Abstract
During thrombopoiesis, maturing megakaryocytes (MKs) migrate within the complex bone marrow stromal microenvironment from the proliferative osteoblastic niche to the capillary-rich vascular niche where proplatelet formation and platelet release occurs. This physiologic process involves proliferation, differentiation, migration, and maturation of MKs before platelet production occurs. In this study, we report a role for the glycoprotein PECAM-1 in thrombopoiesis. We show that following induced thrombocytopenia, recovery of the peripheral platelet count is impaired in PECAM-1–deficient mice. Whereas MK maturation, proplatelet formation, and platelet production under in vitro conditions were unaffected, we identified a migration defect in PECAM-1–deficient MKs in response to a gradient of stromal cell–derived factor 1 (SDF1), a major chemokine regulating MK migration within the bone marrow. This defect could be explained by defective PECAM-1−/− MK polarization of the SDF1 receptor CXCR4 and an increase in adhesion to immobilized bone marrow matrix proteins that can be explained by an increase in integrin activation. The defect of migration and polarization was confirmed in vivo with demonstration of altered spatial localization of MKs within the bone marrow in PECAM-1–deficient mice, following immune-induced thrombocytopenia. This study identifies a novel role for PECAM-1 in regulating MK migration and thrombopoiesis.
Introduction
Megakaryocytopoiesis involves proliferation and differentiation of megakaryocyte (MK) progenitors to a large, terminally differentiated cell with a multi-lobulated, polyploid nucleus. Nuclear maturation, a process known as endoreplication, proceeds in concert with cytoplasmic maturation and expression of platelet surface markers including the glycoprotein receptors IIb/IIIa, GPIb, GPIX, and GPVI. As the MK matures and differentiates, it migrates to sinusoidal bone marrow endothelial cells where it forms transendothelial projections called proplatelets that release 1000 to 5000 platelets per MK into the intravascular space.1–5
Thrombopoietin (TPO) participates in the humoral regulation of thrombopoiesis. TPO is produced constitutively in the liver and by bone marrow stromal cells and its levels are regulated by binding to the receptor c-Mpl expressed on platelets.6 This has the net effect of reducing the concentration of TPO in the circulation and thereby inhibiting differentiation of progenitor cells along the MK lineage. Thus, megakaryocytopoiesis and platelet count are directly regulated by circulating TPO.2
A key step in thrombopoiesis is migration of maturing MKs from the proliferative osteoblastic niche within the bone marrow microenvironment, where hematopoietic stem cells reside, to the capillary-rich vascular niche, where proplatelets are formed.6 This process is regulated by a variety of chemokines and cytokines, as well as by adhesive interactions with interstitial cells and extracellular matrix proteins. For example, the chemokine SDF1 directs movement of MK progenitors through its receptor CXCR4 from the proliferative “osteoblastic niche” to the “vascular niche,” where platelets are formed.6 SDF1 therefore acts in combination with TPO to promote differentiation of MK progenitor cells to mature MKs.7,8 In addition, SDF1 promotes the interaction and transmigration of mature MKs through bone marrow endothelial cells, resulting in augmentation of platelet release.3,6,9,10
The glycoprotein PECAM-1 (also known as CD31) has 6 extracellular immunoglobulin domains, a single transmembrane domain, and a large cytoplasmic domain that contains 2 conserved tyrosines in immunoreceptor-tyrosine–based inhibition motifs (ITIMs). PECAM-1 is expressed at very high levels on the surface of endothelial cells and at moderate levels on most hematopoietic cells, including platelets, T cells, and B cells.11 PECAM-1 acts as an adhesion-dependent signaling molecule and has been shown to have both stimulatory and inhibitory roles that contribute to several important processes in the vasculature. For example, PECAM-1 has a marked inhibitory effect against platelet activation by collagen,12–15 although this is less dramatic under some conditions. In contrast, it directly promotes integrin activation in T cells,16 and PECAM-1 has been shown to contribute to leukocyte transendothelial migration and to regulate movement of neutrophils in response to IL-8.17
The critical role played by PECAM-1 within the vasculature prompted us to consider whether PECAM-1 also contributes to MK development and platelet production, as interactions between MK and bone marrow endothelial cells are critical to this process. The present results demonstrate a key role for PECAM-1 in regulating the rate and direction of migration of MKs and provide evidence that PECAM-1 plays a crucial role in platelet recovery following immune-induced thrombocytopenia. We further demonstrate that the role of PECAM-1 in the control of MK migration is due to the polarization of the SDF1 receptor CXCR4 and the regulation of adhesion to extracellular matrix proteins.
Materials and methods
Materials
The following were obtained from sources shown in brackets: fibronectin (Calbiochem, Darmstadt, Germany); anti–mouse CXCR4 IgG (R&D Systems, Abingdon, United Kingdom); murine TPO, murine SCF, murine SDF1α (PeproTech, London, United Kingdom); anti–mouse GP1bα, Jon/A antibody (Emfret Analytics, Wurzburg, Germany); sheep anti–rat IgG Dynabeads (Dynal Biotech, Paisley, United Kingdom); biotin-conjugated rat anti–mouse CD45R/B220, purified rat anti–mouse CD16/CD32, FITC-conjugated anti–mouse CD41, FITC-conjugated anti–mouse P-selectin monoclonal antibodies (mAbs; BD Pharmingen, Oxford, United Kingdom); anti–mouse Ly-6G, biotin anti–mouse CD11b (eBioscience, Wembley, United Kingdom); anti–mouse CD49e (Serotec, Oxford, United Kingdom). Lotrafiban was a gift from GlaxoSmithKline (Piscataway, NJ). AMD3100 was a kind gift from Dr G Bridger (AnorMed, Langley, BC, Canada). All other reagents were from Sigma (Dorset, United Kingdom) or referenced sources. PECAM-1−/− mice18 were bred from heterozygotes on a C57Bl6 background and housed in pathogen-free conditions as described.13
Megakaryocyte purification and culture
A highly purified preparation of MKs was generated from bone marrow obtained from femora and tibiae of 3- to 4-month-old PECAM-1−/− and control littermate mice as described.19 Bone marrow cells (∼2.5 × 107 cells/mouse) were flushed and CD16/CD32+Gr1+B220+CD11b+ cells (∼90% of cells) were depleted using immunomagnetic beads. The remaining population was cultured in 2.6% serum-supplemented StemPro medium with 2 mM L-glutamine, penicillin/streptomycin, and 20 ng/mL murine SCF at 37°C under 5% CO2 for 2 days. Cells were then cultured for a further 5 days in the presence of 20 ng/mL SCF and 50 ng/mL of murine TPO. Mature MKs were isolated using a 0% to 3% BSA gradient and collected after standing gravity (1g) for 40 minutes at room temperature.
Platelet depletion experiments
PECAM-1−/− mice and PECAM-1+/− mice with the corresponding littermate controls aged 2 to 4 months were tail bled to determine baseline blood cell counts. The mice were given a sterile intraperitoneal injection of anti–mouse GP1bα antibody (2 μg/g of mouse) after 7 days to induce an immune thrombocytopenia. Platelet counts were measured at 48, 72, 120, and 172 hours after injection.
Functional platelet production and proplatelet formation assays
The platelet production assay was adapted from Hamada et al.9 Endothelial cells from human umbilical veins (HUVECs) were isolated by a standard method as previously described.20 HUVECs (2 × 105) were seeded on 5-μm pore transwell microporous membrane (Transwell, Costar, High Wycombe, United Kingdom) in M199 culture medium (Invitrogen, Carlsbad, CA) supplemented with 20% heat-inactivated human serum, 50 U/mL sodium heparin, 1 mg/mL glutamine, and 28 μg/mL gentamicin. Confluency was achieved after 5 days. The transwell inserts with the monolayers of HUVECs were placed in a 24-well tissue-culture plate, thus separating an upper from a lower chamber in each well. MKs (100 000) resuspended in StemPro medium were added onto each HUVEC-covered transwell. Immediately, 600 μL of serum-free chemotactic medium, containing StemPro with 300 ng/mL SDF1α, was placed in the lower chamber and the plate was incubated for 30 hours at 37°C in 5% CO2. In order to identify platelet production in the lower chamber, the cells were centrifugated at 1000g for 6 minutes with PGI2 to avoid platelet preactivation and then resuspended in modified Tyrode buffer (134 mM NaCl; 2.9 mM KCl; 0.34 mM Na2HPO412H2O; 12 mM NaHCO3; HEPES 20 mM; MgCl2 1 mM; glucose 5 mM, pH7.3) and left to rest for 30 minutes at 37°C. The suspension was stimulated with 1 U/mL thrombin for 3 minutes at 37°C. Anti–mouse P-selectin–FITC antibody was added for 30 minutes and P-selectin expression after thrombin stimulation was measured by flow cytometry in order to identify functional platelet-like particles, using the characteristic forward and side scatter pattern of in vitro–generated platelets (morphologic assay) with FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, San Jose, CA). To assess adhesion and proplatelet formation of MKs on the EC monolayer, HUVEC-coated 12-mm coverslips were prepared in a 24-well plate. After 30 hours coculture in StemPro and 300 ng/mL SDF1 medium, the adherent MKs on HUVEC-coated coverslips were fixed in 3.7% paraformaldehyde and stained with anti–mouse CD41–FITC antibody. MKs in the supernatant or adherent to the HUVEC monolayers were counted.
Cell migration assays
Chemotaxis was assessed using the Dunn chamber (Weber Scientific International, Teddington, United Kingdom). MK suspension was applied on fibronectin-coated (10 μg/mL) coverslips in complete medium and left at 37°C for 60 minutes for cells to adhere. The coverslip was then placed directly onto the Dunn chamber where both the inner and outer wells were filled with medium. Using sterile filter paper, the outer well was drained to leave the inner well and the region over the bridge filled with medium. The outer well was refilled with the same medium containing 300 ng/mL SDF1α. The Dunn chamber was placed in a humidified chamber on a heated stage at 37°C within an inverted microscope setup. Time-lapse images were digitally captured every 60 seconds for 4 hours using the Axiovert 200 inverted high-end microscope from Zeiss (Welwyn Garden City, United Kingdom). To track migration paths, a series of images were analyzed using Slide book 4 software (Olympus, Melville, NY)and the data were plotted with Microsoft Excel. Net translocation distance was determined as the straight distance between the end points during the 4-hour period. To visualize the directionality of cell migration, we constructed circular histograms showing the proportion of cells whose final position lay relative to a common origin. The coverslips were removed from the Dunn chambers after the assay, fixed in 3.7% paraformaldehyde, blocked in 3% BSA, and stained with anti–mouse CXCR4–FITC antibody. Confocal fluorescence images were obtained using a Leica DMIRE 2 inverted microscope with a 40× objective (Milton Keynes, United Kingdom).
Measurement of surface glycoproteins and ploidy
The level of expression of CXCR4 and CD41 was measured by flow cytometry. Aliquots of 50 000 MKs were stained with 5 μL of antibody in 50 μL of 1% BSA/PBS for 30 minutes. Irrelevant isotype-matched controls directly conjugated to FITC or PE were used to assess nonspecific fluorescence. Cell DNA content was measured by staining with 0.01 mg/mL propidium iodide (PI) as described.19 Samples were analyzed by FACSCalibur flow cytometer and CellQuest software (Becton Dickinson).
Immunohistochemistry
Cryosections (8 μm) of mouse femur were fixed in acetone, blocked in 1% FCS/PBS, and stained with biotin-conjugated anti–mouse CD105 antibody or irrelevant isotype-matched control (5-10 μg/mL) for 60 minutes at room temperature. Slides were then washed and then incubated in FITC-conjugated anti–mouse CD41 (or FITC-conjugated irrelevant isotype-matched control) and PE-conjugated streptavidin for 30 minutes at room temperature. Three mice were used for each study, and 8 to 10 fields per tissue sample and 3 to 6 tissues were quantified blind by 2 individuals. Confocal fluorescence images were obtained using a Leica DMIRE 2 inverted microscope with a 40× objective.
Cell adhesion assay
Coverslips (22 mm2) were coated in the presence of 10 μg/mL fibronectin, 100 μg/mL collagen, or 100 μg/mL fibrinogen overnight at 4°C. After washing twice with PBS, the coverslips were blocked with 1% BSA/PBS for 1 hour at room temperature. BSA-coated coverslips were prepared for negative control. Lotrafiban (1:100), anti-CD49e antibody (10 μg/mL), or IgG control antibodies (10 μg/mL) were added prior to adhesion and cells were incubated for 30 minutes. Purified PECAM-1−/− MKs and littermate control cells were allowed to adhere on the coated coverslips for 3 hours in the presence of SDF1α (300 ng/mL) at 37°C. After removal of unbound MKs, coverslips were washed and the adherent cells were fixed by 3.7% paraformaldehyde for 30 minutes at room temperature. MKs were visualized by light microscopy using the Axiovert 200 inverted high-end microscope from Zeiss.
Statistical analysis
Experiments were performed a minimum of 3 times and images shown are representative data from 1 experiment. Data are shown as mean ± SEM and statistical analysis was conducted using 2-tailed Student t test. Probability values of P below .05 were selected to be statistically significant.
Results
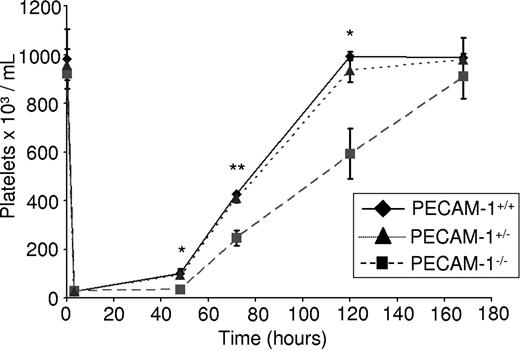
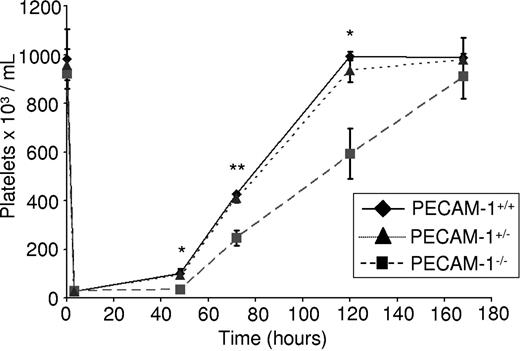
PECAM-1−/− mice have been reported to have normal platelet counts, a result that was confirmed in the present study as shown by measurement of platelet levels compared with littermate control mice (Figure 1). However, we speculated that a partial defect in thrombopoiesis in the absence of PECAM-1 may be masked in nonchallenged mice by the presence of additional regulatory pathways. To investigate this possibility, we monitored platelet recovery in response to thrombocytopenia in the absence of PECAM-1. Complement-mediated immune thrombocytopenia was induced by an intraperitoneal injection of an anti–mouse GP1bα antibody into PECAM-1−/− mice, PECAM-1 heterozygote mice, and littermate control mice as described,21 based on the method of Bergmeier et al22 and Nieswandt et al.23 This treatment reduced the platelet counts by greater than 95% within 4 hours (Figure 1), whereas peripheral leukocyte counts were unaffected (not shown). Indeed, PECAM-1−/− mice have a slightly elevated neutrophil count, although the total number of leukocytes remains unchanged (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), in agreement with an earlier study.18 Platelet recovery was measured at 48, 72, 120, and 172 hours after injection. Consistent with previous observations, recovery of platelet count was achieved at day 5 after injection in littermate control mice. Strikingly, however, a significant delay in platelet recovery was observed at 48, 72, and 120 hours after injection in PECAM-1−/− mice (Figure 1), with full recovery only occurring by day 7 after injection. The recovery of PECAM-1 heterozygote mice was not significantly different to the littermate controls with complete recovery at day 5 after injection (Figure 1). This finding establishes a dynamic role for PECAM-1 in mediating the kinetics of platelet recovery following complement-mediated immune thrombocytopenia and contrasts with the observation that baseline platelet counts are not significantly different from controls in the absence of PECAM-1. This defect in platelet recovery could be due to a role for PECAM-1 in MK maturation, MK adhesion to bone marrow endothelial cells, proplatelet formation, and/or platelet release. Subsequent studies were designed to investigate each of these possibilities.
Complement-mediated immune thrombocytopenia results in delayed thrombopoiesis in PECAM-1−/− mice. Two- to 4-month-old PECAM-1−/− mice (n = 8) and PECAM-1+/− mice (n = 8) with littermate controls (n = 8) were given a sterile intraperitoneal injection of anti–mouse GP1bα antibody (2 μg/g of mouse). Platelet counts were measured at 48, 72, 120, and 172 hours after injection. *P < .05; **P < .005. Error bars indicate standard error.
Complement-mediated immune thrombocytopenia results in delayed thrombopoiesis in PECAM-1−/− mice. Two- to 4-month-old PECAM-1−/− mice (n = 8) and PECAM-1+/− mice (n = 8) with littermate controls (n = 8) were given a sterile intraperitoneal injection of anti–mouse GP1bα antibody (2 μg/g of mouse). Platelet counts were measured at 48, 72, 120, and 172 hours after injection. *P < .05; **P < .005. Error bars indicate standard error.
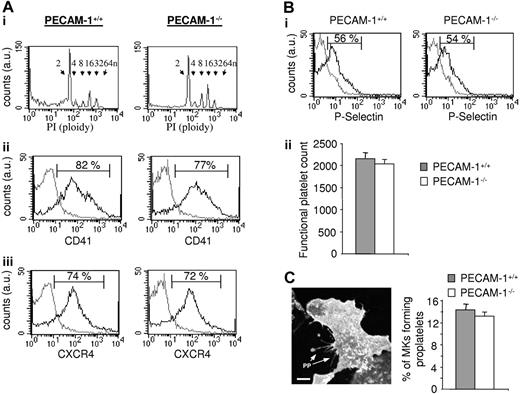
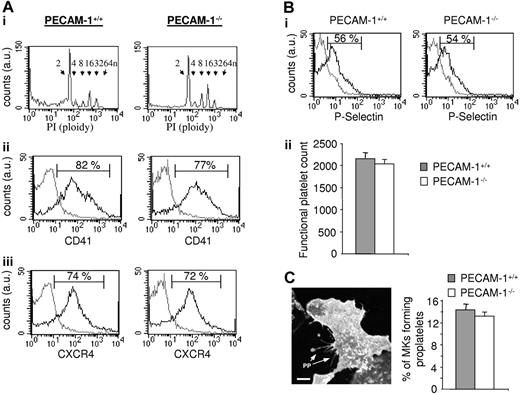
We initially compared the characteristics of control and PECAM-1−/− MKs grown under in vitro conditions as described.19,21 The populations of control and PECAM-1−/− MKs were similar in morphology when examined by differential interference contrast (DIC) light microscopy (not shown). Moreover, there was no major difference in the degree of polyploidy (Figure 2Ai) or in the level of 1 of the subunits of the major platelet integrin GPIIb (also known as CD41 or αIIb), which is a marker of MK differentiation (Figure 2Aii). Further, the level of the SDF1 chemokine receptor CXCR4 was similar in the 2 MK populations (Figure 2Aiii). These results suggest that PECAM-1 does not play a significant role in megakaryocytopoiesis, at least under these experimental conditions.
PECAM-1−/− MKs demonstrate normal in vitro nuclear and cytoplasmic maturation, normal production of functional platelets, and proplatelet formation. (A) Flow-cytometric analysis of MKs. (i) Propidium iodide (PI) fluorescence histogram showing ploidy distribution of MKs (ploidy subclasses are indicated). (ii-iii) Fluorescence histograms of cells stained with FITC-CD41 (ii) or FITC-CXCR4 (iii). Gray line indicates irrelevant antibody control; black line, FITC-CD41 or FITC-CXCR4 antibodies. au indicates arbitrary unit. (B) Normal functional platelet number produced by PECAM-1−/− MKs. In order to isolate the platelet-like particles produced, the whole-cell population from the lower chamber was centrifugated at 1000g in the presence of PGI2 to avoid platelet preactivation. The pellet including transmigrated MKs, proplatelet-forming MKs, some HUVECs, cell debris, and platelets was then resuspended in Tyrode buffer and stimulated with or without thrombin to identify and isolate the functional platelet-like particles. Platelets generated in response to SDF1α and HUVECs from PECAM-1−/− MKs are functional and express P-selectin after thrombin stimulation, as shown by the representative fluorescence-activated cell sorter (FACS) histogram (i; gray line indicates unstimulated platelets; black line, thrombin-stimulated platelets), with the functional platelet counts represented as the mean of 3 experiments ± SEM (ii). (C) Representative CD41 immunofluorescent image of a PECAM-1−/− MKs adhered to a HUVEC monolayer generating proplatelets (PPs). Cumulative analysis shows similar numbers of PECAM-1+/+ MKs and PECAM-1−/− MKs demonstrating proplatelet formation (n = 3). Scale bar = 20 μm. Error bars indicate standard error. Fluorescence images were obtained using a Leica DMIRE 2 inverted microscope (Leica, Milton Keynes, United Kingdom) equipped with a 40×/1.3 NA Plan-Apochromat objective lens and a Coolsnap Photometrics camera (Photometrics, Huntington Beach, CA). Slidebook 4.0 software (Intelligent Imaging Innovations, Denver, CO) and ImageJ software (National Institutes of Health, Bethesda, MD) were used to acquire and process images.
PECAM-1−/− MKs demonstrate normal in vitro nuclear and cytoplasmic maturation, normal production of functional platelets, and proplatelet formation. (A) Flow-cytometric analysis of MKs. (i) Propidium iodide (PI) fluorescence histogram showing ploidy distribution of MKs (ploidy subclasses are indicated). (ii-iii) Fluorescence histograms of cells stained with FITC-CD41 (ii) or FITC-CXCR4 (iii). Gray line indicates irrelevant antibody control; black line, FITC-CD41 or FITC-CXCR4 antibodies. au indicates arbitrary unit. (B) Normal functional platelet number produced by PECAM-1−/− MKs. In order to isolate the platelet-like particles produced, the whole-cell population from the lower chamber was centrifugated at 1000g in the presence of PGI2 to avoid platelet preactivation. The pellet including transmigrated MKs, proplatelet-forming MKs, some HUVECs, cell debris, and platelets was then resuspended in Tyrode buffer and stimulated with or without thrombin to identify and isolate the functional platelet-like particles. Platelets generated in response to SDF1α and HUVECs from PECAM-1−/− MKs are functional and express P-selectin after thrombin stimulation, as shown by the representative fluorescence-activated cell sorter (FACS) histogram (i; gray line indicates unstimulated platelets; black line, thrombin-stimulated platelets), with the functional platelet counts represented as the mean of 3 experiments ± SEM (ii). (C) Representative CD41 immunofluorescent image of a PECAM-1−/− MKs adhered to a HUVEC monolayer generating proplatelets (PPs). Cumulative analysis shows similar numbers of PECAM-1+/+ MKs and PECAM-1−/− MKs demonstrating proplatelet formation (n = 3). Scale bar = 20 μm. Error bars indicate standard error. Fluorescence images were obtained using a Leica DMIRE 2 inverted microscope (Leica, Milton Keynes, United Kingdom) equipped with a 40×/1.3 NA Plan-Apochromat objective lens and a Coolsnap Photometrics camera (Photometrics, Huntington Beach, CA). Slidebook 4.0 software (Intelligent Imaging Innovations, Denver, CO) and ImageJ software (National Institutes of Health, Bethesda, MD) were used to acquire and process images.
The adhesion of MKs to endothelial cells is essential for robust platelet production in vitro, a process that is believed to mimic that in vivo where MKs interact with bone marrow endothelial cells within the vascular niche.6,9 Studies were therefore undertaken to investigate the ability of control and PECAM-1−/− MKs to adhere to a HUVEC monolayer and to generate proplatelets. The ability of the adherent MKs to generate functional platelets was measured using a transwell assay according to the protocol of Hamada et al.9 A similar yield of functional platelets identified by the generation of platelet-like particles showing an increased level of expression of P-selectin in response to thrombin was observed in the 2 populations of platelets (Figure 2Bi-ii), providing evidence that there is no significant difference in platelet production between PECAM-1−/− mice and littermate controls. Moreover, a similar level of proplatelet forming MKs after adhesion of control and PECAM-1−/− MKs to HUVEC-coated coverslips was observed, which amounted to 14.3% ± 2.3% and 13.0% ± 2.1% of added MKs, respectively (n = 3), with no discernable difference in their number or overall pattern (Figure 2C). Thus, these data suggest that the reduction in platelet recovery following complement-mediated immune thrombocytopenia in the absence of PECAM-1 is not due to a defect in MK maturation or platelet production.
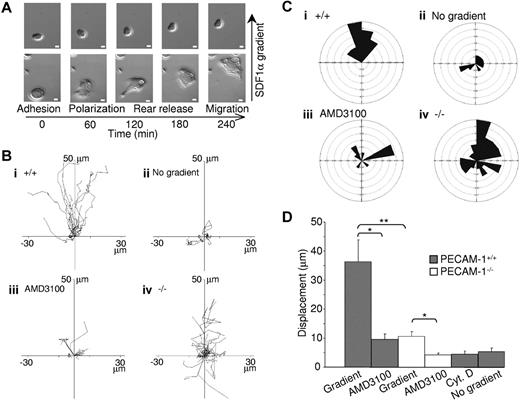
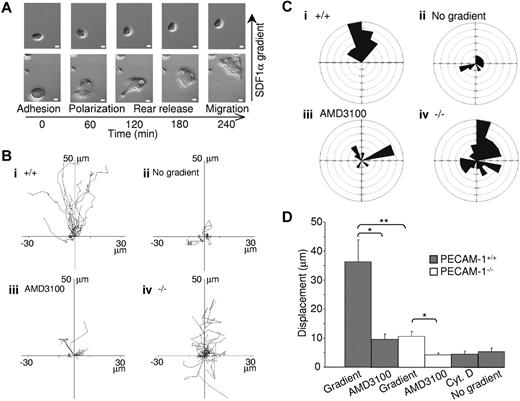
PECAM-1 is known to play a role in migration, as has been shown in several cell types.11 Studies were therefore undertaken to investigate whether this also extends to megakaryocytes. MK migration was performed on fibronectin, one of the major bone marrow extracellular matrix components,24 using a gradient of the chemokine SDF1α in a Dunn chemotaxis chamber.25 For a majority of large wild-type MKs, real-time visualization of migration revealed that movement could be divided into 4 distinct phases, namely adhesion, polarization, rear release, and migration, whereas for smaller cells these phases were less distinct (see example records in Figure 3A and Videos S1 [PECAM-1+/+] and S2 [PECAM-1−/−]). MKs initially polarize and extend protrusions in the direction of migration. These protrusions can be either broad lamellipodia or spike-like filopodia, which are driven by actin polymerization (as shown by the inhibitory effect of cytochalasin D, see below) and stabilized by adhering to the extracellular matrix. These adhesions serve as traction sites for migration as the cell moves forward at the migrating edge and detaches at the rear, allowing movement to take place.
PECAM-1−/− MKs demonstrate defective cell migration toward a gradient of SDF1α. (A) Differential interference contrast (DIC) images of wild-type MKs exposed to an SDF1α gradient within the Dunn chamber. Scale bar = 10 μm. (B) The migration paths over 4 hours of 30 MKs from 8 mice in each graph were traced. The intersection of the x- and y-axis was taken to be the starting point of each cell path, whereas the source of the SDF1α was at the top. (i) PECAM-1+/+ MKs exposed to SDF1α gradient. (ii) PECAM-1+/+ MKs exposed to SDF1α but with no gradient. (iii) PECAM-1+/+ MKs exposed to SDF1α gradient in the presence of AMD3100. (iv) PECAM-1−/− MKs in the presence of SDF1α gradient. (C) Circular histograms showing the proportion of cells whose final position was located within each of 18 equal sectors (20°). The source of SDF1α was at the top. (D) The net translocation distance (displacement from the start to the end point) of each cell in the absence or presence of inhibitors AMD3100 or cytochalasin D (CytD). *P < .01; **P < .001. Error bars indicate standard error. DIC images were visualized using a Zeiss Axiovert 200 inverted high-end microscope (Zeiss, Welwyn Garden City, United Kingdom) equipped with a 20×/0.4 Plan-Neofluor objective lens. Image acquisition was otherwise performed as described for Figure 2C.
PECAM-1−/− MKs demonstrate defective cell migration toward a gradient of SDF1α. (A) Differential interference contrast (DIC) images of wild-type MKs exposed to an SDF1α gradient within the Dunn chamber. Scale bar = 10 μm. (B) The migration paths over 4 hours of 30 MKs from 8 mice in each graph were traced. The intersection of the x- and y-axis was taken to be the starting point of each cell path, whereas the source of the SDF1α was at the top. (i) PECAM-1+/+ MKs exposed to SDF1α gradient. (ii) PECAM-1+/+ MKs exposed to SDF1α but with no gradient. (iii) PECAM-1+/+ MKs exposed to SDF1α gradient in the presence of AMD3100. (iv) PECAM-1−/− MKs in the presence of SDF1α gradient. (C) Circular histograms showing the proportion of cells whose final position was located within each of 18 equal sectors (20°). The source of SDF1α was at the top. (D) The net translocation distance (displacement from the start to the end point) of each cell in the absence or presence of inhibitors AMD3100 or cytochalasin D (CytD). *P < .01; **P < .001. Error bars indicate standard error. DIC images were visualized using a Zeiss Axiovert 200 inverted high-end microscope (Zeiss, Welwyn Garden City, United Kingdom) equipped with a 20×/0.4 Plan-Neofluor objective lens. Image acquisition was otherwise performed as described for Figure 2C.
Individual line tracings (Figure 3Bi) and circular histograms show the overall direction of migration (Figure 3Ci), demonstrating the variation in the rate of movement of individual cells but little deviation away from the direction of the SDF1α gradient. Importantly, there was minimal migration of MKs in the absence of an SDF1α gradient or in the presence of the CXCR4 antagonist AMD310026 (Figure 4B-D). Filopodial and lamellipodia structures and migration were abolished in the presence of cytochalasin D, an inhibitor of actin polymerization (Figure 3D), confirming that these processes are driven by actin remodeling.
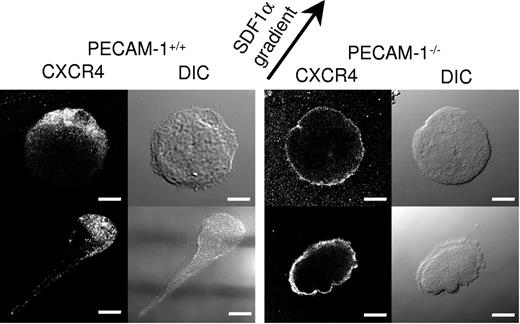
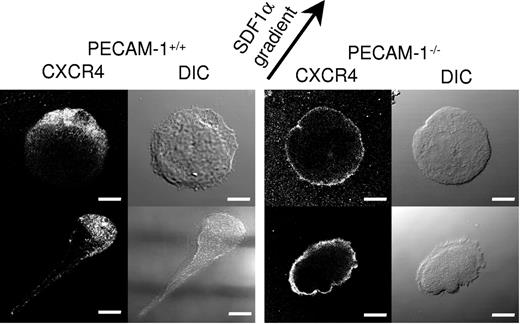
PECAM-1−/− MKs demonstrate defective polarization of the chemokine receptor CXCR4. CXCR4 immunostaining of migrating PECAM-1+/+ MKs and PECAM-1−/− MKs toward the SDF1α source (as indicated by the arrow) demonstrates defective polarization. Scale bar = 20 μm. Image acquisition was performed as described for Figure 2C.
PECAM-1−/− MKs demonstrate defective polarization of the chemokine receptor CXCR4. CXCR4 immunostaining of migrating PECAM-1+/+ MKs and PECAM-1−/− MKs toward the SDF1α source (as indicated by the arrow) demonstrates defective polarization. Scale bar = 20 μm. Image acquisition was performed as described for Figure 2C.
Strikingly, PECAM-1−/− MK migration toward a gradient of SDF1α was 29% that of control cells, as shown by the individual line recordings of 30 cells (Figure 3B) and the pooled data (Figure 3D). This defect was due to a loss of directional movement rather than a generalized decrease in migration (Figure 3C). Specifically, all PECAM-1+/+ MKs move to within a 120° arc facing the SDF1α source, whereas only 47% of PECAM-1−/− MKs are present in this region. Moreover, PECAM-1−/− MKs exhibit a circular motion during which they stretch and retract in different directions with no apparent evidence of polarization. The migration paths demonstrate that PECAM-1−/− MKs move relatively short distances before changing direction (Figure 3Biv) due to a lack of directional persistence. Directional movement of PECAM-1−/− MKs was completely inhibited in the presence of the CXCR4 receptor antagonist AMD3100 (Figure 3D), confirming that migration was mediated through CXCR4.
We first considered that the reduction in rate and direction of migration observed in the absence of PECAM-1 could be due to a defect in polarization of the PECAM-1−/− MKs under the influence of a gradient of the chemokine SDF1. MKs were judged to have undergone polarization when a leading edge of lamellipodia could be readily discerned. Strikingly, quantitative analysis of DIC images of cell migration revealed that 23% of PECAM-1−/− MKs were polarized compared with 68% of littermate controls (Figure 4). This difference prompted us to consider the relationship between the surface expression of the SDF1 receptor CXCR4 and the direction of membrane polarization. Fluorescence microscopic analyses using a specific antibody demonstrated marked polarization of CXCR4 in 70.6% ± 5.3% compared with 20.0% ± 8.1% of control and PECAM-1−/− MKs, respectively, as illustrated by the representative cells in Figure 4. In the majority of PECAM-1−/− MKs, CXCR4 was distributed through all of the MK membrane in contrast to the marked polarization observed in control MKs (Figure 4). A 3-dimensional view of CXCR4 membrane localization is included in Figure S1. Control and PECAM-1−/− MKs express similar levels of CXCR4 (Figure 2Aiii), demonstrating that this does not underlie the inability of the latter to polarize the chemokine receptor.
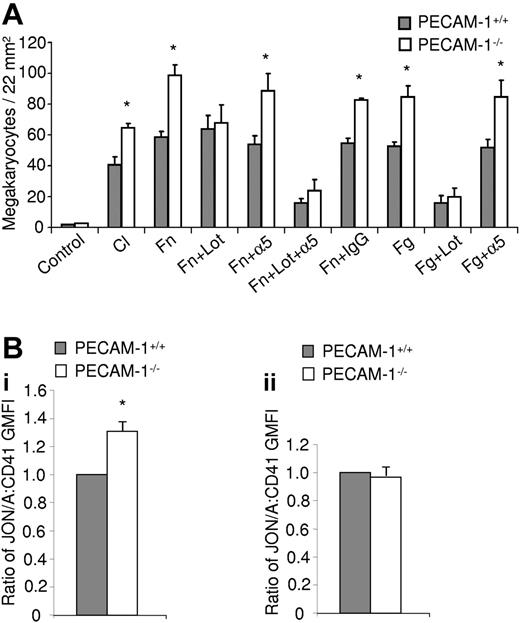
In addition to the change in CXCR4 distribution, there is a marked increase in adhesion of PECAM-1−/− MKs on fibronectin, as well as on 2 other surfaces, fibrinogen and collagen, in the presence of the SDF1α (Figure 5A). In order to determine the relative contribution of integrin activation in the MKs, lotrafiban (a specific inhibitor to the αIIbβ3 integrin) and a blocking antibody to the α5 integrin were also used in the adhesion experiments. This increase in adhesion of PECAM-1−/− MKs to fibronectin is lost in the presence of lotrafiban but unaltered following addition of the α5 integrin blocking antibody, demonstrating that the increase in adhesion is mediated via integrin αIIbβ3 (Figure 5A). On the other hand, adhesion of litter-matched control MKs (PECAM-1+/+) to fibronectin is dependent on both integrin αIIbβ3 and integrin α5β1, as shown using a combination of lotrafiban and α5 integrin blocking antibody (Figure 5A). In comparison, adhesion to fibrinogen of litter-matched control and PECAM-1−/− MKs is mediated via integrin αIIbβ3 and independent of integrin α5β1 (Figure 5A). The observed increase in adhesion of PECAM-1−/− MKs and blockade by lotrafiban provides evidence for activation of integrin αIIbβ3 under basal conditions. To address this directly, we measured binding of the αIIbβ3 activation reporter antibody Jon/A relative to an activation-independent antibody. These experiments confirmed constitutive activation of integrin αIIbβ3 integrin in PECAM-1−/− MKs (Figure 5Bi), although interestingly a similar result was not observed in PECAM-1−/− platelets (Figure 5Bii). These results therefore demonstrate that PECAM-1 plays a major role in regulating MK directional motility and integrin-mediated adhesion on bone marrow extracellular matrix proteins.
PECAM-1−/− MKs demonstrate increased adhesion mediated by overactivation of the αIIbβ3 integrin. (A) PECAM-1−/− MKs demonstrate increased adherence to fibronectin (Fn), collagen (Cl), and fibrinogen (Fg) monolayers. This increased adhesion is maintained in the presence of an α5 integrin blocking antibody (α5) however absent in the presence of the αIIbβ3 integrin inhibitor lotrafiban (Lot). Mean of 3 experiments. *P < .05. (Bi) The ratio of Jon/A to CD41 binding on MKs (mean of 3 experiments) presented as geometric mean fluorescence intensity (GMFI) ± SEM. (ii) The ratio of Jon/A to CD41 binding on platelets (mean of 3 experiments) presented as geometric mean fluorescence intensity (GMFI) ± SEM. *P < .05.
PECAM-1−/− MKs demonstrate increased adhesion mediated by overactivation of the αIIbβ3 integrin. (A) PECAM-1−/− MKs demonstrate increased adherence to fibronectin (Fn), collagen (Cl), and fibrinogen (Fg) monolayers. This increased adhesion is maintained in the presence of an α5 integrin blocking antibody (α5) however absent in the presence of the αIIbβ3 integrin inhibitor lotrafiban (Lot). Mean of 3 experiments. *P < .05. (Bi) The ratio of Jon/A to CD41 binding on MKs (mean of 3 experiments) presented as geometric mean fluorescence intensity (GMFI) ± SEM. (ii) The ratio of Jon/A to CD41 binding on platelets (mean of 3 experiments) presented as geometric mean fluorescence intensity (GMFI) ± SEM. *P < .05.
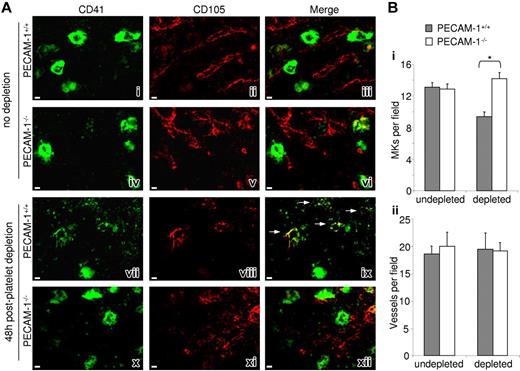
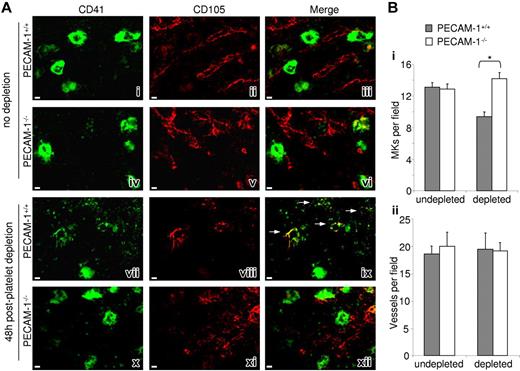
During megakaryocytopoiesis, differentiating MKs migrate within the complex bone marrow stromal microenvironment from the endosteal niche to the bone marrow sinusoidal endothelial cells, the site of platelet formation. Having demonstrated an abnormality in PECAM-1−/− MK motility and adhesion, we hypothesized that there may be altered spatial localization of PECAM-1−/− MKs within the bone marrow in response to induced thrombocytopenia. We therefore prepared cryofrozen bone marrow sections and studied MK and blood vessel localization in mice before and after induced thrombocytopenia. Example fields of bone marrow cellular structure in DIC images with highlighted MKs (white arrows) demonstrating the regions of bone marrow studied have been included in Figure S2. Specific staining and localization of MKs was performed using a fluorescent anti-CD41 antibody for the integrin subunit GPIIb. In mice that have not been subject to platelet depletion, bone marrow sections of PECAM-1−/− and littermate control mice demonstrated similar numbers of MKs, namely 12.9 ± 0.6 and 13.1 ± 0.6 MK per field, respectively (Figure 6A). Costaining of the vascular endothelium using an antibody to endoglin (CD105) demonstrates that a similar proportion of MKs are localized close to vessels in both populations of mice. However, examination of bone marrow 48 hours after platelet depletion revealed a significantly larger number of intact PECAM-1−/− MKs relative to littermate controls, namely 14.2 ± 0.7 and 9.4 ± 0.5 MK per field, respectively (Figure 6Bi), whereas vascular density was similar in both groups (Figure 6Bii). Strikingly, the sections from the platelet-depleted control mice show a significant number of MKs in the process of generating proplatelets and platelets, as illustrated by the diffuse staining at the perivascular region (Figure 6Aix arrows). Such structures were rarely detected in PECAM-1−/− bone marrow. Moreover, no defect in the osteoblastic region of the marrow was observed (data not shown), consistent with the observation of no maturation defect on the PECAM-1−/− MKs. Taken together, these data suggest that in control mice, platelet depletion in the vasculature leads to an increase in the rate of migration of mature MKs within the vascular niche to the perivascular region, but this is severely impaired in the absence of PECAM-1.
PECAM-1−/− MKs show defective bone marrow sinusoidal colocalization 48 hours after platelet depletion. (A) Longitudinal sections of whole murine femora from undepleted PECAM-1+/+ (i-iii) versus PECAM-1−/− (iv-vi) mice 48 hours after platelet depletion (vii-ix and x-xii, respectively). MKs were identified by FITC-CD41 (i, iv, vii, x); marrow sinusoidal vessels were identified by CD105/biotin/PE-streptavidin (ii, v, viii, xi); merged images allow comparison of MK location from vessels (iii, vi, ix, xii); yellow color and white arrows show colocalization. Scale bar = 30 μm. (B) The average number of MKs (i) and blood vessels (ii) per field were determined in CD41 and CD105 stained marrow sections throughout the length of 3 femora. *P < .05. Error bars indicate standard error. Images were obtained as described for Figure 2C.
PECAM-1−/− MKs show defective bone marrow sinusoidal colocalization 48 hours after platelet depletion. (A) Longitudinal sections of whole murine femora from undepleted PECAM-1+/+ (i-iii) versus PECAM-1−/− (iv-vi) mice 48 hours after platelet depletion (vii-ix and x-xii, respectively). MKs were identified by FITC-CD41 (i, iv, vii, x); marrow sinusoidal vessels were identified by CD105/biotin/PE-streptavidin (ii, v, viii, xi); merged images allow comparison of MK location from vessels (iii, vi, ix, xii); yellow color and white arrows show colocalization. Scale bar = 30 μm. (B) The average number of MKs (i) and blood vessels (ii) per field were determined in CD41 and CD105 stained marrow sections throughout the length of 3 femora. *P < .05. Error bars indicate standard error. Images were obtained as described for Figure 2C.
Discussion
The present study provides evidence for a novel role of PECAM-1 in thrombopoiesis as illustrated by the delay in kinetics of platelet recovery after immune-mediated platelet depletion in the PECAM-1−/− mouse. The molecular basis of this defect appears to be due to altered migration of MKs as a consequence of altered polarization of the CXCR4 receptor and an increase in adhesion to matrix proteins. Importantly, this defect in migration is able to account for the in vivo observation of an altered distribution of MKs within the bone marrow of PECAM-1−/− mice under conditions of “accelerated thrombopoiesis” as a consequence of a reduction in movement of mature MKs to the perivascular region. On the other hand, PECAM-1 does not appear to contribute to MK differentiation, maturation, or proplatelet/platelet formation.
During megakaryocytopoiesis, differentiating MKs migrate within the complex bone marrow stromal microenvironment from the endosteal osteoblastic-rich niche where hematopoietic stem cells reside to the capillary-rich vascular niche and eventually to the bone marrow sinusoidal endothelial cells, the site of platelet formation.1–3,6 The chemokine SDF1 augments this motility and promotes the interaction of MK progenitors with the bone marrow vascular niche, localizing them to the junctions between sinusoidal bone marrow endothelial cells and therefore supporting thrombopoiesis. In the present study, the migration of PECAM-1−/− MKs on fibronectin toward a gradient of SDF1 was shown to be defective using real-time videomicroscopy. This is important in that fibronectin is distributed throughout the central region of the bone marrow24 and a role for the fibronectin receptor integrin αIIbβ3 in platelet formation has recently been reported.21 Thus, a defect of migration on fibronectin may underlie the altered distribution of megakaryocytes that is seen in response to thrombocytopenia as described. The defective migration on fibronectin in the PECAM-1−/− MKs can be explained by the altered polarization of the SDF1 receptor CXCR4 in PECAM-1−/− MKs, which is likely to account for the loss in directional motility, and the increase in integrin-dependent adhesion. This is a novel role for PECAM-1 in contributing to the polarization of a chemokine receptor and indeed provides evidence that PECAM-1 may be behaving as a membrane-trafficking protein. It is unclear whether the defect in polarization of CXCR4 and increased adhesion to bone marrow matrix proteins are causally related.
Although this is the first report of a functional role of PECAM-1 in MKs, there is considerable evidence that PECAM-1 regulates migration of other vascular cells. For example, this is illustrated by the role of PECAM-1 in regulating migration of monocytes and neutrophils through endothelial cells, as demonstrated using neutralizing antibodies both in vivo and in vitro.27–30 Similarly, an antibody against human PECAM-1 has been shown to inhibit migration of HUVECs through Matrigel-coated filters or in response to wound injury.31 Furthermore, neutrophils harvested from PECAM-1−/− mice exhibit loss of directionality and have a significantly reduced speed of migration in response to IL-8.17
There are several mechanisms by which PECAM-1 could mediate its effects in MKs, bearing in mind that PECAM-1 has been shown to generate both inhibition and stimulation signals in a cell-dependent manner.11 Perhaps, the most characterized response to PECAM-1 is recruitment of the protein tyrosine-phosphatase SHP-2 to the phosphorylated ITIM in the cytosolic tail of immunoglobulin receptor.11,32 SHP-2 has been shown to stimulate cell migration by promoting turnover of cell-matrix adhesive interactions that are critical for cell motility.33,34 In addition, PECAM-1 has been shown to inhibit ITAM-dependent signaling responses in platelets12–14,35 and this could underlie the up-regulation of integrin activity that is reported in the present study in MKs on a variety of surfaces (fibronectin, fibrinogen, and collagen). Indeed, this effect may also be mediated by recruitment of SHP-2 and possibly the related tyrosine phosphatase SHP-1.11 On the other hand, the small GTPase Rap1 has been shown to critically regulate integrin activity by PECAM-1 in T lymphocytes.16 A complete understanding of the molecular basis of the defect in PECAM-1−/− MKs will therefore require generation and expression of mutant forms of the immunoglobulin receptor and various downstream signaling proteins in protein-deficient MKs.
The novel observation of an abnormal response of PECAM-1−/− mice to immune-mediated thrombocytopenia corresponds with previous reports of these mice demonstrating defective recovery after the onset of other exogenous stresses. As PECAM-1−/− mice age, they develop an autoimmune lupus-like syndrome and exhibit adverse prognoses in conditions such as collagen-induced arthritis, LPS-induced septic shock, encephalomyelitis,36–39 and endothelial X-ray irradiation,40 demonstrating that PECAM-1−/− mice manifest a consistent defective response to stress.
Our study has revealed a novel role for PECAM-1 in thrombopoiesis and the regulation of MK motility within the bone marrow. We have described a novel observation of delayed platelet recovery within the PECAM-1–deficient mouse and directly attributed this defect to a decrease in their migration to the chemokine SDF1 and enhanced ability of MKs to adhere to matrix proteins. On the other hand, MK maturation and proplatelet and platelet formation are normal in PECAM-1−/− mice. Furthermore, we have substantiated our findings in vivo using immunostaining of PECAM-1−/− bone marrow to show that MKs are inappropriately retained following platelet depletion in these mice compared with littermate controls. These findings further substantiate the growing recognition that PECAM-1 is a “compensatory” molecule whose major role is most clearly evident following pathologic stress events. The possibility that PECAM-1 plays a similar role in regulating the recovery of other hematopoietic lineages following depletion is worthy of consideration.
Authorship
Contribution: T.S.D. and C.P. designed and performed research, collected and analyzed data, and wrote the paper. E.A.R. and M.B.P. collected and analyzed data. M.K.L. designed and performed research and collected and analyzed data. C.D.B. designed research and wrote the paper. S.P.W. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
T.S.D. and C.P. contributed equally to this work.
Correspondence: Tarvinder S. Dhanjal, Institute of Biomedical Research, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: t.s.dhanjal@bham.ac.uk.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We would like to thank Dr Rudi Manz for assistance in preparing bone marrow sections and Prof Gerard Nash and Phil Stone for supplying the HUVECs.
This work was funded by the British Heart Foundation (BHF). S.P.W. holds a BHF Chair and T.S.D. holds a BHF Clinical Fellowship.