Abstract
Allogeneic hematopoietic stem cell transplantation (SCT) regimens incorporating the lymphocytotoxic CD52 antibody alemtuzumab demonstrate efficient engraftment and reduced graft-versus-host disease (GVHD). However, these protocols substantially impair posttransplantation antiviral and antitumor immunity. To accelerate immune reconstitution after alemtuzumab-based reduced-intensity SCT, we administered prophylactic CD8-depleted donor lymphocyte infusions (DLIs) starting on days 60 and 120 after transplantation. DLIs were processed in an immunomagnetic good manufacturing practice depletion procedure resulting in a 2.5- to 6-log reduction in CD8 T cells. Of 23 high-risk patients with hematologic malignancies, 11 received a total of 21 CD8-depleted DLIs. Five patients developed transient grade I acute GVHD following transfer. Only 2 patients with HLA-C–mismatched donors showed grade II and III acute GVHD and subsequently progressed to limited chronic GVHD. Following DLIs, 4 patients with declining hematopoietic donor chimerism converted to full chimeras. A 2.1-fold median increase of circulating CD4 T cells was observed within 2 weeks after infusion. Non-DLI patients did not show a comparable rise in CD4 counts. Four patients demonstrated enhanced frequencies of cytomegalovirus-specific CD4 and CD8 T cells following transfer. Our results suggest that prophylactic CD8-depleted DLIs accelerate immune reconstitution after lymphodepleted HLA-matched SCT and carry a low risk of inducing severe GVHD.
Introduction
In patients undergoing allogeneic hematopoietic stem cell transplantation (SCT), T-cell depletion (TCD) prevents graft-versus-host disease (GVHD) but carries the risks of graft failure, opportunistic infections, and disease relapse.1 Investigators in the United Kingdom previously reported on an in vivo TCD approach using the anti-CD52 antibody alemtuzumab within a reduced-intensity fludarabine (Flu)/melphalan (Mel)–containing conditioning regimen.2 This protocol demonstrated impressive results with regard to hematopoietic engraftment and control of severe GVHD, even in unrelated donor and HLA-mismatch settings.3 Pharmacokinetic analysis in alemtuzumab/Flu/Mel–treated patients revealed that the antibody is detectable at lympholytic serum concentrations for up to 56 days after transplantation.4 Not surprisingly, patients receiving alemtuzumab show a poor immune reconstitution, particularly with very slow recovery of the CD4 T-cell subset.5 This leads to a strong and long-lasting impairment of antiviral immunity associated with significant morbidity and mortality.6,7 In addition, the higher relapse rate inherent to this protocol requires the frequent use of donor lymphocyte infusions (DLIs) to promote graft-versus-malignancy activity.3 While unmanipulated DLIs induce considerable antitumor responses in this setting, significant GVHD toxicity is observed after transfer.8
Several groups have demonstrated that depletion of CD8 T cells from either the allotransplant or from DLIs efficiently reduces the incidence and severity of GVHD.9-12 Nevertheless, CD8-depleted DLIs can induce significant disease remissions in patients with myeloma or chronic myeloid leukemia (CML) relapse.11-13 Randomized studies confirmed that CD8-depletion of DLIs or bone marrow maintains considerable antileukemic activity while reducing GVHD.14,15 Contamination with CD8 cells in the preparations used in most of these studies was substantial, leaving the hope that a more rigorous depletion might further improve the risk-benefit balance. We hypothesized that the combination of extensively CD8-depleted DLIs with a TCD reduced-intensity SCT regimen should promote antiviral and antitumor immunity at lower risks of treatment-related toxicity and GVHD. We report herein the results of a pilot study where patients conditioned with the alemtuzumab/Flu/Mel protocol were scheduled to receive prophylactic CD8-depleted DLIs early after transplantation.
Patients, materials, and methods
Eligibility criteria and study endpoints
This phase 1 clinical trial was performed at 2 different SCT centers. The protocol was approved by the local ethics committees and the national authorities. All patients gave written informed consent to participate. Patients with hematologic malignancies were eligible if they had (1) a poor response to prior standard treatment and/or a high risk of relapse or progressive disease, and (2) a contraindication to myeloablative allogeneic SCT. Patients' characteristics are shown in Table 1 Donor selection involved molecular typing for HLA-A, -B, -C, -DRB1, and -DQB1.
The primary study endpoints were the safety and feasibility of early cyclosporine A (CsA) taper and subsequent prophylactic CD8-depleted DLIs. In particular, we investigated toxicity, GVHD, graft failure, chimerism, and immune reconstitution. GVHD was graded according to consensus criteria.16
Transplantation and supportive care
Patients were treated with a conditioning regimen consisting of the anti-CD52 monoclonal antibody alemtuzumab (20 mg on days −8 to −4), fludarabine (30 mg/m2 on days −7 to −3), and melphalan (140 mg/m2 on day −2).2,3 They subsequently received unmanipulated donor peripheral blood stem cells (PBSCs) mobilized with granulocyte colony-stimulating factor (G-CSF). Leukapheresis products contained a median of 5.8 × 106 (range, 4.8 × 106–10.0 × 106) CD34 PBSCs/kg body weight (bw). The content of CD4 and CD8 T cells varied from 4.3 × 107 to 6.7 × 108 (median, 1.5 × 108) and 2.7 × 107 to 3.0 × 108 (median, 7.2 × 107) per kilogram bw, respectively. GVHD prophylaxis consisted of 3 mg/kg CsA starting on day −1 with a serum target level of 200 ng/mL.
Patients received routine prophylaxis against herpes virus reactivation and fungal infection consisting of acyclovir and itraconazole, respectively. They were also treated with G-CSF at 5 μg/kg bw daily starting day +7 until the absolute neutrophil count (ANC) exceeded 0.5 × 109/L. After the ANC reached 1 × 109/L, cotrimoxazole or pentamidine was administered as Pneumocystis carinii prophylaxis until CD4 T cells exceeded 0.2 × 109/L. Patients with a serum IgG value below 5 g/L received intravenous polyvalent immunoglobulin at a weekly dose of 10 g until day +100 after transplantation.
Peripheral blood screening for infectious agents was performed weekly using aspergillus galactomannan enzyme immunoassay, adenovirus DNA polymerase chain reaction (PCR), and cytomegalovirus (CMV) DNA PCR and pp65 staining. Patients with signs of CMV reactivation received preemptive therapy consisting of intravenous ganciclovir or foscarnet according to standard guidelines.
Administration of CD8-depleted DLIs
In the absence of acute GVHD, gradual CsA taper was started at day +35 and day +75 after related and unrelated donor transplantations, respectively. Immunosuppressive medication was stopped at least 2 weeks before administration of CD8-depleted DLIs. The latter were only applied if there was (1) no evidence of active GVHD, and (2) no previous episode of grade II to IV GVHD. The first DLI dose (1 × 106 CD4 T cells/kg bw) was scheduled on day +60 and day +120 after related and unrelated donor transplantations, respectively. Subsequent escalating doses (3 × 106, 1 × 107, and 3 × 107 CD4 T cells/kg) were administered at 60- to 90-day intervals in the absence of GVHD. DLIs were postponed or cancelled if tapering of immunosuppression was impossible due to GVHD. After thawing, DLI products were infused intravenously over a 5- to 10-minute period in the outpatient department. Patients did not receive medication prior or concomitant to infusion. No prophylactic immunosuppressive medication was administered after DLIs.
Depletion procedure using CliniMACS CD8 reagent
Leukaphereses were harvested from the original stem cell donors without prior G-CSF treatment. CD8 depletion of leukapheresis products was performed using the CliniMACS Plus Instrument (Miltenyi Biotec, Bergisch Gladbach, Germany) in a good manufacturing practice (GMP) procedure. Briefly, the leukapheresis product was diluted 1:3 in CliniMACS PBS/EDTA buffer and was centrifuged at 300g. After resuspension in 95 mL buffer, 7.5 mL of clinical grade CliniMACS CD8 reagent was added. Subsequently, the leukapheresis product was mixed for 30 minutes at 21°C on an orbital shaker at 25 rotations/minute. After adjusting the pellet to 4 × 108 cells/mL, the leukapheresis bag was connected to a CliniMACS Tubing Set LS. The depletion procedure was performed using the DEPLETION 2.1 program (software 2.31; Miltenyi Biotec).
As accompanying quality controls, cell counts and 5-color flow cytometry analyses were performed on samples obtained from the original leukapheresis, prior to connection to the tubing set (precolumn), the positive (target) fraction, and the negative fraction, respectively. The CD8-depleted target fraction was divided into appropriate aliquots according to CD4 T cells per kilogram bw of the patient. Preparations were cryopreserved in 10% DMSO following standard procedures and were stored in liquid nitrogen until use.
Isolation of leukocyte subsets and chimerism analysis
Isolation of pure CD4, CD8, CD14, CD15, CD19, and CD56 subpopulations from peripheral blood for chimerism analyses was performed using Whole Blood MicroBeads (Miltenyi Biotec) and the autoMACS device (Miltenyi Biotec). Labeled leukocyte subpopulations were automatically isolated using the POSSELD2 program (Miltenyi Biotec). Results were regarded as reliable if purity exceeded 85%. The degree of donor-recipient chimerism was determined by a semiquantitative genotype analysis as previously described.17 The assay has a sensitivity for detecting 5% of donor or recipient cells, respectively.
Isolation of Langerhans cells
For Langerhans cell preparation, 6-mm-punch biopsies were taken from uninvolved skin. The biopsy was subsequently incubated with 2.4 U/mL Dispase II (Roche, Mannheim, Germany) for 2 hours at 37°C. The epidermal layer was carefully distracted. Single-cell suspension was generated by incubation in 0.05% Trypsin/EDTA for 2 hours at 37°C and subsequent mechanical disintegration. Langerhans cells were purified using anti-CD1a Microbeads (Miltenyi Biotec). If feasible, isolated cells were controlled manually by staining cytospin preparations with a FITC-labeled CD1a antibody (Beckman Coulter, Miami, FL) reaching a purity of more than 60%. Cell nuclei were counterstained with the viability dye 7-AAD (BD Biosciences, Heidelberg, Germany).
T-cell receptor (TCR) Vβ analysis
TCR variable (V) β repertoire diversity was investigated by complementarity-determining region (CDR) 3 spectratyping analysis as previously described.18 Briefly, total RNA was extracted from 10 × 106 peripheral blood mononuclear cells (PBMCs). After conversion to cDNA by reverse transcriptase reaction, each TCR Vβ segment was amplified across the CDR3-encoding region by PCR with 1 of 24 unlabeled TCR Vβ subfamily-specific primers and a fluorophore-labeled TCR constant (C) β-specific primer.19 The size distribution of each TCR Vβ CDR3 fluorescent PCR product was determined on a 310 ABI DNA sequencer, and data were analyzed by GeneScan software (ABI, Weiterstadt, Germany). The distribution pattern of each Vβ family was further evaluated for complexity by 2 different scoring systems based on the total number of peaks per subfamily20 or the contribution of major peaks representing at least 10% of the total peak height for a given subfamily.21
IFN-γ ELISPOT assay
A 20-hour IFN-γ enzyme-linked immunospot (ELISPOT) assay including automated spot evaluation was performed as recently described.22 ELISPOT results are expressed as means of duplicates or triplicates analyzed for each experimental condition. To determine anti-HLA mismatch reactivity, K562 cells transfected with single HLA–class I alleles were used as antigen-presenting cells (APCs). Cloning of allelic HLA–class I coding cDNA and stable transfection of K562 cells were performed as previously described.23 To determine anti-CMV reactivity, peptide pools containing CD4 and CD8 epitopes of CMVpp65 were applied (PepMix; Jerini, Berlin, Germany).24 Monocyte-derived dendritic cells (DCs) were used for peptide presentation.25 Controls consisted of T cells seeded with unloaded DCs.
Results
Engraftment and toxicity
Twenty-three high-risk patients with a median age of 55 years received PBSC allografts obtained from either unrelated donors with HLA match (n = 13) or mismatch (n = 7), or donated by HLA-matched siblings (n = 3). Details on patients and disease characteristics are listed in Table 1. All patients had neutrophil recovery exceeding 0.5 × 109/L within a median of 12 days (range, 2-16 days; data not shown). Sustained platelet-transfusion independence with a platelet count higher than 20 × 109/L was achieved in 20 of 22 evaluable patients (91%) within a median of 11 days (range, 7-15 days). All but one patient showed 100% donor chimerism of peripheral blood leukocytes on day +30. Donor chimerism of bone marrow cells on day +50 was 100% in 16 of 18 evaluable patients. To evaluate lineage-specific chimerism, CD4 and CD8 T cells, B cells, NK cells, and granulocytes were purified from peripheral blood at regular 30-day intervals during the first 6 months after transplantation. Initial full donor chimerism for NK cells persisted in all cases. Some patients developed transient mixed chimerism that involved the granulocytic (n = 1), T-cell (n = 7), or B-cell lineages (n = 6).
During a median follow-up of 359 days after transplantation (range, 90-691 days), 4 patients died of recurrent or refractory disease (patients 1, 2, 13, and 21; Table 1). One patient died of multiorgan failure (patient 3), and 2 died of bacterial infections (patients 5 and 16). In the entire study population the 1-year actuarial incidences of overall and disease-free survival were 79.7% (95% confidence interval [95-CI], 61.1-98.3) and 52.5% (95-CI, 28.5-76.5), respectively. Corresponding values for the DLI patient subgroup were 85.7% (95-CI, 71.5-99.9) for overall survival and 68.2% (95-CI, 37.3-99.1) for disease-free survival. Patient 7 lost the allograft during pulmonary legionellosis 3 weeks after primary engraftment. This patient recovered with autologous hematopoiesis. Of 14 CMV-seropositive (CMVpos) patients, 12 developed CMV reactivation. No patient had CMV or adenovirus disease.
GVHD before DLIs and acquisition of Langerhans cell chimerism
In none of the 23 transplantation patients was severe acute GVHD (aGVHD) of overall grade II to IV observed prior to DLIs. However, 9 patients developed persistent aGVHD of the skin (overall grade I) that prevented CsA taper according to schedule. In these cases, prophylactic DLIs were either postponed (patient 4) or cancelled (patients 1, 2, 6, 12, 19, 20, 22, and 23). Four of 8 patients not receiving CD8-depleted DLIs progressed to limited chronic GVHD and none developed extensive chronic GVHD.
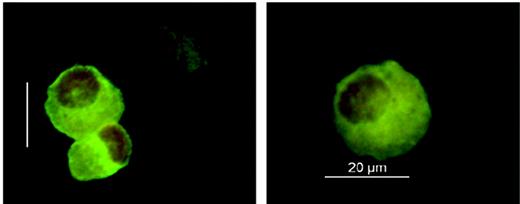
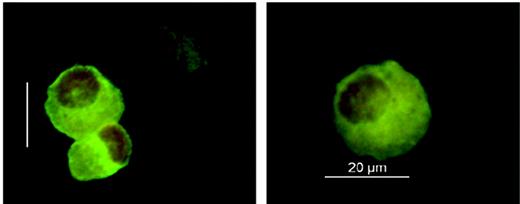
Considering the causative role of host Langerhans cells in the pathogenesis of acute skin GVHD, we established a protocol allowing Langerhans cell isolation from fresh skin punch biopsies for chimerism analysis (Figure 1) In 4 patients, Langerhans cells isolated after day +110 showed complete donor chimerism, whereas Langerhans cells purified before day +110 were predominantly of either full recipient (2 patients) or mixed donor-recipient (9 patients) origin (Table 2). In contrast to this delayed switch in the Langerhans cell lineage, all patients analyzed demonstrated early complete and persisting donor chimerism of blood monocytes during the first 4 months after transplantation (Table 2).
Immunofluorescence analysis of Langerhans cells. Langerhans cells were isolated from 6-mm-punch biopsies of uninvolved skin using magnetic CD1a beads. Cells were stained on cytospin preparations using an FITC-labeled CD1a antibody and cell nuclei counterstaining with 7-AAD (DMR microscope equipped with an HCX PL FLUOTAR 40 ×/0.75 non-oil objective lens, DFC-camera, and IM500 V4.0 software; Leica, Wetzlar, Germany).
Immunofluorescence analysis of Langerhans cells. Langerhans cells were isolated from 6-mm-punch biopsies of uninvolved skin using magnetic CD1a beads. Cells were stained on cytospin preparations using an FITC-labeled CD1a antibody and cell nuclei counterstaining with 7-AAD (DMR microscope equipped with an HCX PL FLUOTAR 40 ×/0.75 non-oil objective lens, DFC-camera, and IM500 V4.0 software; Leica, Wetzlar, Germany).
Immune reconstitution prior to DLIs
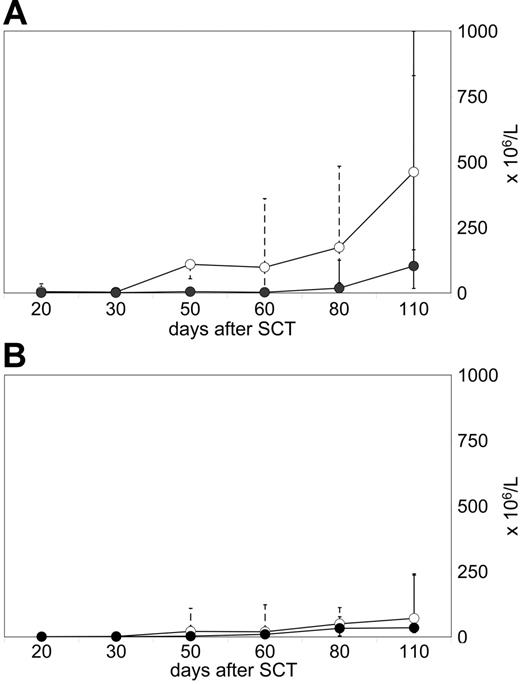
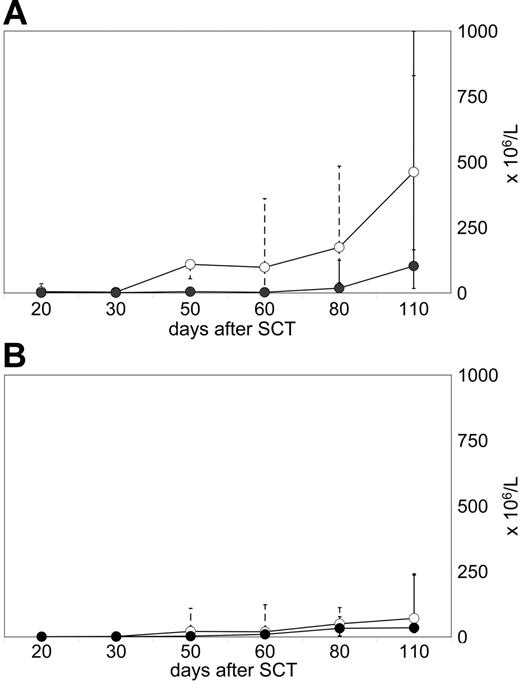
Two different patterns of early T-cell reconstitution were observed and were associated with the lack or the manifestation of GVHD. Seven of 16 evaluated patients demonstrated a spontaneous increase of circulating T cells, and reached more than 200 × 106/L CD3 cells until day +110 after transplantation (median, 481 × 106/L; range, 235-1874 × 106/L; Figure 2A) Among CD3 T cells, CD4 cell numbers usually exceeded CD8 counts. These CD4 T lymphocytes were predominantly CD45RO+/CD45RA−/HLA-DR+/TCRαβ+/CD25−, consistent with a phenotype of memory helper cells (data not shown). All 7 patients with an early rise in T-cell counts developed persistent aGVHD. These patients did not qualify for prophylactic CD8-depleted DLIs.
Different T-cell reconstitution patterns prior to DLIs. Flow cytometry analyses were performed on CD4 T cells (median, -○-; range, dotted line) and CD8 T cells (median, -•-; range, solid line) of peripheral blood samples. (A) Early reconstitution pattern in 7 patients who all developed persistent grade I aGVHD and, therefore, did not receive subsequent DLI therapy. (B) Failure to reconstitute circulating T cells in 9 patients who did not develop spontaneous GVHD and received CD8-depleted DLIs later on.
Different T-cell reconstitution patterns prior to DLIs. Flow cytometry analyses were performed on CD4 T cells (median, -○-; range, dotted line) and CD8 T cells (median, -•-; range, solid line) of peripheral blood samples. (A) Early reconstitution pattern in 7 patients who all developed persistent grade I aGVHD and, therefore, did not receive subsequent DLI therapy. (B) Failure to reconstitute circulating T cells in 9 patients who did not develop spontaneous GVHD and received CD8-depleted DLIs later on.
In contrast, 9 of 16 evaluated patients showed a poor recovery of circulating T cells until day +110 after transplantation (median, 121 × 106/L; range, 56-385 × 106/L; Figure 2B). None of them developed persistent aGVHD. Patients with this pattern of T-cell reconstitution received CD8-depleted DLIs after CsA taper according to schedule (n = 7), or slightly postponed (n = 2).
Most patients had a fast NK-cell recovery, with median numbers of 189 × 106/L (range, 67-860 × 106/L) at day +110. Monocytes and B lymphocytes recovered poorly, and remained below 50 × 106/L until day +110.
CD8 depletion of DLIs
CD8-depleted DLIs were processed from unmobilized leukapheresis products in a GMP procedure using clinical grade magnetic CD8 beads. No serious technical problems were observed. The median recovery of CD4 T cells in the final products was 68% (range, 48%-96%) compared with the original leukaphereses. CD8 T cells were removed from leukapheresis products with a depletion efficiency of 2.5- to 6-log (median, 3.5) after a single round of depletion. In contrast, the procedure did not change the proportion of other cell populations within the leukaphereses. DLI products contained a median of 250 CD8 cells (range, 20-3790) per 106 CD4 cells after processing (Table 3) Of total CD4 cells, a median of 0.8% (range, 0.3%-6.3%) coexpressed CD25high, a marker found on regulatory T cells26 and activated effector CD4 T cells.
Toxicity and chimerism after CD8-depleted DLIs
Eleven patients received a total of 21 CD8-depleted DLIs. The median follow-up after first DLI was 300 days (range, 133-646 days). No adverse event attributable to the CD8-depletion procedure was observed after transfers. No episode of bone marrow aplasia occurred. Seven patients developed aGVHD within a median time of 21 days (range, 13-59 days) after CD8-depleted DLIs (Table 4) Five patients presented with de novo grade I aGVHD of the skin. This GVHD resolved either spontaneously (n = 1) or after short-term systemic (n = 2) or topical (n = 2) immunosuppressive treatment. Patients 4 and 9 developed severe aGVHD after the first dose of 1 × 106/kg CD4 lymphocytes (Table 4). In contrast to the grade I GVHD patients, both had received transplants from HLA-C–mismatched donors and had suffered from transient skin GVHD prolonging CsA taper. Following DLIs, patient 4 developed grade III GVHD of skin, gut, and liver, which went into remission under systemic immunosuppressive treatment. Patient 9 had grade II aGVHD of skin and liver that responded to systemic immunosuppression. The actuarial incidences of postinfusional aGVHD were 54.5% (95-CI, 25-84) for grades I to IV and 18.2% (95-CI, 0-40) for grades II to IV over the total observation period. Of 11 patients receiving CD8-depleted DLIs, only patients 4 and 9 progressed to limited chronic GVHD.
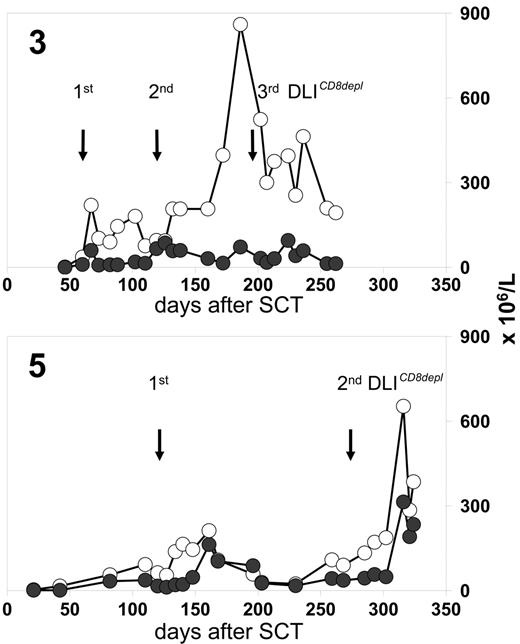
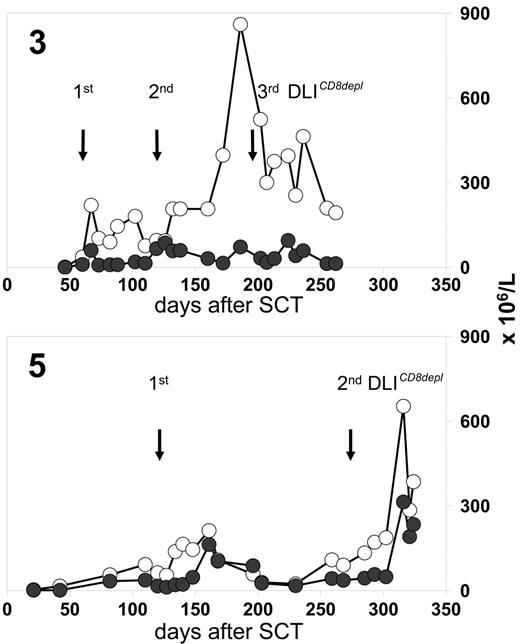
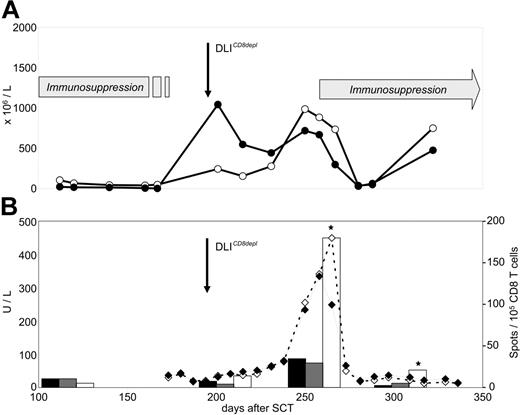
Patients 3, 8, 10, and 17 received CD8-depleted DLIs during a period when preceding full lineage-specific donor chimerism for T cells (n = 3) or granulocytes (n = 1) had significantly dropped. This declining chimerism completely reverted in all cases after CD8-depleted DLIs were administered (Figure 3) In 3 of these patients, the conversion back to full donor chimerism was associated with transiently occurring mild skin GVHD.
Chimerism response following CD8-depleted DLIs. Donor chimerism results (%) were obtained from unseparated peripheral blood leukocytes (-□-), purified CD4 T cells (-⋄-), and purified CD8 T cells (-♦-) of patients 3, 8, and 10. Stars indicate the time of first GVHD diagnosis. Longitudinal bars indicate duration of systemic immunosuppressive treatment (CsA, cyclosporine A). The dotted lines are CD3 T-cell counts in peripheral blood. Mixed T-cell chimerism was not detectable in unseparated peripheral blood leukocytes if the proportions of CD4 and CD8 T cells were below the detection limit of the chimerism assay (5%).
Chimerism response following CD8-depleted DLIs. Donor chimerism results (%) were obtained from unseparated peripheral blood leukocytes (-□-), purified CD4 T cells (-⋄-), and purified CD8 T cells (-♦-) of patients 3, 8, and 10. Stars indicate the time of first GVHD diagnosis. Longitudinal bars indicate duration of systemic immunosuppressive treatment (CsA, cyclosporine A). The dotted lines are CD3 T-cell counts in peripheral blood. Mixed T-cell chimerism was not detectable in unseparated peripheral blood leukocytes if the proportions of CD4 and CD8 T cells were below the detection limit of the chimerism assay (5%).
Immune reconstitution following CD8-depleted DLIs
Measurable increases of circulating CD4 T cells were observed after 13 of 16 analyzed CD8-depleted DLIs (Figure 4) The median rise in CD4 numbers was 2.1-fold (range, 0.3- to 52.0-fold) within 2 weeks following transfer. Increasing CD4 T cells predominantly expressed the CD45RO+/CD45RA−/CD25− phenotype. Expansion of the CD4 compartment was accompanied by lower increases of circulating CD8 T cells (median, 1.4-fold), NK cells (median, 1.4-fold), and B lymphocytes (median, 1.3-fold). In contrast, cell counts of the 7 patients not receiving DLIs did not increase in a comparable manner during the same time period. Median multiplication factors were only 1.1, 1.1, 1.0, and 0.9 for CD4, CD8, NK, and B cells, respectively (data not shown).
Rapid increase of circulating CD4 T cells after CD8-depleted DLIs. Flow cytometry analyses were performed on CD4 T cells (-○-) and CD8 T cells (-•-) within peripheral blood of representative patients 3 and 5 receiving CD8-depleted DLIs.
Rapid increase of circulating CD4 T cells after CD8-depleted DLIs. Flow cytometry analyses were performed on CD4 T cells (-○-) and CD8 T cells (-•-) within peripheral blood of representative patients 3 and 5 receiving CD8-depleted DLIs.
We subsequently performed T-cell receptor (TCR) Vβ spectratyping analysis to investigate changes in the diversity of the TCR Vβ repertoire in PBMCs following DLIs. The spectratypes showed increased peak numbers for several individual TCR Vβ subfamilies 50 days after the first dose of CD8-depleted DLIs. However, this trend toward a more diverse TCR repertoire was not confirmed when evaluating at least 20 different Vβ subfamilies per each sample according to 2 previously described complexity scoring systems.20,21 Similarly, no significant changes in TCR Vβ diversity were observed in noninfused control patients during the same observation period (data not shown).
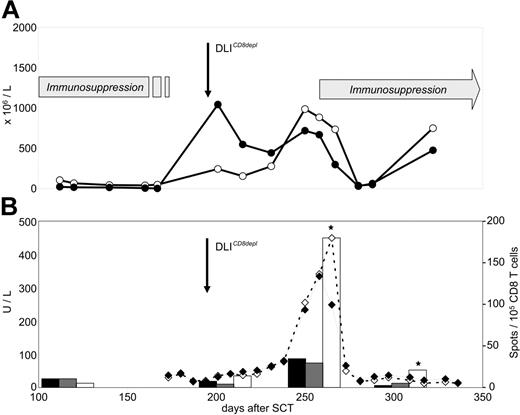
The pattern of T-cell recovery after CD8-depleted DLIs was strikingly different in patient 4, who developed grade III GVHD after the first DLI dose. This patient showed a strong boost of both CD4 and CD8 numbers after transfer (Figure 5A) Circulating CD4 T cells did not demonstrate any reactivity against host PBMCs (data not shown). To characterize the specificity of expanding CD8 T cells, we transfected HLA-negative K562 cells with cDNA coding for HLA-Cw*0501 which represented the single GVH mismatch in this donor-recipient pair. K562 cells expressing the matching HLA-A*0301 allele and untransfected K562 cells served as controls. We observed a strong expansion of circulating CD8 T cells recognizing the HLA-Cw*0501 mismatch allele after DLIs (Figure 5B). This alloreactivity was not detectable in pre-DLI blood samples. Maximum ex vivo reactivity of Cw*0501-specific CD8 T cells (155 per 105) coincided with the onset of grade III GVHD, as demonstrated by the course of liver enzyme levels in this patient.
Ex vivo detection of HLA-Cw*0501–specific CD8 T cells during onset of severe GVHD in patient 4. (A) Course of CD4 (-○-) and CD8 T cells (-•-) measured by flow cytometry analysis in peripheral blood. (B) Course of liver transaminases AST (-⋄-) and ALT (-♦-), and frequencies of HLA-Cw*0501 mismatch–specific T cells measured by IFN-γ ELISPOT assays. ELISPOT responders were purified CD8 blood lymphocytes. Targets were parental K562 cells (▪), and K562 transfectants expressing either the matched HLA-A*0301 (⊡) or the mismatched HLA-Cw*0501 (□) recipient alleles. Stars indicate significant HLA-Cw*0501 reactivity compared with controls (P < .05).
Ex vivo detection of HLA-Cw*0501–specific CD8 T cells during onset of severe GVHD in patient 4. (A) Course of CD4 (-○-) and CD8 T cells (-•-) measured by flow cytometry analysis in peripheral blood. (B) Course of liver transaminases AST (-⋄-) and ALT (-♦-), and frequencies of HLA-Cw*0501 mismatch–specific T cells measured by IFN-γ ELISPOT assays. ELISPOT responders were purified CD8 blood lymphocytes. Targets were parental K562 cells (▪), and K562 transfectants expressing either the matched HLA-A*0301 (⊡) or the mismatched HLA-Cw*0501 (□) recipient alleles. Stars indicate significant HLA-Cw*0501 reactivity compared with controls (P < .05).
CMV-specific T cells after CD8-depleted DLIs
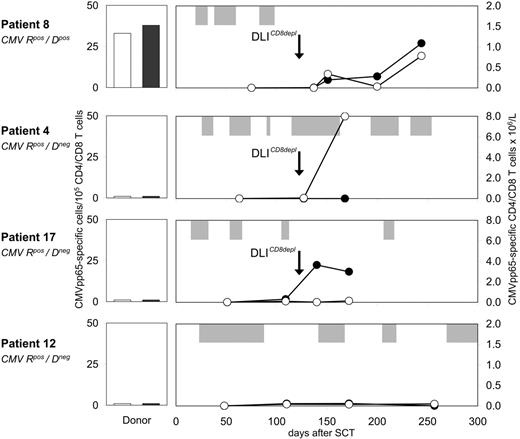
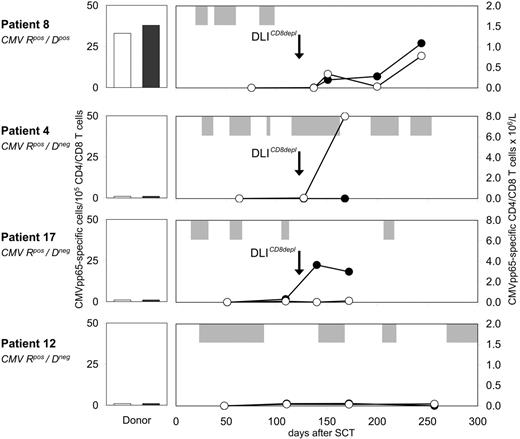
Four of 4 analyzed patients showed enhanced CMV-specific T-cell immunity following prophylactic CD8-depleted DLIs. CMVpos patient 8, who received a CMVpos allograft, developed 3 episodes of CMV reactivation early after transplantation (Figure 6) After the first dose of CD8-depleted DLIs, an expansion of CMVpp65-specific T cells up to 0.8 × 106/L and 1.1 × 106/L was detected in the CD4 and CD8 peripheral blood subsets, respectively. Subsequently, no further episode of CMV reactivation occurred. In CMVpos patients 3, 4, and 17 receiving allografts from CMVneg donors, isolated CMVpp65-specific CD4 or CD8 T-cell responses were observed after transfer. Anti-CMV CD4 and CD8 frequencies increased up to 8.0 × 106/L in patient 4 and 3.8 × 106/L in patient 17, respectively (Figure 6). Similar results were obtained from patient 3 (data not shown). The blood samples containing CMV-specific T cells were drawn at time points when these patients were complete donor T-cell chimeras. This observation suggested de novo generation of anti-CMV T-cell immunity, which was insufficient to completely prevent CMV recurrence in patients 4 and 17 (Figure 6). Anti-CMV response analysis was also performed in patients not receiving DLIs. No comparable changes in CMVpp65-specific T-cell numbers were observed in CMVpos recipients of either CMVneg (patient 12, Figure 6) or CMVpos allografts (data not shown).
Expansion of CMV-specific CD4 and CD8 T cells after CD8-depleted DLIs. Before and after CD8-depleted DLIs, the frequencies of CMVpp65-specific T cells were determined within purified CD4 and CD8 blood lymphocytes of CMVpos patients 8, 4, 17, and 12 by IFN-γ ELISPOT assays. Numbers indicate CMVpp65-specific CD4 (-○-) and CD8 (-•-) T cells × 106/L. All donors were unrelated volunteers with full HLA match, and were either seropositive (patient 8) or seronegative (patients 4, 17, 12) for CMV. Donor PBMCs were also screened for CMVpp65-specific CD4 (□) and CD8 (▪) T cells in IFN-γ ELISPOT assays. Longitudinal bars (⊡) indicate duration of CMVpp65 antigenemia. R, recipient; D, donor.
Expansion of CMV-specific CD4 and CD8 T cells after CD8-depleted DLIs. Before and after CD8-depleted DLIs, the frequencies of CMVpp65-specific T cells were determined within purified CD4 and CD8 blood lymphocytes of CMVpos patients 8, 4, 17, and 12 by IFN-γ ELISPOT assays. Numbers indicate CMVpp65-specific CD4 (-○-) and CD8 (-•-) T cells × 106/L. All donors were unrelated volunteers with full HLA match, and were either seropositive (patient 8) or seronegative (patients 4, 17, 12) for CMV. Donor PBMCs were also screened for CMVpp65-specific CD4 (□) and CD8 (▪) T cells in IFN-γ ELISPOT assays. Longitudinal bars (⊡) indicate duration of CMVpp65 antigenemia. R, recipient; D, donor.
Discussion
Our results confirm that in unrelated donor PBSCT, the Flu/Mel conditioning regimen incorporating in vivo administration of alemtuzumab efficiently reduces the risk of severe GVHD.2,3 However, 8 of 22 engrafted patients suffered from persistent manifestations of acute skin GVHD, which precluded prophylactic CD8-depleted DLIs. These patients demonstrated early increases in T-cell numbers that potentially contributed to increased alloreactivity in the skin. We further considered the impact of locally persisting host APCs for aGVHD induction. Before day +30, skin-derived Langerhans cells showed predominant recipient chimerism and gradually switched to full donor status beyond day +110 after transplantation. These observations are in line with a recent study on Langerhans cell chimerism after reduced-intensity PBSCT.27 In contrast, blood monocytes as the main precursors of circulating DCs demonstrated complete donor chimerism throughout the first 4 months after transplantation. Several groups reported that alemtuzumab rapidly depletes circulating monocyte-derived DCs but not tissue-resident DCs, such as Langerhans cells.28-30 In mice, the persistence of host Langerhans cells in vivo can trigger early skin GVHD.31 This confirms previous animal data on the crucial role of host APCs for GVHD induction.32 Thus, strategies promoting the switch to full donor Langerhans cell chimerism prior to T-cell reconstitution might help to prevent skin GVHD. Nevertheless, whether the chimerism status of tissue-resident APCs is predictive for organ-related GVHD in humans merits further evaluation.
Slow and defective lymphocyte recovery is associated with higher risks of relapse and severe viral and fungal infections following allogeneic SCT.33-35 Particularly, poor CD4 counts have been correlated with an increase in infection-related mortality.36,37 Thus, lymphopenic patients may benefit from prophylactic CD4− DLIs. Results in patients with CML emphasize that GVL responses following DLIs clearly depend on early disease stage.38 This suggests using DLI therapy for the treatment of minimal residual disease early after transplantation. Such a strategy, however, bears a significant risk of inducing severe GVHD.39 Based on their reduced GVHD potential,11-13,15,40,41 we investigated the early prophylactic transfer of CD8-depleted donor lymphocytes in high-risk patients treated with the strongly immunosuppressive alemtuzumab/Flu/Mel conditioning regimen.
With the use of clinical grade magnetic CD8 beads, we first established a GMP procedure for CD8-depletion of donor leukocyte products. The CD8 T-cell content was reduced by 2.5- to 6-log after a single passage over the depletion column (Table 3), thereby exceeding the depletion efficiency of previous methods.9-12,40 Accordingly, all patients treated in our study definitely received fewer than 104 CD8 cells/kg bw at the first DLI dose level.
We administered a total of 21 CD8-depleted DLIs to 11 patients within a phase 1 study. Five patients developed de novo grade I aGVHD of the skin, which resolved spontaneously or after short-term immunosuppressive treatment. Considering the observation that patients suffering from moderate aGVHD have an improved leukemia-free survival,42 induction of mild alloreactivity in previously GVHD-free patients seems tolerable, if not desirable. Only 2 patients with prior grade I aGVHD developed grade II to III disease following DLIs. Although their GVHD responded to intensive immunosuppression, both patients progressed to limited chronic GVHD. Since these patients received allografts from HLA-C–mismatched donors, caution should be taken when applying prophylactic CD8-depleted DLIs in HLA-incompatible SCT. In vivo amplification of HLA-C mismatch–specific donor CD8 T cells was demonstrated and correlated with the onset and severity of GVHD in patient 4 (Figure 5), suggesting their direct involvement in tissue destruction.43 This observation confirms a previous report demonstrating that almost pure CD4 cells provide help to endogenous CD8 cells in mediating alloreactivity.44 Our novel approach to readily identify T-cell–mediated anti-HLA mismatch reactivity using K562/HLA transfectants in an ex vivo IFN-γ ELISPOT assay is easily applicable to any HLA mismatch donor/recipient pair. It might help to detect alloreactive T cells in single HLA mismatch or even haploidentical SCT. For an ‘off-the-shelf’ application, we have already generated 20 different K562 transfectants, each expressing a single HLA-class I allele (C.M.B. and W.H., unpublished results, October 2005).
In lymphopenic patients receiving CD8-depleted DLIs, we consider both the cyclosporine withdrawal and the subsequent DLI therapy as 2 independent possible reasons for the rising T-cell numbers and improved CMV immunity observed in this study. However, the fact that, 2 weeks after CD8-depleted DLIs, increasing peripheral blood cell counts were found predominantly for CD4 compared with CD8 T cells suggests an important DLI-induced rather than an exclusive cyclosporine taper effect. Such CD4 expansions were not observed in previous studies that applied CD8-depleted DLIs later than 6 months after transplantation.45 Apparently, CD4 lymphocytes given earlier after transplantation seem to have an increased capacity to proliferate in lymphodepleted hosts.46 Repopulation of the T-cell compartment after allogeneic SCT occurs initially from expansion of infused memory T cells.5,47 After CD8-depleted DLIs, we also observed rising CD45RO+ memory CD4 T cells in the absence of CD45RA+ naive counterparts.
A similar increase of CD4 T-cell numbers was not detected in patients not receiving DLIs. Comparing T-cell reconstitution data between the DLI and non-DLI groups, however, appears inappropriate since the noninfused patients developed spontaneous aGVHD which can lead to peripheral T-cell expansion.48 At the same time, the need for immunosuppressive treatment might result in stable or even decreasing T-cell numbers. Therefore, randomized trials are necessary to determine whether CD8-depleted DLIs result in targeted reconstitution of non-CD8 lymphocyte subsets compared with control groups receiving either unmanipulated DLI or no DLI therapy.
Although considerable expansions of overall CD4 as well as CMV-reactive T cells were observed after CD8-depleted DLIs, we failed to detect a consistent increase in TCR repertoire diversity except for single individual Vβ subfamilies. There are several possible explanations for this result. First, the spectratyping analysis could have been of insufficient sensitivity, either because of methodologic limitations or the low posttransplantation peripheral T-cell counts that precluded the isolation of pure CD4 T cells for separate analysis. A second explanation could be the inappropriate timing of blood sampling for detecting DLI-induced changes in TCR repertoire diversity. This is supported by observations in T-cell–depleted allograft recipients showing that TCR spectratypes were characterized by instability over time49 with no immediate changes in CDR3 size profiles after unmanipulated DLIs.49,50 A third possible explanation would be that CD8-depleted DLIs do not have a direct effect on the TCR repertoire, but provide help to pre-existing or de novo–generated CD8 T-cell clones, as suggested for the anti-CMVpp65 reactivity in patients 8 and 17 (Figure 6), for the anti–HLA-Cw5 mismatch reactivity in patient 4 (Figure 5), and for anti–minor histocompatibility antigen mismatch reactivity as shown by Zorn et al.44
A further potential benefit of CD8-depleted DLIs was demonstrated in several patients who developed a complete reversion of declining donor T-cell chimerism from a maximum low of 30% following infusion. Thus, CD8-depleted DLIs might be a valuable alternative compared with unmanipulated DLIs for treating patients with a secondary drop of donor chimerism and a significant risk profile for GVHD. We also observed increased frequencies of circulating CMV-specific CD4 and CD8 T cells following CD8-depleted DLIs. The simultaneous recovery of CMV-specific CD4 and CD8 T cells in the CMVpos/pos donor/patient setting was not followed by further CMV reactivations (Figure 6). In CMVpos recipients of CMVneg allografts, however, we only detected either anti-CMV CD4 or CD8 reactivities following CD8-depleted DLIs (Figure 6). These isolated CMV-specific CD4 or CD8 responses were insufficient to completely prevent CMV recurrence.51-53
In conclusion, our results demonstrate that CD8 depletion of donor leukocyte products in a GMP procedure is feasible and highly efficient. The prophylactic transfer of CD8-depleted donor lymphocytes into T-cell–depleted reduced-intensity SCT patients did not cause any acute infusional toxicity or bone marrow aplasia. CD8-depleted DLIs were followed by increased circulating CD4 T-cell numbers as well as enhanced frequencies of CMV-specific CD4 and CD8 T cells in several lymphopenic patients. Considering the high proportion of older patients and unrelated donors in our study population, we observed a low incidence of DLI-associated acute and chronic GVHD. Severe forms of GVHD occurred rarely and only in patients receiving HLA-mismatch unrelated allografts. Several patients developed a complete reversion of declining hematopoietic donor chimerism after CD8-depleted DLIs. Nevertheless, only randomized phase 2/3 trials are able to finally determine whether CD8-depleted DLIs lead to an improved clinical outcome with regard to GVHD, infections, and disease relapse.14,15 Depending on the study design and endpoints examined, such trials should include control groups that receive either unmanipulated DLIs or no DLI therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: W.H., R.G.M., K.K., C.H., and A.J.U. designed research; R.G.M., D.W., K.B., G.H., A.K., U.F.H., T.C.W., and C.G. performed research; C.M.B. and L.U. contributed vital reagents; and R.G.M. and W.H. analyzed data; W.H., R.G.M., and C.H. wrote the paper.
Acknowledgments
We thank Dr Karl Peggs, University of London, United Kingdom, for discussing the study protocol prior to initiation. We further thank Dr Esther von Stebut for her advice concerning Langerhans cell isolation. The technical assistance of Ines Steinert, Elke Schnürer, Uschi Gerhards, Christiane Schön, and Uschi Wollscheid is gratefully acknowledged.
This work was supported by grants 70-2427-HuI and 70-2428/IVB (W.H.) from the German Cancer Aid, and by provision of CD8-depletion reagents from Miltenyi Biotec, Bergisch Gladbach, Germany.