Abstract
We tested the hypothesis that oral beclomethasone dipropionate (BDP) would control gastrointestinal graft-versus-host disease (GVHD) in patients with anorexia, vomiting, and diarrhea. Patients were randomized to prednisone for 10 days and either oral BDP 8 mg/d (n = 62) or placebo (n = 67) tablets for 50 days. At study day 10, prednisone was rapidly tapered while continuing study drug. On an intent-to-treat basis, the risk of GVHD-treatment failure was reduced for the BDP group at study day 50 (hazard ratio [HR] 0.63, 95% confidence interval [CI] 0.35-1.13) and at 30 days follow-up (HR 0.55, 95% CI 0.32-0.93). Among patients eligible for prednisone taper at study day 10, the risk of GVHD-treatment failure was significantly reduced at both study days 50 and 80 (HR 0.39 and 0.38, respectively). By day 200 after transplantation, 5 patients randomized to BDP had died compared with 16 deaths on placebo, a 67% reduction in the hazard of mortality (HR 0.33, P = .03). In 47 recipients of unrelated and HLA-mismatched stem cells, mortality at transplantation day 200 was reduced by 91% in the BDP group compared with placebo (HR 0.09, P = .02). The survival benefit was durable to 1 year after randomization. Oral BDP prevents relapses of gastrointestinal GVHD following tapering of prednisone; survival is statistically significantly better among patients receiving BDP.
Introduction
Gastrointestinal graft-versus-host disease (GVHD) affects up to 60% of patients after allogeneic hematopoietic cell transplantation.1 Intestinal GVHD involves release of cytokines within the mucosa, infiltration of donor T lymphocytes, and crypt-cell apoptosis.2,3 Clinical manifestations include anorexia, nausea, vomiting, diarrhea, and, in patients with severe involvement, fever, cholestasis, protein-losing enteropathy, and sloughing of mucosa.4–8 In animal studies, the extent of intestinal involvement and T-cell activation in Peyer patches are determinants of survival.2,9 Initial treatment is with prednisone 1 to 2 mg/kg/d, followed by a prednisone tapering schedule to prevent GVHD relapses and allow recovery of the hypothalamic-pituitary-adrenal axis.4 Another approach to GVHD treatment involves a 5-day course of prednisolone at 2 mg/kg/d; the response after 5 days is prognosis determining.10 Prednisone-related side effects are common, particularly fatal infections, weakness, hyperglycemia, hypertension, osteopenia, and psychiatric symptoms
We tested the hypothesis that a topically active corticosteroid (beclomethasone dipropionate [BDP, or Bec]), taken orally, allows rapid tapering of prednisone while maintaining control of intestinal GVHD. The results show that a 50-day course of treatment with oral BDP reduces the frequency of relapses of GVHD following accelerated withdrawal of prednisone therapy and results in better survival at transplantation day 200 and at 1 year after randomization compared with placebo. Because of this survival benefit, we also examined outcomes from a previous randomized trial of a shorter 30-day course of oral BDP.11
Patients, materials, and methods
Patient selection
Patients with symptoms of GVHD were evaluated with endoscopy and mucosal biopsy. If biopsy specimens demonstrated histologic findings of GVHD12,13 and stool and mucosal biopsy cultures were negative for pathogens,14,15 patients were invited to participate. Patients were excluded if diarrhea exceeded 1 L/d or if skin or liver GVHD were present. All patients received medications for GVHD prophylaxis; patients receiving corticosteroids within 30 days of study entry were excluded. Patients signed informed consent documents, in accordance with the Declaration of Helsinki, approved by the institutional review boards of all participating institutions.
Formulation of BDP
Both immediate-release tablets and enteric-coated tablets contained 1 mg of BDP (DOR BioPharma, Miami, FL). The dosing regimen was 1 immediate-release and 1 enteric-coated tablet taken orally, 4 times daily (total daily dose, 8 mg BDP).
Stratification and randomization
A blocked stratified allocation scheme was used to balance the treatment groups within study centers (Table 1). Additional stratifying variables were HLA-matched sibling and use of cutaneous corticosteroids at baseline. Patients were randomized to receive either BDP or identical placebo tablets in a 1:1 allocation.
Treatment plan
Therapy consisted of study drugs plus 10 days of prednisone. The initial 16 patients were administered prednisone 2 mg/kg/d for 10 days; the remaining 113 patients received an initial prednisone dose of 1 mg/kg/d after hypothalamic-pituitary-adrenal axis suppression was observed at the higher dose at the time of study day–50 testing. In patients with control of GVHD symptoms at study day 10, prednisone was tapered over 7 days (0.25 mg/kg twice daily on study days 11 and 12; 0.125 mg/kg twice daily on study days 13 and 14; 0.0625 mg/kg twice daily on study days 15 and 16), after which patients were maintained on physiologic replacement doses of prednisone (0.0625 mg/kg daily). Patients who did not demonstrate adequate control of GVHD by study day 10 were considered treatment failures. Patients received study drug for 50 days, until they met the treatment-failure end point, or until they were withdrawn from the study. Patients who were declared treatment failures had study drug discontinued; subsequent treatment for GVHD was dictated by their physicians.
Definitions of treatment failure and efficacy end points
Treatment failure was a worsening or recurrence of GVHD that required additional immunosuppressive therapy. The primary efficacy end point was the time to treatment failure through study day 50. Prospectively defined secondary efficacy end points included time to treatment failure 30 days after discontinuation of study drug and survival at day 200 after transplantation. Survival at 1 year after randomization was evaluated in a retrospective manner.
Evaluation of drug safety and adverse events
Safety assessment was based on cumulative prednisone exposure, the incidence and degree of hypothalamic-pituitary-adrenal axis suppression, and rates of adverse events.
Statistical methods
A total sample size of approximately 130 patients (65 in each group) with 48 treatment-failure events was determined to provide 80% power for a comparison of time to treatment failure between treatment groups using the log-rank test.16 The sample-size calculation was based on a 2-sided significance level of 0.05 and assumed treatment failure rates at study day 50 of 0.30 and 0.55 for the BDP and placebo groups, respectively. These assumptions were predicated upon the results of a previous randomized trial of BDP in the same patient population.11 The study was planned to enroll approximately 130 patients to compensate for loss to follow-up for the secondary end points and to provide adequate safety data. Analysis of the time to treatment failure and survival end points includes all randomized patients. The primary analysis of time to treatment failure and survival after randomization was based on the Kaplan-Meier method and log-rank test, stratified by donor type (HLA-matched sibling vs unrelated or HLA-mismatched donor). For each end point, the hazard ratio (HR) for treatment was estimated based on a stratified Cox proportional hazards model that included a term for treatment group.16 Because of the variable length of time among patients between transplantation and randomization, randomization to BDP treatment was defined in the proportional hazards model as a time-dependent covariate for the day-200 posttransplantation survival end point.17 Under this model, the comparison between BDP and placebo was made using the Wald chi-square test. For the primary analysis, the time to treatment failure was right-censored for patients who discontinued study drug during the first 50 days without meeting the criteria for treatment failure, provided the reason for discontinuation was not related to uncontrolled GVHD or study drug–related toxicity. This determination was made in real time and prior to analysis by the study's medical monitor who was blinded to the patients' randomly assigned treatment. For each survival end point, a variety of multivariate models was used to determine if the reduced mortality risk attributed to BDP in the univariate model was still present after accounting for subject-, disease-, and transplantation-related factors. Hypothesis tests of the primary and secondary end points were performed using a 2-sided significance level of .05. No adjustments were made to the significance level for inferential tests of the secondary end points. All patients who received at least 1 dose of BDP or placebo were included in the assessment of safety. In addition to the primary analysis of the treatment failure and survival end points, an exploratory analysis was performed to assess the impact of the 12 patients who experienced treatment failure shortly after randomization (ie, during the first 10 days of protocol treatment during which oral BDP is unlikely to affect initial responses to concomitant high-dose corticosteroids). Patients who experienced treatment failure during this period were right-censored at the time of early treatment failure. Analyses were performed using SAS (version 8.2; SAS Institute, Cary, NC), R (freeware; version 2.0.1), and S-Plus (version 6.2; Insightful, Seattle, WA) software.18,19
Results
Patient demographics
Between July 2001 and July 2004, 129 patients were enrolled. With the exception of the transplant conditioning regimen (myeloablative or nonmyeloablative), no major imbalances were noted between the treatment groups for baseline transplantation-related characteristics. The percentage of patients who received a nonmyeloablative conditioning regimen was approximately 2-fold higher in the BDP group compared with placebo (Table 1).
Analysis of treatment efficacy
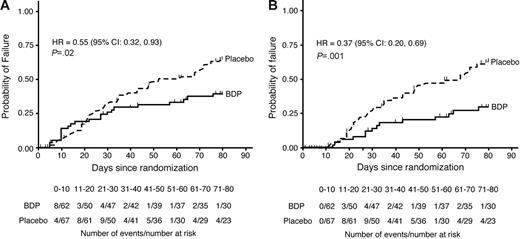
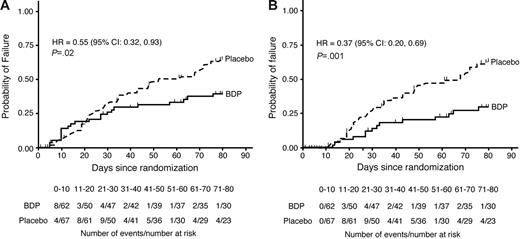
By study day 50, there were 30 GVHD-treatment failures in the placebo group and 18 in the BDP group (Figure 1). Fourteen patients (7 in each group) discontinued from study drug early for reasons not related to uncontrolled GVHD or study drug–related toxicity and were right-censored on the day of their last dose of study drug. The cumulative rate of GVHD-treatment failure was 31% for BDP versus 48% for placebo; the hazard of treatment failure was reduced in the BDP group relative to placebo, although not statistically significantly so (HR 0.63; 95% CI, 0.35-1.13; P = .12, stratified log-rank test; Figure 1A).
Time to GVHD-treatment failure through study day 80 with hazard ratios and confidence intervals. Panel A shows product-limit estimates on an intent-to-treat basis; panel B shows estimates with a guarantee period for the first 10 days of treatment. Estimates are based on the Kaplan-Meier method. The hazard ratios at study day 50 were (A) 0.63 (95% CI, 0.35-1.13; P = .12) and (B) 0.39 (95% CI, 0.19-0.81; P = .009), respectively.
Time to GVHD-treatment failure through study day 80 with hazard ratios and confidence intervals. Panel A shows product-limit estimates on an intent-to-treat basis; panel B shows estimates with a guarantee period for the first 10 days of treatment. Estimates are based on the Kaplan-Meier method. The hazard ratios at study day 50 were (A) 0.63 (95% CI, 0.35-1.13; P = .12) and (B) 0.39 (95% CI, 0.19-0.81; P = .009), respectively.
The majority of patients with GVHD-treatment failure had recurrent gastrointestinal symptoms: 3 patients had gastrointestinal and skin GVHD, 6 had skin GVHD, 2 had liver GVHD, and 1 had bronchiolitis obliterans–organizing pneumonia. GVHD-treatment failure occurred during the first 10 days of prednisone treatment in 12 of the 48 patients with GVHD-treatment failure by study day 50 (8 BDP, 4 placebo). The impact of early treatment failures on the primary end point was assessed by designating the first 10 days of treatment as a guarantee period (see “Patients, materials, and methods,” “Statistical methods”). Patients with treatment failure during the guarantee period were right-censored on the day of GVHD-treatment failure. For this analysis, the risk of GVHD-treatment failure by study day 50 was statistically significantly reduced for the BDP group relative to placebo (HR 0.39; 95% CI, 0.19-0.81; P = .009, stratified log-rank test; Figure 1B).
By study day 80, 30 days after discontinuation of study drug, a total of 22 patients in the BDP group and 39 patients in the placebo group were GVHD-treatment failures. Six patients (4 BDP, 2 placebo) withdrew from study during the 30-day posttreatment observation period who did not experience treatment failure prior to their withdrawal. Based on blinded review, the reason for withdrawal was classified as unrelated to uncontrolled GVHD or study drug–related toxicities. The time to treatment failure was right-censored for the 6 patients on the day of their last study visit. The cumulative treatment failure rates were 39% for BDP and 65% for placebo. For the entire 80-day study period, the risk of treatment failure was statistically significantly reduced for patients in the BDP group relative to placebo (HR 0.55; 95% CI, 0.32-0.93; P = .02, stratified log-rank test; Figure 1A). In an analysis using a 10-day guarantee period, the risk of treatment failure was statistically significantly reduced for the BDP arm relative to placebo for the 80-day study period (HR 0.37; 95% CI, 0.20-0.69; P = .001, stratified log-rank test; Figure 1B).
Survival analysis at transplantation day 200
By day 200 after transplantation, 5 patients (8%) who had been randomized to BDP had died compared with 16 deaths (24%) among patients who had been randomized to placebo (Table 2). Based on a stratified time-dependent Cox proportional hazards model, the risk of mortality during the 200-day posttransplantation period was 67% lower with BDP treatment compared with placebo treatment (HR 0.33; 95% CI, 0.12-0.89; P = .03, Wald chi-square test). The number of deaths (21) does not allow the inclusion into the regression model of more than 1 or 2 variables in addition to the treatment group variable. The only factor that was largely imbalanced between the treatment groups was the planned intensity of the transplant conditioning regimen (Table 1). Adjustment for this factor did not, however, alter the estimated hazard ratio for mortality for BPD treatment versus placebo (adjusted HR 0.33; 95% CI, 0.12-0.91; P = .03, Wald chi-square test). Moreover, the estimated treatment effect also remained generally unchanged after adjusting for various combinations of other factors (resulting in models with no more than 2 factors in addition to treatment group), including study center, patient age and sex, primary diagnosis (high relapse risk or not), source of donor cells (marrow, peripheral blood stem cells), and donor-recipient HLA match (data not shown).
The most common proximate causes of death by transplantation day 200 were relapse of the underlying malignancy and infection (Table 2). Relapse of the hematologic malignancy had contributed to the deaths of 9 (13.4%) of 67 patients in the placebo arm and 3 (4.8%) of 62 patients in the BDP arm. Infection contributed to the deaths of 9 (13.4%) of 67 patients in the placebo arm and 3 (4.8%) of 62 in the BDP arm. Acute or chronic GVHD was the proximate cause of death in 3 (4.5%) of 67 patients in the placebo arm and in 1 (1.6%) of 62 in the BDP arm.
There was a statistically significant interaction (P = .05) between donor type (HLA-matched sibling vs other donors) and use of BDP for the outcome day-200 mortality, suggesting the need for separate analyses according to donor type. Among 47 patients who had received stem cells from unrelated or HLA-mismatched donors, 1 (4%) of 23 patients who had been randomized to BDP had died compared with 10 deaths (42%) among 24 patients who had been randomized to placebo, leading to a statistically significantly reduced risk of day-200 mortality (HR 0.09; 95% CI, 0.01-0.70; P = .02, Wald chi-square test). On the other hand, among 82 patients who had received stem cells from an HLA-matched sibling, 4 (10%) of 39 patients randomized to BDP had died by day 200 compared with 6 (14%) of 43 deaths among patients randomized to placebo, leading to a lower risk of mortality but not statistically significantly so (HR 0.83; 95% CI, 0.23-2.93; P = .77, Wald chi-square test).
Survival analysis at 1 year after randomization
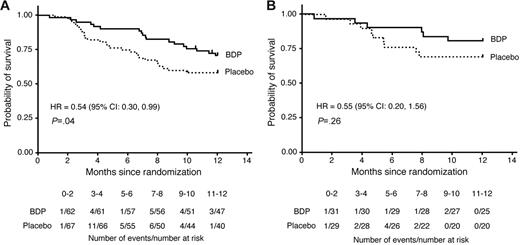
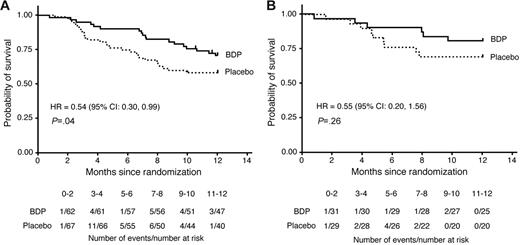
Of 129 patients randomized, 2 were lost to follow-up within 1 year of randomization (last contacted at 321 and 354 days after randomization; both patients had been randomized to BDP); in the survival analysis these patients were right-censored on the date of last contact. All other surviving patients were followed a minimum of 1 year after randomization. Overall, 28 patients (42%) in the placebo group and 18 patients (29%) in the BDP group died within 1 year of randomization (HR 0.54; 95% CI, 0.30-0.99; P = .04, stratified log-rank test; Figure 2A).
Survival of patients to 1 year after randomization to either BDP or placebo. (A) The current patient cohort (N = 129). (B) Patients from the previous randomized trial (N = 60).11
Survival of patients to 1 year after randomization to either BDP or placebo. (A) The current patient cohort (N = 129). (B) Patients from the previous randomized trial (N = 60).11
Safety and adverse events
The frequencies of severe adverse events, adverse events related to study drug, and adverse events resulting in study-drug discontinuation were all higher in the placebo group. Patients who remained on BDP until study day 50 had a higher likelihood of having biochemical evidence of abnormal HPA function compared with patients on placebo.
Survival analysis of patients in a previous randomized trial of oral BDP
This trial enrolled 60 patients, 31 randomized to oral BDP and 29 to placebo. The duration of treatment with study drug was 30 days, with a 10-day follow-up period. The treatment response rate at study day 30 was 71% for BDP versus 41% for placebo (P = .02).11 Analysis of survival at day 200 after transplantation is shown in Table 2. Three patients (10%) who had been randomized to BDP had died compared with 6 deaths (21%) among patients who had been randomized to placebo, leading to a reduced hazard of day-200 mortality although not statistically significantly different. By transplantation day 200, relapse of hematologic malignancy had contributed to the deaths of 4 (14%) of 29 patients in the placebo arm and 1 (3%) of 31 patients in the BDP arm. Infection contributed to the deaths of 5 (17%) of 29 patients in the placebo arm and 2 (6%) of 31 in the BDP arm. By 1 year after randomization, 9 of 29 patients in the placebo group and 6 of 31 patients in the BDP group had died (Figure 2B).
Long-term survival
As of September 1, 2005, median follow-up of patients in the 2 randomized trials of BDP was 3.5 and 3.6 years for patients in placebo and BDP groups, respectively, with an overall range of 10.6 months to 11.1 years. The risk of mortality was 37% lower for patients randomized to BDP compared with placebo (HR 0.63; P = .03, stratified log-rank test).
Discussion
This placebo-controlled trial demonstrates that oral BDP allows prednisone to be rapidly tapered with fewer recurrences of GVHD. Although the end point time to treatment failure by study day 50 did not reach statistical significance, the outcome of all clinically important secondary end points, including study day–80 efficacy, day-200 survival, and survival at 1 year after randomization, was statistically significantly better in the BDP group. These results confirm those of a smaller, single-center, placebo-controlled trial of BDP.11 In patients who had been randomized to BDP in the current trial, there were reductions in the hazard of mortality of 66% and 46% at day 200 and at 1 year after randomization, respectively. The earlier 60-patient randomized trial11 showed similar reductions in the hazard of mortality. The current trial was undertaken to extend BDP treatment from 30 days to 50 days and to demonstrate efficacy in centers with disparate practices. In an analysis of patients in the current study who had received cells from unrelated or HLA-mismatched donors, the reduction in the hazard of mortality at day 200 was 91% among patients randomized to BDP compared with placebo. Patients with abdominal pain, secretory diarrhea in excess of 1 L daily, intestinal bleeding, or liver and skin GVHD were not included in this study, as patients who were more likely to develop severe GVHD were not optimal candidates for a strategy that attempted to minimize prednisone exposure.5,10,20–22 The patients enrolled are representative of most patients now presenting with acute GVHD, as more severe GVHD has become less common than in the past.1,23
The beneficial effects of topical corticosteroids for mucosal inflammatory diseases of the intestinal tract, lungs, and nasopharynx have been known for over 30 years.24–27 Exacerbations of asthma, chronic obstructive pulmonary disease, and Crohn disease respond to high-dose prednisone therapy, but maintenance therapy is often accomplished with topical corticosteroids.28,29 Because GVHD involves the mucosa from the stomach to the rectum, formulations of oral BDP were composed of an immediate-release tablet (bioavailable to gastric, duodenal, and jejunal mucosa) and an enteric-coated tablet (for jejunal, ileal, and colonic mucosa).30,31 Oral BDP is biologically active as an immunosuppressive drug in vivo32 ; the parent compound is metabolized in intestinal mucosa and the liver to beclomethasone-17-monopropionate (17-BMP), which has an approximately 25-fold greater glucocorticoid receptor binding activity than BDP.33 BDP does not appear in the systemic circulation because of its metabolism in intestinal mucosa and the liver, but 17-BMP can be detected in the blood stream.34 It is believed that the primary anti-inflammatory effect of oral BDP occurs in the gastrointestinal mucosa, as both BDP and 17-BMP are present in high concentrations there. Compared with prolonged prednisone exposure to control GVHD, any systemic effect of 17-BMP in predisposing patients to infection must be relatively minor, as patients randomized to BDP had infrequent fatal infections and better day-200 posttransplantation survival.
A treatment that controls the signs and symptoms of GVHD while avoiding prolonged systemic immunosuppression is likely to result in fewer serious infections. High-dose glucocorticoids decrease immune responses to CMV35 and increase the risk of uncontrolled CMV viremia during antiviral therapy,36 increase the risk of invasive aspergillosis and mold infection–related death after hematopoietic cell transplantation (HCT) with nonmyeloablative conditioning regimens,37,38 and greatly increase the risk of blood stream infections following reduced-intensity cord-blood transplantations.39 We speculate that the frequency of leukemic relapse may be higher in the placebo arm because protracted exposure to prednisone to control symptoms of GVHD abrogates T-cell responses; however, the study was not designed to address this question.
Other potential mechanisms by which a highly potent topical corticosteroid might improve outcomes are blunting of inflammatory cytokine production by T cells in intestinal mucosa, inhibition of T-cell–mediated apoptosis of epithelial cells, induction of apoptosis in activated effector T cells, and deviation of T-cell responses toward tolerance or nonresponsiveness. Several studies have shown that glucocorticoids inhibit the differentiation40 and maturation41 of dendritic cells in vitro. These effects might help to preserve integrity of the mucosal surface, thereby reducing activation of innate immune mechanisms.
With the exception of adrenal axis suppression, we could not identify adverse reactions to oral BDP in the current study or in prior studies that specifically examined infection as an adverse event.11,31 The use of BDP inhalers has been associated with oropharyngeal and esophageal candidiasis,42,43 but oral delivery of BDP did not result in an increased incidence of fungal or bacterial colonization or infections after HCT.11,31 Metabolites of BDP are systemically bioavailable, resulting in decreased adrenal responsiveness over time of drug exposure.31,44–47 Two recent studies of long-term use of oral, topically active corticosteroids in doses similar to those used in this trial demonstrated little evidence of clinical adrenal insufficiency.45,48
Two randomized trials have shown that oral BDP prevents relapses of acute gastrointestinal GVHD after accelerated withdrawal of prednisone therapy. The effect is durable even following discontinuation of BDP. We hypothesize that topical therapy with BDP improved survival by limiting GVHD-related gastrointestinal epithelial injury, preserving the mucosal barrier, reducing the need for systemic glucocorticoid treatment, and reducing the frequency of life-threatening infections. The duration of the survival benefit in patients randomized to BDP, however, will require longer follow-up.
Authorship
Contribution: D.M.H. entered patients into the trial and cowrote the manuscript; S.C. performed statistical analyses and assisted in the preparation of the manuscript; T.C.R. was the medical director for the trial, assisted in the analysis, and assisted in the preparation of the manuscript; T.G. performed statistical analyses; F.S., S.D.R., D.D., M.B., B.B., and S.A. entered patients into the trial; M.B. collated long-term survival data; G.B.M. designed the research and cowrote the manuscript. Note that 9 additional members of the OrBec GVHD Study Group are listed as entering patients into the trial.
Conflict-of-interest disclosure: S.C., T.C.R., and G.B.M. have declared a financial interest in a company whose potential product was studied in the present work. Each of these authors is a consultant to DOR BioPharma, Inc, the sponsor of the study. G.B.M. has an equity position in DOR BioPharma, Inc.
A complete list of the members of the orBec GVHD Study Group appears as Document S1 (available on the Blood website; see the Supplemental Document link at the top of the online version of the article).
Correspondence: David M. Hockenbery, Clinical Research Division (D2-190), Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, Seattle WA 98109-1024; e-mail: dhockenb@fhcrc.org.
An Inside Blood analysis of this article appears at the front of this article.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by an Orphan Products Development Grant from the US Food and Drug Administration (FD-R 02599) and by Enteron Pharmaceuticals, a subsidiary of DOR BioPharma, Miami, FL.