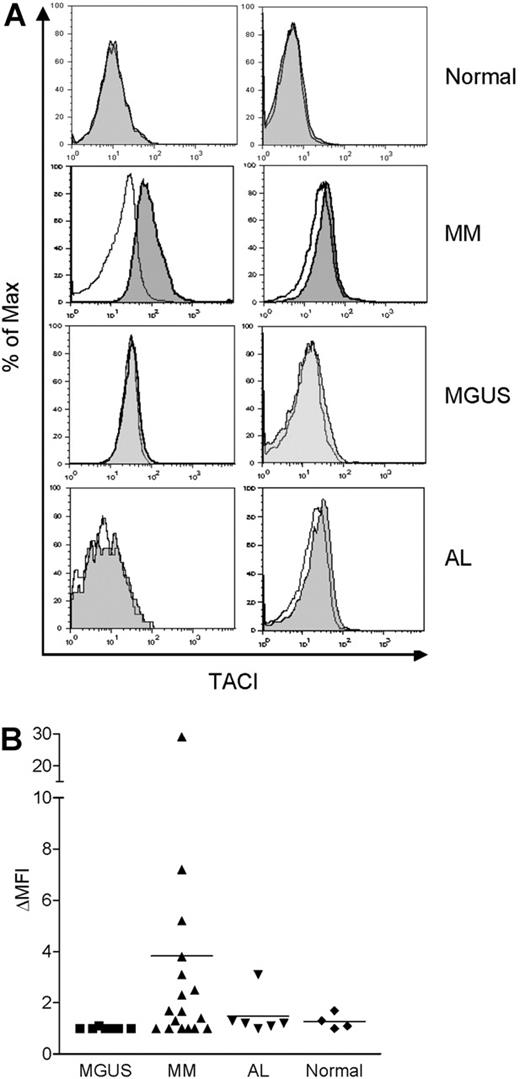
As mentioned earlier, we observed that BLyS augmented monocyte survival when bone marrow MNCs from MM patients were cultured with BLyS (data not shown). Previous reports also showed that high levels of BLyS are present in the serum31 and bone marrow of MM patients.32 Thus, we examined whether freshly isolated monocytes from the bone marrow of MM patients express TACI on the cell surface. We also tested monocytes obtained from patients with 2 other plasma cell disorders, MGUS and AL. TACI expression was not detected in monocytes from normal bone marrow (Figure 7A-B). By contrast, surface TACI was evident in monocytes obtained from some, but not all, MM patients and when present, absolute levels of TACI expression were variable. With one exception, monocytes obtained from a limited number of MGUS and AL patients did not express detectable TACI. These findings correlate with our previous report that there is variable expression of BLyS in bone marrows from MM patients. Thus, these data suggest that bone marrow microenvironmental factors in some MM patients (eg, elevated BLyS) may up-regulate surface TACI expression by monocytes.
TACI expression in monocytes. (A) Surface staining of TACI, BCMA, and BAFF-R on freshly isolated monocytes. Specific receptor expression is indicated by the gray shaded curves, and isotype control staining is indicated by the open curves. (B) Intracellular staining of TACI, BCMA, and BAFF-R in freshly isolated monocytes. Fluorescence levels (FL) for each antibody are shown on the ordinate and forward light scatter (FSC) is shown on the abscissa. (C) Expression of TACI by Western blot analysis in freshly isolated CD14+ monocytes, B cells (B; positive control), or eosinophils (Eos; negative control). β-Actin was used as a loading control. (D) Cells were cultured with or without 200 ng/mL LBLyS overnight, and then analyzed for surface expression of TACI, BCMA, or BAFF-R on viable cells as determined by FSC versus SSC. Isotype controls and fluorescence controls were performed for each sample (open curves).
TACI expression in monocytes. (A) Surface staining of TACI, BCMA, and BAFF-R on freshly isolated monocytes. Specific receptor expression is indicated by the gray shaded curves, and isotype control staining is indicated by the open curves. (B) Intracellular staining of TACI, BCMA, and BAFF-R in freshly isolated monocytes. Fluorescence levels (FL) for each antibody are shown on the ordinate and forward light scatter (FSC) is shown on the abscissa. (C) Expression of TACI by Western blot analysis in freshly isolated CD14+ monocytes, B cells (B; positive control), or eosinophils (Eos; negative control). β-Actin was used as a loading control. (D) Cells were cultured with or without 200 ng/mL LBLyS overnight, and then analyzed for surface expression of TACI, BCMA, or BAFF-R on viable cells as determined by FSC versus SSC. Isotype controls and fluorescence controls were performed for each sample (open curves).
TACI is expressed in monocytes from MM patients. Bone marrow MNCs from 4 healthy controls, 17 MM patients, 7 MGUS patients, and 6 AL patients were costained with anti-TACI and anti-CD14 antibodies, and analyzed by flow cytometry. Representative histograms are shown in panel A. TACI is indicated by the gray shaded curves, and isotype control staining is indicated by the open curves in CD14+ gated cells. (B) Surface TACI levels are indicated by ΔMFI in CD14+ gated cells. The solid line represents the mean value for each group.
TACI is expressed in monocytes from MM patients. Bone marrow MNCs from 4 healthy controls, 17 MM patients, 7 MGUS patients, and 6 AL patients were costained with anti-TACI and anti-CD14 antibodies, and analyzed by flow cytometry. Representative histograms are shown in panel A. TACI is indicated by the gray shaded curves, and isotype control staining is indicated by the open curves in CD14+ gated cells. (B) Surface TACI levels are indicated by ΔMFI in CD14+ gated cells. The solid line represents the mean value for each group.
Discussion
Previous studies on the functional effects of BLyS have focused on adaptive immune cells. To our knowledge, there are no reports investigating the effects of BLyS on the function of innate immune cells, such as monocytes. In this study, we present evidence that BLyS strongly induced monocyte survival and activation/maturation as revealed by NF-κB activation and induction of proinflammatory cytokine secretion and expression of costimulatory molecules. We have also identified TACI as the BLyS binding receptor in monocytes. Thus, our findings suggest that BLyS regulates the function of innate as well as adaptive immune cells.
Although the 2 different molecular forms of soluble BLyS used in this study both promoted monocyte survival, LBLyS was more effective than SBLyS. In this regard, previous reports showed that other TNF ligands, such as FasL and CD40L, had higher activity in a membrane-bound form or in a soluble form if it included the stalk region.44,45 The stalk region may facilitate stabilization of this molecule as a trimer, which is the functional form of members of the TNF ligand family. Even though LBLyS is not believed to exist naturally10 because it contains the extracellular domain including the stalk region, it may mimic the membrane-bound form of BLyS and suggest that, in vivo, monocytes may be activated by cell-cell interaction mediated by BLyS when expressed as an intrinsic membrane protein. It would not be advantageous for circulating peripheral blood monocytes to become activated by normal serum levels of BLyS, thus monocytes may need a higher threshold to respond to the soluble form of BLyS compared with the membrane-bound form of BLyS.
BLyS promoted both classical and nonclassical NF-κB activation in monocytes. Our observation that BLyS stimulates p100 expression in monocytes differs from previous studies in B cells showing that BLyS reduces the level of p100 molecules.37,40 However, it has been shown that LPS, IL-1β, and TNF-α induce p100 expression at mRNA and protein levels.46 An additional study demonstrated that TNF stimulates p100 synthesis through activation of the classical NF-κB pathway.47 At this point, it remains unclear as to whether increased p100 levels in monocytes resulted from direct activation of the classical pathway by BLyS, or as a secondary response to inflammatory cytokines, such as TNF-α, induced by BLyS. In additional studies not shown, we have also demonstrated that BLyS is similar to TNF-α in its ability to stimulate activation of p38 and MAP kinase. Of interest, the kinetics of p38 and MAPK activation were delayed in comparison with LPS-mediated activation of these signaling intermediates. Additional studies examining downstream signaling consequences are warranted to determine whether there are any signals that are uniquely triggered by BLyS. However, it is also possible that BLyS-mediated signals are not unique from other members of this cytokine superfamily, but instead BLyS adds another level of redundancy to this family of molecules.48
Monocytes activated by BLyS secreted significant amounts of IL-6, TNF-α, and IL-1β. Even though M-CSF and GM-CSF maintain monocyte survival and support monocyte differentiation, they are not able to induce detectable secretion of TNF-α, or IL-6 protein in monocytes by themselves.49,50 Thus BLyS-stimulated monocyte secretion of IL-6, TNF-α, and IL-1β did not simply reflect higher monocyte viability, indicating that BLyS is uniquely capable of activating monocytes to produce the proinflammatory cytokines.
When BLyS receptor expression was previously screened among normal human peripheral blood nucleated cells, monocytes appeared not to express receptors for BLyS.9 Of interest, another study has shown that TACI mRNA is expressed in monocytes and increased in IL-10-stimulated monocytes, although the expression levels are much lower compared with B cells.51 In our study, we too observed that freshly isolated monocytes did not express detectable levels of surface BAFF-R, TACI, or BCMA by flow cytometry, however, following overnight culture with BLyS or IL-10, we found that monocytes acquired detectable levels of cell surface TACI. Despite the FACS data, our results demonstrating BLyS binding by Western blotting and NF-κB activation in freshly isolated monocytes support the possibility that low levels of surface TACI are indeed present, and that following activation, these levels increase perhaps as a result of activation-induced increased shuttling of cytoplasmic TACI to the cell surface. It is also possible that a yet-to-be-identified receptor might be involved in the initial effect of BLyS and surface TACI expression in monocytes. Many studies have shown that recruitment of monocytes into inflammatory sites requires interaction with endothelial cells and extracellular matrix proteins by adhesion molecules. Interaction of those adhesion molecules with their ligands is known to play a role in monocyte activation and maturation, including priming cells to respond to stimuli.52,53 During transmigration into inflamed tissues, monocytes may acquire increased cell surface TACI expression thereby allowing monocytes to respond to BLyS produced in inflamed tissues. After they arrive at the inflammatory site, they also begin to express more BLyS in response to known inflammatory cytokines.14,18 Moreover, other types of cells, such as neutrophils,16 dendritic dells,14 and some epithelial cells,26 produce BLyS. In this cytokine-rich environment, monocytes may respond to elevated BLyS through TACI by secreting inflammatory cytokines such as IL-6, TNF-α, and IL-1β and finally differentiate into tissue macrophages. However, it remains unclear how TACI is up-regulated on cell surface by BLyS or IL-10, but not by IFN-γ and TNF-α. Even though BLyS is produced by IFN-γ- and TNF-α-stimulated monocytes,14,18 the level of BLyS production after overnight culture may not be sufficient to up-regulate surface TACI expression. Alternatively, IL-10 and BLyS may deliver unique intracellular signals required to activate transportation of cytoplasmic TACI to the cell surface. Additional studies are required to elucidate the precise mechanism of cytokine-induced expression of cell surface TACI.
A recent study suggested that BLyS augments Th1-mediated delayed-type hypersensitivity reactions and a high degree of inflammation.54 Monocyte/macrophages release immunoregulatory cytokines, such as IL-10 and IL-12, which are critically involved in the regulation of the Th1 or Th2 differentiation of the lymphocyte response.55 In addition, macrophages play a key role in the initiation, maintenance, and resolution of inflammation by producing a wide range of cytokines or chemokines that participate in both beneficial and detrimental outcomes in inflammation.6 Our data demonstrate that monocytes cultured with BLyS differentiated into macrophage-like cells. Thus, macrophages differentiated by BLyS could be involved in Th1-type inflammatory diseases. It has been shown that BLyS is highly expressed in various autoimmune diseases.27-30 For example, in rheumatoid arthritis, synovial inflammatory infiltrates typically include T cells, B cells, macrophages, and DCs.29 BLyS is highly expressed by a subset of CD68+ macrophages in rheumatoid synovitis.29 In those microenvironments, recruited monocytes may be activated and survive well by responding to the membrane-bound form (LBLyS) as well as the soluble form (SBLyS) that would be abundantly present in this environment. It would therefore be interesting to look at the contribution of monocytes/macrophages in BLyS-related autoimmune diseases.
Of interest, freshly isolated bone marrow monocytes from some MM patients expressed TACI. By contrast, bone marrow monocytes obtained from MGUS and primary amyloidosis patients and from healthy adults did not express detectable cell surface TACI by flow cytometry. We have previously shown that BLyS is highly expressed in the bone marrow of some patients with MM compared with healthy controls.32 Surface TACI expression by monocytes may therefore depend on the bone marrow microenvironment, and in the case where BLyS levels are high, this may result in monocyte activation as revealed by TACI expression. This speculation is consistent with our in vitro results demonstrating the ability of BLyS to stimulate TACI expression. In addition, Moreaux et al31 showed that serum levels of BLyS from most myeloma patients were in the range of 10 to 100 ng/mL. From our in vitro data, 100 ng/mL BLyS strongly induced monocyte survival and even 15.6 ng/mL LBLyS promoted monocyte survival up to 50%. Thus, we suggest that monocytes may be activated through a BLyS-TACI interaction and thereby secrete proinflammatory cytokines, including IL-6, which is a known survival and growth factor for myeloma cells. Monocytes may therefore also play a role in multiple myeloma disease progression. However, additional studies examining a larger cohort of patients are clearly warranted to more rigorously test this hypothesis.
In conclusion, our findings demonstrate that BLyS regulates innate immune responses through monocyte activation and maturation, in addition to playing a role in adaptive immune responses. The dysregulation of BLyS production may contribute to induction of abnormal monocyte/macrophage activation, resulting in exacerbating certain inflammatory autoimmune disorders, as well as MM.
Prepublished online as Blood First Edition Paper, July 6, 2006; DOI 10.1182/blood-2005-12-017319.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Yoon for eosinophils and discussion and R. C. Tschumper for technical assistance.