Abstract
We investigated the role of c-Kit and the membrane-bound ligand (mbKitL) in endothelial progenitor cell (EPC) recruitment by microvascular endothelial cells (ECs). We demonstrated that inflammatory activation induced the expression of the mbKitL on ECs both in vitro and in vivo, and that recruitment of EPCs depended on c-Kit/mbKitL interaction. Depletion of endogenous c-Kit or inhibition of c-Kit enzymatic activity by imatinib mesylate prevented adhesion of EPCs to activated ECs both in vitro and in vivo, indicating that a functional c-Kit on EPCs is essential. We also demonstrate that Akt was the downstream molecule regulating cell adhesion. A potential role of the c-Kit/mbKitL interaction in pathological settings is sustained by the expression of the mbKitL on ECs lining intraplaque neovessels. Thus, our results provide new insights into the mechanisms underlying EPC recruitment and the bases for novel strategies to hinder pathological angiogenesis.
Introduction
Signal transduction initiated by the interaction of growth factors with specific receptors is an important mechanism regulating normal cell growth and differentiation. The c-kit proto-oncogene encodes a 145-kDa membrane-spanning receptor that belongs, both structurally and functionally, to a subclass of receptor tyrosine kinases (RTKs) that includes the platelet-derived growth factor (PDGF) receptor.1 Hematopoietic and other stem cells including cardiac, endothelial, and epithelial cells express c-Kit.2,3 Mutations of c-Kit as well as of the ligand (KitL) produce defects in germ cell and melanocyte development, impairment of hematopoiesis, and increased sensitivity to radiation and chemotherapy,4 suggesting that a common signaling cascade activated by KitL might govern proliferation, recruitment, and homing of stem cells. The KitL is normally expressed in 2 spliced variants, both of which are initially localized to the cell surface. The larger variant contains an extracellular proteolytic cleavage site, which permits the release of KitL from the cell surface.5 The spliced smaller variant is usually not cleaved and normally remains associated with the cell surface. Stromal cells in bone marrow, by means of their specific expression of the cell-bound KitL, anchor and maintain stem cells in a quiescent state.5 However, under stress conditions, matrix metalloproteinases-9–mediated membrane-bound KitL processing leads to the release of soluble KitL, and allows the transfer of endothelial and hematopoietic stem cells from a quiescent to a proliferative niche and their mobilization to circulation.6
Production of cytokines and growth factors by endothelial cells plays a crucial role in complex inflammatory processes. These soluble factors, which include the soluble KitL,7 are mainly involved in the generation and regulation of phagocyte effector cells and in their accumulation within sites of inflammation.8 However, the recent observation that the soluble KitL, by activating the Akt signaling pathway, promotes endothelial cell migration and survival9 sustains the possibility that both in physiological and pathological neoangiogenesis, c-Kit can also support the angiogenic performance of mature endothelial cells.
Circulating endothelial progenitor cells (EPCs) by definition not only have the potential to instigate new vessel formation via angiogenesis and vasculogenesis but also, after mobilization, have the potential to provide ongoing endothelial repair by homing to sites of endothelial damage.10 Although their contribution to human vasculature has been reported,11 the mechanisms that allow EPCs to insert themselves into neoformed vessels is poorly understood. Several lines of evidence suggest that mechanisms involved in the recruitment of inflammatory cells within sites of inflammation might also regulate EPC homing. Recruitment of inflammatory cells requires a coordinated sequence of multistep adhesive and signaling events, including selectin-mediated rolling, leukocyte activation by chemokines, integrin-mediated firm adhesion, and diapedesis.12 Indeed, selectins13,14 and integrins11 have been shown to regulate EPC rolling and migration.
In the present study, we evaluated the contribution of c-Kit and of its membrane-bound ligand to EPC recruitment to the activated endothelium. We demonstrated that, both in vitro and in vivo, microvascular endothelial cells challenged with inflammatory stimuli expressed the membrane-bound form of KitL and recruited EPCs via a c-Kit–mediated activation of the Akt signaling pathway. Moreover, in an in vivo model of angiogenesis, we demonstrated that EPCs endogenously depleted of c-Kit could not be recruited to neoformed vessels.
Materials and methods
Reagents and antibodies
M199 medium (endotoxin tested), bovine serum albumin, MEM-D-val medium, Dulbecco modified Eagle medium (DMEM), collagenase I, and LPS were from Sigma-Aldrich (St Louis, MO). Bovine calf serum (BCS; endotoxin tested) was from HyClone (Logan, UT). Trypsin was purchased from Difco (Detroit, MI). TNFα, IL-1β, and KitL were from Chemicon International (Temecula, CA). Nitrocellulose filters, HRP-conjugated anti–rabbit IgG and anti–mouse IgG, molecular weight markers, and chemiluminescence reagent (ECL) were from Amersham (Braunschweig, Germany). EGM endothelial growth medium was from Cambrex (Walkersville, MD). Lipofectin reagents were purchased from Life Technologies (Gaithersburg, MD). The presence of endotoxin contamination was tested by the Limulus amebocyte assay (concentration was < 0.1 ng/mL). Imatinib mesylate was from Novartis (West Sussex, United Kingdom). 1L-6-hydroxymethyl-ciro-inositol-2-(R)-2-methyl-3-O-octadecylcarbonate (Akt inhib) was from Alexis (Lausen, Switzerland). Anti–β-actin, anti–ICAM 1, anti–E-selectin, and anti–VCAM 1 antisera were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). Anti–VE-cadherin and anti-CD34 were from New England Biolabs (Beverly, MA). Anti–p-Akt, anti-Akt, anti-ERK1/ERK2, anti–p-ERK1/ERK2, and anti–p–c-Kit were from Cell Signaling Technology (Beverly, MA). Monoclonal anti-CD146 antibody was from BioCytex (Marseille, France). Anti–c-Kit (K213), K44, and K4515 were kindly provided by Yosef Yarden. Anti-KitL antibody, recombinant human KitL (rhKitL), PDGF, neutralizing anti-PDGFRβ antibody, and the KitL enzyme-linked immunosorbent assay (ELISA) assay kit and anti-CD31 were from R&D Systems (Minneapolis, MN).
Cell lines
CDC-HMEC-1 cells and M07e cells were cultured as previously described.16,17 Bone marrow fibroblasts (fibroblasts) were obtained as described by Shehata et al.18 EPCs were isolated from peripheral blood mononuclear cells of healthy donors using CD34 or CD133+ isolation Kit (MINIMACS system; Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions and cultured as previously described.19 Human tumor endothelial cells (TECs), kindly provided by Prof Camussi, were grown as previously described.20
Human arterial samples and isolation of vascular cells
The institutional review board of the Ospedale Molinette approved the study. Human nonatherosclerotic and atherosclerotic arteries (renal and carotid arteries) were obtained with informed consent from patients who underwent transplantation or endoarteriectomy. By histologic analysis, we classified these into nondiseased (n = 5), fibromuscular plaques (n = 6), and atheromatous plaques (n = 5) according to the American Heart Association histologic criteria. Neointima from fresh samples of advanced specimens or intima from nonatherosclerotic specimens were finely minced with scissors and then digested by incubation for 1 hour at 37°C in DMEM containing collagenase I. After washing in medium plus 10% BCS, the cell suspension was forced through a graded series of meshes to separate the cell component from stroma and aggregate. Cells were pelleted and resuspended, and endothelial cells were isolated via anti-CD105 Ab coupled to magnetic beads, by magnetic cell sorting using the magnetic-activated cell sorter (MACS) system (Miltenyi Biotech). Briefly, cells were labeled with the anti-CD105 mAb for 20 minutes and then were washed twice and resuspended in MACS buffer (PBS without Ca2+ and Mg2+, supplemented with 1% bovine serum albumin and 5 mM EDTA) at a concentration of 1 × 106 cells/80 μL. After washing, cells were separated on a magnetic stainless steel wool column (Miltenyi Biotech) according to the manufacturer's recommendation. CD105+ cells were plated into 1% gelatin-coated plates in M199 medium supplemented with 20% BCS and bFGF (10 ng/mL). The endothelial phenotype of cells was verified by immunohistochemistry using an anti-CD31 antibody (data not shown). Isolation of smooth muscle cells was obtained by culturing CD105− cells in MEM-D-val medium containing 20% BCS and PDGF (10 ng/mL). The smooth muscle cell phenotype was confirmed by immunohistochemistry using an anti–α-actin antibody (data not shown). The cells were used for real-time quantitative polymerase chain reaction (PCR) and for adhesion experiments.
FACS analysis
TECs overexpressing or not the mbKitL, fibroblasts, or CDC-HMEC-1 cells, untreated or treated as indicated, were put in suspension; labeled with mbKitL, E-selectin, ICAM 1, or a preimmune mAb for 30 minutes at 4°C; washed twice in PBS; and incubated with fluorescein-labeled anti–mouse or anti–rabbit IgG for the same time. The expression of cell surface molecules was evaluated by flow cytometry (FACScan; Becton Dickinson, San Jose, CA). Fluorescence-activated cell sorting (FACS) analysis was also used for analyzing VE-cadherin, CD146, or CD34 expression on EPCs.
Silencing of endogenous c-Kit and Akt by small interfering RNAs (siRNAs)
To obtain inactivation of endogenous c-Kit or Akt, EPCs cultured as described in “Cell lines” were transiently transfected with purified siRNA for c-Kit (K1) or Akt and with duplex siRNAs purchased by Qiagen (Valencia, CA) as scramble controls. Transfection was performed using Lipofectamine PLUS reagent (Invitrogen, Frederick, MD) according to the vendor's instructions.19 Twenty-four, 48, and 72 hours later, whole cell extracts were prepared, separated on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted with antibodies against c-Kit or Akt. Cell viability was evaluated at the end of the experiment. In selected experiments, bone marrow fibroblasts were also transiently transfected with purified Akt siRNA.
Transfection of EPCs with c-Kit mutants
EPCs were transiently transfected with 2 different c-Kit mutants containing a phenylalanine substitution at the tyrosine residue 719 (Y719F) or at the tyrosine residue 821 (Y821F), gently provided by Dr Kitamura.21 The 2 constructs differ for their ability to activate PI3-K (the Y821F did not; the Y719F did). Transfections were performed by the lipofectin method.19
Adhesion assay
Adhesion of EPCs to CDC-HMEC-1, fibroblasts, or TECs was assayed in static conditions.14 In selected experiments, endothelial cells (ECs) from intima or neointima of arterial specimens were also used. Briefly, EPCs were labeled with the red fluorescent PKH26 (Sigma-Aldrich) and, after centrifugation at 1400g for 10 minutes, were resuspended in medium free containing 0.25% BSA. Cells were then added (at 2 × 105 cells/well) to confluent monolayer of CDC-HMEC-1 cells, untreated or treated with TNFα (10 ng/mL), fibroblasts, or TECs, stably transfected with the vector or with the mbKitL construct, plated on 6-well plates. EPC cocultures were incubated at 37°C for 4 hours, and nonadherent cells were removed by washing 3 times with PBS. Samples were then fixed with 4% formaldehyde/PBS and observed under an epifluorescence microscope. Bound fluorescent red EPCs were counted. Experiments were performed in triplicate, and 10 fields at × 400 magnification per sample were evaluated. Experiments were also performed with anti–c-Kit monoclonal antibodies, able (K44) or unable (K45) to block ligand-binding domain15 ; a KitL-blocking monoclonal antibody; a pharmacological inhibitor of Akt (20 μM) or of c-Kit enzymatic activity (imatinib mesylate)22 ; a neutralizing anti-PDGFRβ; and an anti–E-selectin, anti–ICAM 1, or anti–VCAM 1 antibody. In selected experiments, the blocking effect of the anti-PDGFRβ antibody was evaluated. To this end, PDGFBB (30 ng/mL) was added to EPCs in the presence of different concentrations of the anti-PDGFRβ–neutralizing antibody. We found that 30 μg/mL of the blocking antibody was able to inhibit PDGFRβ phosphorylation (data not shown).23 Thus, 30 μg/mL of the antibody was used in adhesion experiments. EPCs depleted of endogenous c-Kit or Akt, or transfected with the c-Kit mutants, were also used. For biochemical studies, EPCs were let to adhere to fibroblasts for different times as described.24 In selected experiments, adhesion was performed on plates coated with rhKitL. To this end, tissue culture plates were coated with 20 μg/mL rhKitL by overnight incubation at 4°C and postcoated with 1% BSA for 1 hour at 37°C. For adhesion experiments, EPCs were detached by 10 mM EDTA treatment in PBS and after washing in PBS containing 1 mM CaCl2 and 1 mM MgCl2 resuspended in prewarmed DMEM medium pretreated or not with K44 or K45 and plated on the tissue culture plates for 45 minutes at 37°C. BSA was used as negative control.
Adhesion assay for electron microscopy
Samples were fixed in 2.5% paraformaldehyde containing 2% sucrose, and postfixed in 2.5% glutaraldehyde, dehydrated in alcohol, dried, and coated with gold by spatter coating. The specimens were examined in scanning electron microscope (Jeol T300 electron microscope; Tokyo, Japan). Images were obtained at working distance of 15 to 25 mm and accelerating voltage of 14 to 26 kV.
Western blot analysis
Cells were lysed (50 mM Tris HCl [pH 8.3], 1% Triton X-100, 10 mM PMSF, 100 U/mL aprotinin, 10 μM/mL leupeptin) and protein concentrations were obtained as previously described.19 Proteins (50 μg) were subjected to SDS-PAGE, transferred into nitrocellulose membranes, and revealed by chemiluminescence detection system (ECL).
Real-time quantitative (RQ)–PCR
LPS (100 ng/mL), IL-1β (10 ng/mL), or TNFα (10 ng/mL) was used to stimulate microvascular endothelial cells. In selected experiments, endothelial cells and smooth muscle cells from neointima were also used. mbKitL, E-selectin, and ICAM 1 mRNA quantification by RQ-PCR was performed using the ABI PRISM 7700 Sequence detection system and the SYBR Green Master Mix Kit (both from Applied Biosystem, Foster City, CA). β-Glucuronidase (GUS) gene was used as standard reference. The relative expression of mbKitL, E-selectin, and ICAM 1 was calculated by using comparative threshold cycle methods. The primer sequences were as follows: sense (5′-CCATTGATGCCTTCAAGGAC-3′) and antisense (5′-GGCTGTCTCTTCTTCGAGTA-3′) for mbKitL; sense (5′-CAGTGACCATCTACAGCTTTCCGG-3′) and antisense (5′-GCTGCTACCACAGTGATGATGACAA-3′) for ICAM 1; and sense (5′-CCAGTGCTTATTGTCAGC-3′) and antisense (5′-CACATTGCAGGCTGGAAT-3′) for E-selectin.
In vivo experiments
The mbKitL cDNA (gently provided by P. Leder) was Pst1 and BamHI digested and cloned in pcDNA3.1/Zeo (+) plasmid and then sequenced. Stable transfection of TECs was obtained after 500 μg/μL Zeocin selection. FACS analysis was performed to evaluate the expression of the mbKitL construct. One of these clones was used to inoculate severe combined immunodeficient (SCID) mice. For in vivo study, tumor-derived endothelial cells expressing the mbKitL were harvested by trypsin EDTA and washed in PBS, counted, and resuspended in DMEM (2 × 106 in 250 μL DMEM). Cells were chilled in ice, added to 250 μL Matrigel at 4°C, and injected subcutaneously into the left back of SCID mice. Five days later, untransfected EPCs or EPCs transfected with the scramble siRNA, the c-Kit siRNA, or pretreated with imatinib mesylate (10 μM/L) were labeled with fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CSFE; Molecular Probes).25 Labeled EPCs were injected in the tail vein of the mice and 2 days after mice were killed, and endothelial plugs were recovered, fixed in 10% buffered formalin, and embedded in paraffin for immunohistochemistry. The vessel area and the total Matrigel area were planimetrically assessed from stained sections as previously described.16 Considered vessels were only those structures possessing a patent lumen and containing red blood cells (RBCs). Angiogenesis was expressed as the percentage ± SD of the vessel area to the total Matrigel area. Quantification of neoformed microvessel density was also evaluated by CD31 staining of vascular endothelial cells. Any stained endothelial cell or endothelial cell cluster, clearly separated from connective tissue elements, was considered as a single microvessel and counted, according to Weidner et al.26 In selected experiments, CDC-HMEC-1 cells were added to Matrigel in the presence of IL-1β or TNFα (20 ng/mL) and injected subcutaneously into the left back of SCID mice (4 mice). After 3 days, mice were killed and endothelial plugs were recovered, fixed, and processed for immunofluorescence.16
Animal procedures conformed to the Guide for Care and Use of Laboratory Resources.27 To evaluate the expression of the mbKitL in neovessels, Matrigel plugs were subjected to RNA extraction and RQ-PCR was performed as described in “Real-time quantitative (RQ)–PCR”. Cell viability of EPCs depleted of endogenous c-Kit or treated with imatinib mesylate (after 24 and 48 hours) was evaluated by trypan blue exclusion staining.
Immunohistochemistry and immunofluorescence
Sections from paraffin-embedded blocks of Matrigel plugs were collected onto poly-lysine–coated slides. Endogenous peroxidase activity was blocked with 6% H2O2 for 8 minutes at room temperature. To detect cells labeled with fluorescent dye CSFE, anti–Fluorescein/Oregon Green polyclonal Abs (Molecular Probes) were applied to slides overnight at 4°C. Horseradish peroxidase–labeled antirabbit Envision polymer (DakoCytomation, Carpinteria, CA) was incubated for 30 minutes. The reaction product was developed using 3,3-diaminobenzidine. Omission of the primary Ab or substitution with an unrelated rabbit serum IgG served as negative control. The percentage of positive cells was counted in 4 nonsequential sections for each experiment at × 100 magnification. For immunofluorescence assay, specimens from normal vessels, atherosclerotic plaques, or Matrigel plugs, fixed and embedded in paraffin, were stained with antibodies against CD31 and mbKitL and processed as previously described.16 Images were acquired with a Zeiss LSM 5 Pascal confocal laser-scanning microscope (Carl Zeiss, Jena, Germany) equipped with a helium/neon laser (543 mm), an argon laser (450-530 mm), and an EC planar Neofluar 40×/1.3 oil-immersion DIC objective lens. Images were analyzed using Zeiss LSM 5 version 3.2 software.
ELISA assay
Cell cultures in serum-free media from untreated or treated CDC-HMEC-1 cells or from fibroblasts were collected and centrifuged to remove cell debris. Soluble KitL levels were measured in full-strength conditioned media using a colorimetric sandwich enzyme immunoassay (Quantikine ELISA kit; R&D Systems) as described by the manufacturer's instructions.
Statistical analysis
In vitro and in vivo results are representative of at least 3 independent experiments. The in vitro experiments were performed in triplicate. Densitometric analysis using a Bio-Rad GS 250 molecular imager (Hercules, CA) was used to calculate the differences in the fold induction of mRNA expression protein activation or expression (both * and § indicate P < .05, statistically significant between experimental and control values; data not shown). Significance of differences between experimental and control values (including adhesion assays) was calculated using analysis of variance with Newman-Keuls multicomparison test. Similar statistical analysis was performed in the in vivo experiments.
Results
mbKitL expression depends on endothelial cell activation
When exposed to inflammatory stimuli, macrovascular endothelial cells produce the soluble KitL.7 To evaluate the effects of inflammation on mbKitL expression, FACS analysis was performed on microvascular endothelial cells, CDC-HMEC-1.28 We found that, unlike fibroblasts, CDC-HMEC-1 cells did not constitutively express the mbKitL (Figure 1A), however, when exposed to inflammatory mediators such as LPS, IL-1β, and TNFα both the mbKitL transcript (Figure 1B) and the protein (Figure 1C) were clearly detectable. Moreover, as shown in Figure S1A (available on the Blood website; see the Supplemental Materials link at the top of the online article), the expression of the mbKitL was temporally related to that of E-selectin and ICAM 1
Endothelial cell activation is required for mbKitL expression. (A) Fibroblasts and CDC-HMEC-1 cells were analyzed for the expression of the mbKitL by FACS analysis. Dark lines indicate preimmune mouse IgG used as negative control; light lights, mbKitL expression. (B) RQ-PCR was performed on CDC-HMEC-1 cells treated with LPS, IL-1β, and TNFα for the indicated times. Fibroblasts were used as positive control (+). White lines indicate where irrelevant lanes of the gels have been deleted. (C) CDC-HMEC-1 cells treated with LPS (6 hours), IL-1β, and TNFα (18 hours) were analyzed for the expression of the mbKitL, E-selectin, and ICAM 1 by FACS analysis. Light lines indicate preimmune mouse IgG used as negative control; dark lines, mbKitL, E-selectin, or ICAM 1 expression. Three different experiments were performed with similar results.
Endothelial cell activation is required for mbKitL expression. (A) Fibroblasts and CDC-HMEC-1 cells were analyzed for the expression of the mbKitL by FACS analysis. Dark lines indicate preimmune mouse IgG used as negative control; light lights, mbKitL expression. (B) RQ-PCR was performed on CDC-HMEC-1 cells treated with LPS, IL-1β, and TNFα for the indicated times. Fibroblasts were used as positive control (+). White lines indicate where irrelevant lanes of the gels have been deleted. (C) CDC-HMEC-1 cells treated with LPS (6 hours), IL-1β, and TNFα (18 hours) were analyzed for the expression of the mbKitL, E-selectin, and ICAM 1 by FACS analysis. Light lines indicate preimmune mouse IgG used as negative control; dark lines, mbKitL, E-selectin, or ICAM 1 expression. Three different experiments were performed with similar results.
Endothelial cell activation is required for adhesion of EPCs
KitL consists of 2 spliced variants: the soluble and the membrane-bound KitL (mbKitL).5 While a motogenic/survival effect has been recognized for the soluble KitL,6 a specific biologic function for the mbKitL is not clearly defined. Our finding that, upon activation, microvascular endothelial cells expressed the mbKitL suggested the possibility that, behaving as a membrane-bound/adhesion molecule for c-Kit, the mbKitL could contribute to EPC recruitment. To validate this hypothesis, we first demonstrated that ex vivo–expanded EPCs, bearing VE-cadherin– and CD146-specific differentiation markers (Figure 2A), expressed c-Kit (Figure 2B). Static adhesion was then performed on untreated or TNFα-treated CDC-HMEC-1 cells. Time course experiments demonstrated that the number of EPCs adherent to activated microvascular cells started to significantly increase after 20-minute incubation and reached the plateau after 4 hours (Figure S1B). Therefore, cell adhesion was routinely evaluated after 4-hour incubation. Evaluation of adhesion by immunoscanning electron microscopy was thus performed. Data reported in Figure 2C show that EPCs were able to adhere only when CDC-HMEC-1 cells were activated. To assess the role of c-Kit/mbKitL interaction in regulating adhesion, CDC-HMEC-1 cells were preincubated with an anti-KitL antibody and EPCs with 2 different anti–c-Kit monoclonal antibodies, respectively. The results reported in Figure 2D demonstrated that the blockade of the mbKitL as well as of c-Kit with an anti–c-Kit monoclonal antibody (K44),15 raised against the KitL binding domain, prevented EPC adhesion. In contrast, EPC adhesion was not affected by the K45 monoclonal antibody,15 an anti–c-Kit antibody that does not recognize the KitL-binding domain. Similar results were obtained when adhesion was assessed after 30-minute incubation (Figure S1B). Likewise, immunogold-labeled EPCs failed to adhere to activated microvascular cells when preincubated with K44 (Figure 2C). CDC-HMEC-1 cells treated with different stimuli were analyzed for the presence of soluble KitL. The results reported in Figure S2 demonstrated that IL-1β, LPS, and TNFα were able to stimulate the release of both the soluble KitL (Figure S2A) and the mbKitL (Figure S2B) from microvascular endothelial cells. To exclude the possibility that adhesion of EPCs to the activated endothelium depended on the release of the soluble KitL, an ELISA assay was performed in the same experimental conditions used for adhesion experiments. As shown in Figure S2C, no soluble KitL was detected in the supernatants of microvascular endothelial cells after cytokine deprivation. On the contrary, CDC-HMEC-1 cells still retained mbKitL expression upon TNFα deprivation (Figure S2D). Similar results were obtained with IL-1β or LPS deprivation (data not shown). To further confirm the role of c-Kit in mediating adhesion, the ability of EPCs to bind to immobilized rhKitL was investigated. Figure 2E shows that EPCs were able to adhere to immobilized rhKitL and that K44, but not K45, prevented this event.
c-Kit/mbKitL interaction mediates EPC adhesion. (A) The indicated markers were analyzed on EPCs by FACS. Left curves indicate preimmune mouse IgG used as negative control; right curves, CD146 or VE-cadherin expression. (B) EPC extracts were subjected to SDS-PAGE and the filter was immunoblotted (IB) with an anti–c-Kit (K213) antiserum. M07e cells were used as positive control. (C) A representative scanning electron microscope micrograph of EPCs adherent to untreated or TNFα-treated CDC-HMEC-1 cells is reported. K44 was used as indicated (× 1000 magnification). Adherent cells to untreated or TNFα-treated CDC-HMEC-1 cells were also counted for statistical analysis (left panel; data are the mean ± SD; *P < .05 untreated vs TNFα-treated cells). (D) EPCs, treated or not with K44 or K45, were let to adhere to activated CDC-HMEC-1 cells. The anti-KitL antibody was used to pretreat CDC-HMEC-1 cells before the adhesion assay. Adherent cells were counted for statistical analysis and data reported are the mean ± SD (*P < .05 control vs experimental groups). (E) EPCs were added to rhKitL-coated plates and adhesion was evaluated after 45 minutes of incubation. K44 or K45 was added where indicated. Adherent cells were counted and statistical analysis was performed (data are the mean ± SD; *P < .05 BSA vs experimental groups; §P < .05 rhKitL + K44 vs rhKitL). (F) Adhesion was performed on activated CDC-HMEC-1 cells preincubated with an anti–ICAM 1, an anti–E-selectin, or an anti–VCAM 1 antibody. K44 was added to EPCs where indicated. (*P < .05 control vs experimental groups; anti–ICAM 1 + K44 vs anti–ICAM 1; anti–E-selectin + K44 vs anti–E-selectin; anti–VCAM 1 + K44 vs anti–VCAM 1). Four different experiments were performed with similar results.
c-Kit/mbKitL interaction mediates EPC adhesion. (A) The indicated markers were analyzed on EPCs by FACS. Left curves indicate preimmune mouse IgG used as negative control; right curves, CD146 or VE-cadherin expression. (B) EPC extracts were subjected to SDS-PAGE and the filter was immunoblotted (IB) with an anti–c-Kit (K213) antiserum. M07e cells were used as positive control. (C) A representative scanning electron microscope micrograph of EPCs adherent to untreated or TNFα-treated CDC-HMEC-1 cells is reported. K44 was used as indicated (× 1000 magnification). Adherent cells to untreated or TNFα-treated CDC-HMEC-1 cells were also counted for statistical analysis (left panel; data are the mean ± SD; *P < .05 untreated vs TNFα-treated cells). (D) EPCs, treated or not with K44 or K45, were let to adhere to activated CDC-HMEC-1 cells. The anti-KitL antibody was used to pretreat CDC-HMEC-1 cells before the adhesion assay. Adherent cells were counted for statistical analysis and data reported are the mean ± SD (*P < .05 control vs experimental groups). (E) EPCs were added to rhKitL-coated plates and adhesion was evaluated after 45 minutes of incubation. K44 or K45 was added where indicated. Adherent cells were counted and statistical analysis was performed (data are the mean ± SD; *P < .05 BSA vs experimental groups; §P < .05 rhKitL + K44 vs rhKitL). (F) Adhesion was performed on activated CDC-HMEC-1 cells preincubated with an anti–ICAM 1, an anti–E-selectin, or an anti–VCAM 1 antibody. K44 was added to EPCs where indicated. (*P < .05 control vs experimental groups; anti–ICAM 1 + K44 vs anti–ICAM 1; anti–E-selectin + K44 vs anti–E-selectin; anti–VCAM 1 + K44 vs anti–VCAM 1). Four different experiments were performed with similar results.
It has been reported that E-selectin and ICAM 1 by β2 integrin regulate EPC homing.11,13 Moreover, VCAM 1 expression has been reported to promote homing of EPCs by ischemic tissues.29 Therefore in selected experiments an anti–ICAM 1, an anti–E-selectin, and an anti–VCAM 1 antibody, alone or in combination with K44, were used. As depicted in Figure 2F, anti–ICAM 1, anti–VCAM 1, and anti–E-selectin were able to significantly reduce the number of EPCs adherent to activated CDC-HMEC-1 cells. However, full inhibition of cell adhesion was achieved only when K44 was used in combination with anti–ICAM 1, anti–VCAM 1, or anti–E-selectin antibodies, suggesting that these adhesion molecules cooperate with c-Kit to stabilize adhesion.
c-Kit mediates EPC adhesion
To confirm the role of c-Kit in mediating EPC adhesion, EPCs were endogenously depleted of c-Kit by siRNA (K1) (Figure 3A). As shown in Figure 3B, adhesion of EPCs was completely prevented when the cells were transfected with the c-Kit siRNA. By contrast, no changes in EPC adhesion could be detected when scramble siRNA was used. To evaluate whether c-Kit enzymatic activity was required for adhesion, EPCs were first incubated with different concentrations of the chemotherapeutic drug imatinib mesylate, an inhibitor of c-Kit kinase activity.22 The results reported in Figure 3C demonstrated that 10 μM imatinib mesylate fully abrogated the ability of the soluble KitL to induce c-Kit tyrosine phosphorylation. Similarly 10 μM imatinib mesylate was able to prevent EPC adhesion (Figure 3D), indicating that adhesion strictly depended on c-Kit functional activity. The inhibitory effect of imatinib mesylate and K44 was also evaluated on fibroblasts. To this end, EPCs treated with imatinib mesylate or K44 were let to adhere to fibroblasts expressing the mbKitL but not c-Kit (Figure 3E). Consistent with the results obtained with microvascular cells, adhesion to fibroblasts was abrogated by both K44 and imatinib mesylate (Figure 3F). These data further sustain the role of the mbKitL in mediating EPC adhesion.
Depletion of endogenous c-Kit inhibits EPC adhesion. (A) EPCs were transfected with c-Kit siRNA (K1) or with the scrambled sequence (scramble) and lysed. The filter was IB with an anti–c-Kit and an anti–β-actin antibody. (B) Adhesion was performed with EPCs transfected with the scramble or the K1 siRNA (48h). (C) EPCs pretreated with 5 or 10 μM imatinib mesylate were untreated or treated with the soluble KitL (10 ng/mL). The filter was IB with an anti–p–c-Kit and an anti–c-Kit antibody. M07e cells were used as positive control (+). (D) Adhesion was performed with EPCs untreated or treated with 5 or 10 μM imatinib mesylate or with a neutralizing anti-PDGFRβ antibody (30 μg/mL).23 Adherent cells were counted for statistical analysis (data in B and D are the mean ± SD; *P < .05 control vs experimental groups). (E) Fibroblasts were analyzed for c-Kit and mbKitL expression by FACS analysis. Light lines indicate preimmune mouse IgG used as a negative control; dark lines, c-Kit or mbKitL expression. (F) Adhesion was performed on fibroblasts with EPCs pretreated with 5 or 10 μM imatinib mesylate or K44. Adherent cells were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). Four different experiments were performed with similar results.
Depletion of endogenous c-Kit inhibits EPC adhesion. (A) EPCs were transfected with c-Kit siRNA (K1) or with the scrambled sequence (scramble) and lysed. The filter was IB with an anti–c-Kit and an anti–β-actin antibody. (B) Adhesion was performed with EPCs transfected with the scramble or the K1 siRNA (48h). (C) EPCs pretreated with 5 or 10 μM imatinib mesylate were untreated or treated with the soluble KitL (10 ng/mL). The filter was IB with an anti–p–c-Kit and an anti–c-Kit antibody. M07e cells were used as positive control (+). (D) Adhesion was performed with EPCs untreated or treated with 5 or 10 μM imatinib mesylate or with a neutralizing anti-PDGFRβ antibody (30 μg/mL).23 Adherent cells were counted for statistical analysis (data in B and D are the mean ± SD; *P < .05 control vs experimental groups). (E) Fibroblasts were analyzed for c-Kit and mbKitL expression by FACS analysis. Light lines indicate preimmune mouse IgG used as a negative control; dark lines, c-Kit or mbKitL expression. (F) Adhesion was performed on fibroblasts with EPCs pretreated with 5 or 10 μM imatinib mesylate or K44. Adherent cells were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). Four different experiments were performed with similar results.
PDGFRβ has been recently shown to contribute to neovascularization by regulating stem cell differentiation.23 It is known that imatinib mesylate prevents both c-Kit and PDGFR kinase activity.22 Our in vitro experiments demonstrated that PDGFRβ blockade (Figure 3D) did not affect EPC adhesion to inflamed endothelium, ruling out the possibility that the effect of imatinib mesylate depended on inhibition of PDGFR kinase activity.
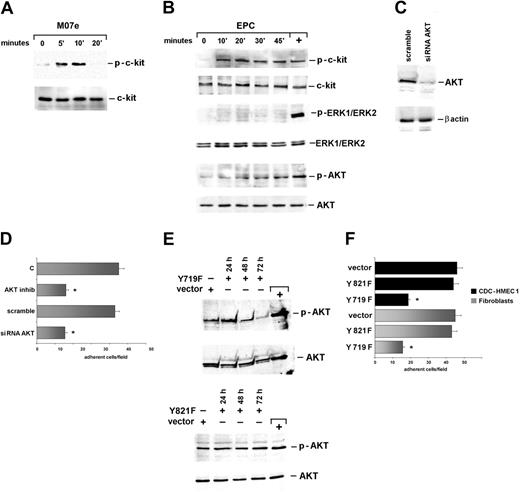
EPC adhesion occurs via Akt-mediated signaling pathway
c-Kit engagement by its soluble ligand triggers Erk1/2 and Akt signaling pathways that modulate macrovascular cell survival and migration.9 To assess the role of these signaling pathways in c-Kit/mbKitL-mediated adhesion, EPCs were seeded for different times on fibroblasts and evaluated for c-Kit activation. Parallel experiments were performed using the M07e cells challenged with soluble KitL. Time course experiments depicted in Figure 4 demonstrated that, unlike the soluble KitL (Figure 4A), the mbKitL induced a persistent activation of c-Kit (Figure 4B). Moreover, when cell extracts from EPCs adherent to fibroblasts were evaluated for Erk1/2 MAPK and Akt activation only Akt was found activated (Figure 4B), indicating that the soluble and the membrane-bound KitL elicited different downstream signaling events. The role of Akt in regulating EPC adhesion was confirmed by the finding that no Akt activation could be detected when EPCs were depleted of endogenous c-Kit (Figure S3). To assess the role of Akt in c-Kit–mediated cell adhesion, different strategies were adopted. Indeed, both Akt kinase inhibition (Figure 4D) and endogenous Akt depletion (Figure 4C) were able to prevent EPC adhesion (Figure 4D). In addition, in EPCs expressing the Y719F c-Kit mutant, unable to bind and activate PI3 kinase, we failed to detect Akt activation (Figure 4E) and adhesion of EPCs to both fibroblasts and activated CDC-HMEC-1 cells (Figure 4F). Consistently, Akt activation and adhesion occurred in EPCs expressing the Y821F c-Kit mutant (Figure 4E-F). Hence these data, together with the observation that depletion of endogenous Akt in fibroblasts did not affect EPC adhesion (Figure S4), demonstrated that the integrity of EPC signaling machinery is required for adhesion to occur and identify Akt as the major signaling event regulating c-Kit/mbKitL–mediated adhesion.
Akt mediates EPC adhesion. (A) M07e cells stimulated with the KitL were lysed. (B) EPCs let to adhere to fibroblasts were lysed. Cell extracts from M07e cells (A) and from EPCs adherent to fibroblasts (B) were analyzed with anti–p–c-Kit, anti–c-Kit, anti–p-ERK1/ERK2 MAPK, anti-ERK1/ERK2, anti–p-Akt, or anti-Akt antibodies as indicated. (C) EPCs transfected with an Akt siRNA or with the scrambled sequence (scramble) were lysed. The filter was IB with an anti-Akt and anti–β-actin antibody. (D) Adhesion on fibroblasts was performed using EPCs depleted or not of the endogenous Akt. Experiments were also performed by preincubating EPCs with the Akt kinase inhibitor. Adherent cells were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). (E) EPCs were transfected with the c-Kit mutants or the empty vector. After 24, 48, and 72 hours of transfection, EPCs were let to adhere to fibroblasts for 30 minutes and lysed. The filters were IB with an anti–p-Akt and an anti-Akt antibody. M07e cells were used as positive control (+). (F) Fibroblasts and activated CDC-HMEC-1 cells were used for adhesion of EPCs transfected (72 hours) with the Y821F and Y719F mutants or the empty vector. Adherent cells were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). Five different experiments were performed with similar results.
Akt mediates EPC adhesion. (A) M07e cells stimulated with the KitL were lysed. (B) EPCs let to adhere to fibroblasts were lysed. Cell extracts from M07e cells (A) and from EPCs adherent to fibroblasts (B) were analyzed with anti–p–c-Kit, anti–c-Kit, anti–p-ERK1/ERK2 MAPK, anti-ERK1/ERK2, anti–p-Akt, or anti-Akt antibodies as indicated. (C) EPCs transfected with an Akt siRNA or with the scrambled sequence (scramble) were lysed. The filter was IB with an anti-Akt and anti–β-actin antibody. (D) Adhesion on fibroblasts was performed using EPCs depleted or not of the endogenous Akt. Experiments were also performed by preincubating EPCs with the Akt kinase inhibitor. Adherent cells were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). (E) EPCs were transfected with the c-Kit mutants or the empty vector. After 24, 48, and 72 hours of transfection, EPCs were let to adhere to fibroblasts for 30 minutes and lysed. The filters were IB with an anti–p-Akt and an anti-Akt antibody. M07e cells were used as positive control (+). (F) Fibroblasts and activated CDC-HMEC-1 cells were used for adhesion of EPCs transfected (72 hours) with the Y821F and Y719F mutants or the empty vector. Adherent cells were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). Five different experiments were performed with similar results.
Depletion of endogenous c-Kit prevents EPC recruitment to neoformed vessels
It has been shown that TECs are able to form vascular structures in SCID mice.20 Thus, to confirm the role of c-Kit in mediating EPC recruitment in vivo, TECs were transfected with the mbKitL (Figure 5A) and used as a model of inflamed endothelium. As shown in Figure 5B, adhesion of EPCs to TECs was strongly enhanced by the expression of mbKitL, and this event was abolished by the presence of K44 or the anti-KitL antiserum, but not by K45. For in vivo experiments, 2 × 106 TECs constitutively expressing the mbKitL were added to Matrigel and implanted subcutaneously into SCID mice. After 5 days, untransfected or transfected (c-Kit siRNA) EPCs were injected intravenously through the tail vein. In selected experiments, EPCs pretreated with imatinib mesylate were also injected. The results reported in Figure 5C demonstrated that untreated EPCs (Figure 5Ci-ii) or EPCs transfected with the scramble siRNA (data not shown) were recruited to vascular structures. On the contrary, depletion of endogenous c-Kit (Figure 5Ciii-iv) or imatinib mesylate pretreatment (Figure 5Cv-vi) dramatically reduced EPC recruitment to neoformed vessels (8% ± 0.7% vs 0.7% ± 0.04% and 0.9% ± 0.08%, respectively). No significant reduction of vascular density was observed in the different experimental conditions (Figure 5D: vessel area/total Matrigel area) or by immunostaining for CD31 (control: 6.1 ± 1.2; K1: 5.7 ± 1.3; imatinib mesylate: 5.8 ± 1.5 per 200 × field). To exclude the possibility that inactivation or depletion of c-Kit could affect cell viability, in vitro experiments were performed. To this end, in selected experiments the number of viable EPCs was evaluated following 24 and 48 hours of c-Kit siRNA transfection or imatinib mesylate treatment (control: 93% ± 3%; K1: 91% ± 2%; imatinib mesylate: 90% ± 2% cell viability/after 48 hours of treatment). These results demonstrated that more than 88% of EPCs were still viable after imatinib mesylate treatment or c-Kit depletion. The presence of mbKitL-expressing TECs was confirmed by RQ-PCR on digested Matrigel plugs (Figure 5E). These data, together with the finding that less than 1% of EPCs could be detected when wild-type TECs (not expressing the mbKitL) were used for the in vivo experiments (data not shown), indicated that in our experimental conditions a functional c-Kit/mbKitL interaction was required for EPC recruitment.
Depletion of endogenous c-Kit prevents in vivo EPC recruitment to neoformed vessels. (A) TECs transfected with the empty vector (left panel) or with the mbKitL construct (right panel) were analyzed by FACS. Light lines indicate preimmune mouse IgG used as negative control; dark lines, mbKitL expression. (B) Fluorescent EPCs were let to adhere to TECs, transfected with the empty vector or with the mbKitL construct. EPCs or transfected TECs were pretreated with K44, K45 (EPC), or anti-KitL (TEC) as indicated. Adherent EPCs were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). (C) Sections of Matrigel plugs from SCID mice injected with untreated EPCs (i-ii), EPCs depleted of c-Kit (iii-iv), or EPCs treated with imatinib mesylate (v-vi) were analyzed by immunohistochemistry. To detect CSFE-positive EPCs, anti–Fluorescein/Oregon Green polyclonal Abs and horseradish peroxidase–labeled antirabbit were used as described in “Materials and methods.” Black arrows indicate vessels; red arrows indicate some positive EPCs. Magnification × 200 (i,iii,v); × 400 (ii,iv,vi). (D) The quantification of neovascularization was performed on hematoxylin-eosin–stained sections, and the results were expressed as percentage ± SD of the vessel area to the total Matrigel area. Five different sections from each individual experimental group (4 mice per group) were analyzed. (E) Matrigel plugs from different experimental groups (lane 1: untreated EPCs; lane 2: c-Kit–depleted EPCs; lane 3: imatinib mesylate–treated EPCs) were subjected to RNA extraction, and RQ-PCR was performed to assess the expression of the mbKitL on injected TECs. Fibroblasts were used as positive control (+). Three groups of mice (each of 4 mice) were used for the in vivo experiments.
Depletion of endogenous c-Kit prevents in vivo EPC recruitment to neoformed vessels. (A) TECs transfected with the empty vector (left panel) or with the mbKitL construct (right panel) were analyzed by FACS. Light lines indicate preimmune mouse IgG used as negative control; dark lines, mbKitL expression. (B) Fluorescent EPCs were let to adhere to TECs, transfected with the empty vector or with the mbKitL construct. EPCs or transfected TECs were pretreated with K44, K45 (EPC), or anti-KitL (TEC) as indicated. Adherent EPCs were counted for statistical analysis (data are the mean ± SD; *P < .05 control vs experimental groups). (C) Sections of Matrigel plugs from SCID mice injected with untreated EPCs (i-ii), EPCs depleted of c-Kit (iii-iv), or EPCs treated with imatinib mesylate (v-vi) were analyzed by immunohistochemistry. To detect CSFE-positive EPCs, anti–Fluorescein/Oregon Green polyclonal Abs and horseradish peroxidase–labeled antirabbit were used as described in “Materials and methods.” Black arrows indicate vessels; red arrows indicate some positive EPCs. Magnification × 200 (i,iii,v); × 400 (ii,iv,vi). (D) The quantification of neovascularization was performed on hematoxylin-eosin–stained sections, and the results were expressed as percentage ± SD of the vessel area to the total Matrigel area. Five different sections from each individual experimental group (4 mice per group) were analyzed. (E) Matrigel plugs from different experimental groups (lane 1: untreated EPCs; lane 2: c-Kit–depleted EPCs; lane 3: imatinib mesylate–treated EPCs) were subjected to RNA extraction, and RQ-PCR was performed to assess the expression of the mbKitL on injected TECs. Fibroblasts were used as positive control (+). Three groups of mice (each of 4 mice) were used for the in vivo experiments.
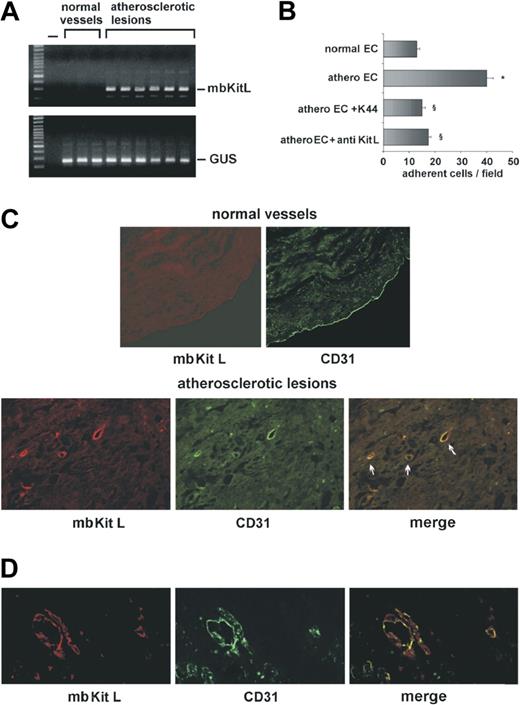
The mbKitL is expressed on endothelial cells lining the atherosclerotic plaques
To investigate the role of the mbKitL in human diseases, ECs were separated from atherosclerotic or nonatherosclerotic vessels and analyzed by real-time PCR. As shown in Figure 6A, unlike ECs from nonatherosclerotic arteries, ECs from atherosclerotic vessels expressed the mbKitL. On the contrary, we failed to detect the mbKitL in smooth muscle cells both in normal vessels and atherosclerotic lesions (data not shown). The role of c-Kit/mbKitL interaction in EPC recruitment was evaluated by adhesion experiments performed on ECs from normal vessels or from atherosclerotic plaques. Figure 6B shows that EPCs adhered to only ECs derived from atherosclerotic plaques and that both K44 and anti-KitL antibodies were able to prevent their adhesion. Consistently, immunoreactivity for the mbKitL could be detected in neointima vessels but not in ECs lining normal vessels (Figure 6C). TNFα and IL-1β are abundantly present in plaque microenvironment.30 The potential in vivo role of such inflammatory stimuli in supporting this event is sustained by the observation that neovessels formed in response to TNFα (Figure 6D) and IL-1β (data not shown) expressed the mbKitL.
The mbKitL is expressed in vivo by inflamed endothelium and by neointima vessels. (A) RQ-PCR was performed on ECs derived from normal vessels or from different grade of atherosclerotic plaques. GUS gene was used to normalize the samples. (B) EPCs were let to adhere to ECs derived from normal or atherosclerotic vessels. In selected experiments, ECs from atherosclerotic plaques were pretreated with anti-KitL or EPCs were pretreated with K44. Adherent cells were counted and statistical analysis was performed (data are the mean ± SD; *P < .05 control vs experimental group: §P < .05 ECs from atherosclerotic vessels + anti-KitL or + K44 vs ECs from atherosclerotic vessels). (C) Specimens from normal vessels or atherosclerotic lesions were double immunostained with an anti-KitL or an anti-CD31 as primary antibody. TRITC- or FITC-conjugated goat anti–mouse IgG was used as secondary antibody, respectively. Colocalization of mbKitL and CD31 is represented in merge. Arrows indicated atherosclerotic vessels. Similar results were obtained with 6 different samples. (D) Representative immunofluorescence microscopy of Matrigel plugs containing human microvascular endothelial cells and TNFα (20 ng/mL) is reported. An anti-KitL or an anti-CD31 antibody was used as primary antibody. TRITC- or FITC-conjugated IgG was used as secondary antibody, respectively. Colocalization of mbKitL and CD31 in neoformed vessels is reported in merge. Four different sections per mice (4 mice) were analyzed.
The mbKitL is expressed in vivo by inflamed endothelium and by neointima vessels. (A) RQ-PCR was performed on ECs derived from normal vessels or from different grade of atherosclerotic plaques. GUS gene was used to normalize the samples. (B) EPCs were let to adhere to ECs derived from normal or atherosclerotic vessels. In selected experiments, ECs from atherosclerotic plaques were pretreated with anti-KitL or EPCs were pretreated with K44. Adherent cells were counted and statistical analysis was performed (data are the mean ± SD; *P < .05 control vs experimental group: §P < .05 ECs from atherosclerotic vessels + anti-KitL or + K44 vs ECs from atherosclerotic vessels). (C) Specimens from normal vessels or atherosclerotic lesions were double immunostained with an anti-KitL or an anti-CD31 as primary antibody. TRITC- or FITC-conjugated goat anti–mouse IgG was used as secondary antibody, respectively. Colocalization of mbKitL and CD31 is represented in merge. Arrows indicated atherosclerotic vessels. Similar results were obtained with 6 different samples. (D) Representative immunofluorescence microscopy of Matrigel plugs containing human microvascular endothelial cells and TNFα (20 ng/mL) is reported. An anti-KitL or an anti-CD31 antibody was used as primary antibody. TRITC- or FITC-conjugated IgG was used as secondary antibody, respectively. Colocalization of mbKitL and CD31 in neoformed vessels is reported in merge. Four different sections per mice (4 mice) were analyzed.
Discussion
The conversion of the KitL from a membrane-bound adhesion/survival-promoting molecule to a soluble survival/motogenic factor has been reported.6 Our results provide evidence on the role of c-Kit and of the membrane-bound form of the ligand in EPC recruitment to activated endothelium. Moreover, in vivo experiments indicate that in pathological settings this mechanism can contribute to EPC recruitment to neoformed vessels. In a condition such as atherosclerosis, intimal angiogenesis occurs as a part of the adaptive changes known as vasculature remodeling.31 It is commonly accepted that this process is accompanied by, and possibly requires, inflammation. Indeed, the complex interplay among different inflammatory cytokines and growth factors can trigger a vicious circle that sustains neointimal vasculature and contributes to plaque evolution. Inhibition of intraplaque neoangiogenesis significantly reduces plaque growth,32 and alternative targeting treatments able to prevent disease progression have been investigated. Recently, a protective role of imatinib mesylate relying on inhibition of inflammatory cytokine expression and PDGF receptor–mediated signals has been reported.33 It has been reported that EPCs may contribute to intraplaque neovascularization, a critical step in lesion development and progression.34 Herein, we show that endothelial cells lining atherosclerotic vessels express the mbKitL and that in vivo this event can be sustained by inflammatory cytokines such as TNFα and IL-1β. c-Kit targeting is a well-established therapeutic strategy in some neoplastic diseases. The results of the present study suggest that this strategy could also exert a protective role by efficiently modulating EPC recruitment and by affecting pathological angiogenesis.
Authorship
Contribution: P.D. performed biologic experiments; A.R. performed transfections; A.B. and S.C.B. performed isolation and characterization of EPCs; A.Z. performed in vivo experiments; M.P. provided surgery specimens; G.C. performed electron microscopy; L.P. served as senior advisor; and M.F.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Felice Brizzi, Department of Internal Medicine, University of Torino, Corso Dogliotti 14, 10126, Torino, Italy; e-mail: mariafelice.brizzi@unito.it.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants of the Italian Association for Cancer Research (AIRC) (M.F.B. and G.C.) and MIUR (Ministero dell'Università e Ricerca Scientifica, cofinanziamento MURST and fondi ex-60%) (M.F.B., G.C., and L.P.).
We thank Drs Kitamura and Leder for kindly providing the c-Kit mutants and the mbKitL construct, respectively. We also thank Prof Yarden for his helpful advice.