Abstract
The immunoreceptor PD-1 is significantly up-regulated on exhausted CD8+ T cells during chronic viral infections such as HIV-1. However, it remains unknown whether PD-1 expression on CD8+ T cells differs between typical progressors (TPs) and long-term nonprogressors (LTNPs). In this report, we examined PD-1 expression on HIV-specific CD8+ T cells from 63 adults with chronic HIV infection. We found that LTNPs exhibited functional HIV-specific memory CD8+ T cells with markedly lower PD-1 expression. TPs, in contrast, showed significantly up-regulated PD-1 expression that was closely correlated with a reduction in CD4 T-cell number and an elevation in plasma viral load. Importantly, PD-1 up-regulation was also associated with reduced perforin and IFN-γ production, as well as decreased HIV-specific effector memory CD8+ T-cell proliferation in TPs but not LTNPs. Blocking PD-1/PD-L1 interactions efficiently restored HIV-specific CD8+ T-cell effector function and proliferation. Taken together, these findings confirm the hypothesis that high PD-1 up-regulation mediates HIV-specific CD8+ T-cell exhaustion. Blocking the PD-1/PD-L1 pathway may represent a new therapeutic option for this disease and provide more insight into immune pathogenesis in LTNPs.
Introduction
CD8+ T cells play a critical role in clearing human immunodeficiency virus (HIV) infection in vivo.1 In general, typical progressors (TPs) have insufficient CD8+ T-cell responses to HIV and fail to maintain durable control of the HIV load, whereas long-term nonprogressors (LTNPs) preferentially maintain functional HIV-specific CD8+ T cells that can persistently control viral replication.2–10 During the CD8+ T-cell response to HIV, there is a complex process of memory CD8+ T-cell differentiation.11 On the basis of CD45RA and CCR7 surface marker expression and relative function, 4 subpopulations of memory CD8+ T cells are proposed to exist: naive CD45RA+CCR7+ T cells, central memory CD45RA−CCR7+ T (TCM) cells, effector memory CD45RA−CCR7−T (TEM) cells, and terminally differentiated effector CD45RA+CCR7− T (TEMRA) cells.12–15 The TCM subpopulation readily differentiates into proliferating effector cells and homes to T-cell areas of the secondary lymphoid organs, but it has little or no effector function. The TEM subset has selectively lost constitutive CCR7 expression and produces high levels of perforin, IFN-γ, and IL-4 within hours of antigenic stimulation. The TEMRA subset, the TEM subpopulation that expresses CD45RA, can produce high levels of perforin. Thus, TEM cells are characterized by rapid effector function. Champagne et al16 have demonstrated that chronic HIV-1 infection affects the differentiation pattern of different CD8+ T-cell subsets; however, the molecular mechanisms of how this process occurs remain undefined
Memory T-cell responses are an important component of protective immunity against viral infection. However, memory T-cell responses generated during chronic viral infection often show defects in antiviral function and skewed differentiation of the memory T-cell subsets.16–18 For example, the proportion of HIV-specific CD45RA−CCR7−CD8+ T (TEM) cells is shown to increase significantly more than corresponding CMV-specific CD8+ T cells during chronic HIV-1 infection.16 This HIV-specific CD8+ TEM population is shown to release low levels of IL-2 and high levels of IFN-γ and have reduced proliferative capability. Importantly, perforin expression, the predominant antiviral mechanism used by HIV-specific CD8+ T lymphocytes, is significantly impaired or exhausted.2,3 The accumulation of exhausted virus-specific T cells may correlate with the inability of the immune response to control viral replication during chronic HIV infection.3–10,17 As a result, extensive focus has been placed on understanding the mechanism underlying the exhaustion of memory T-cell subsets in this disease.
PD-1 (CD279, encoded by Pdcd1) belongs to the CD28 family and is inducibly expressed on activated T cells, natural killer T cells, B cells, and myeloid cells.19–22 Its ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273), are broadly expressed on professional and nonprofessional antigen-presenting cells (APCs) as well as nonhematopoietic cell types.23,24 Binding of PD-1 to PD-L1 or PD-L2 activates a critical immunosuppressive pathway.23–25 It is shown that PD-L1 and PD-L2 expression on APCs can switch off autoreactive T cells and induce peripheral tolerance, whereas expression on virus-infected cells can suppress functional effector T cells and prevent destruction of virus-infected cells.26,27 Several studies have recently demonstrated that the PD-1/PD-L pathway can mediate exhaustion of virus-specific CD8+ T cells during chronic viral infection of mice28 and HIV-infected humans.29–31 These findings illustrate that the level of PD-1 expression correlates with the extent of HIV-specific CD8+ T-cell exhaustion and that high HIV antigen levels may drive PD-1 expression. These studies also demonstrate that blocking the PD-1/PD-L pathway increases T-cell proliferation and effector cytokine production. Collectively, these studies suggest that the PD-1/PD-L pathway may be operating during chronic HIV infection.32 However, it remains unclear whether PD-1 expression on CD8+ T cells differs between LTNPs and TPs.
In this study, we found that LTNPs exhibited markedly lower PD-1 expression on functional HIV-specific memory CD8+ T cells than TP patients, in whom significant up-regulation of PD-1 expression mediated HIV-specific effector memory CD8+ T-cell exhaustion. Blocking PD-1/PD-L1 interactions efficiently restored effector function and proliferation of HIV-specific CD8+ T cells in TP patients. These findings further confirmed the impact of PD-1 up-regulation on HIV-specific CD8+ T-cell exhaustion and supported the idea that blocking PD-1/PD-L1 may represent a new therapeutic option for this disease. In addition, these results provide more insight into understanding immune pathogenesis in LTNPs.
Materials and methods
Subjects
Sixty-three HIV-infected patients were enrolled in our study. Their infection status was defined, and they were divided into 3 groups according to previous reports,2,7 including a cohort of 18 LTNPs (who had a durable maintenance of peripheral CD4 T-cell counts > 500 cells/μL, and a plasma viral RNA concentration < 500 copies/mL in the absence of antiviral treatment or opportunistic infection for duration of infection), 26 TPs (who exhibited a typical progressive disease with peripheral CD4 T-cell counts < 500 cells/μL and plasma viral RNA > 1000 copies/mL without receiving antiviral treatment), and 19 complete responders (CRs) to antiviral therapy (who effectively controlled disease with plasma HIV-1 RNA < 500 copies/mL) (Table 1). The majority of these individuals were paid blood donors who had become infected through illegal blood collection between 1994 and 1995. Eighteen uninfected age- and sex-matched subjects were used as healthy controls (HCs). All the samples are collected with the approval of The Beijing 302 Hospital Research Ethnics Committee, and written informed consent was obtained from each subject. All HIV-infected subjects were serologically identified as having the HLA-A2+ genotype, and this was further confirmed using polymerase chain reaction.9
Isolation of PBMCs, purification of CD8+ T cells, and cell sorting
Peripheral blood mononuclear cells (PBMCs) were isolated from freshly heparinized blood by centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Cells were maintained in RPMI1640 (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated human AB serum, 2 mM L-glutamine, 20 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 M 2-mercaptoethanol. CD8+ T cells were obtained by positive selection using the MiniMACS system (Miltenyi Biotech, Bergisch-Gladbach, Germany) according to the manufacturer's instructions. CD8+ T-cell purity exceeded 95% as shown by flow cytometric analysis. PD-1+ and PD-1− CD8+ T-cell sorting was further performed from 3 TP patients using FACSVantage (Becton Dickinson Systems, San Jose, CA).
Antibodies and reagents
All antibodies were purchased from BD Biosciences (San Jose, CA), except anti–PD-1-PE (clone MIH4), which was from eBioscience (San Diego, CA), anti–CCR7-FITC (clone 150503), which was from R&D Systems (Minneapolis, MN), and PE-labeled HLA-A2 pentamer complexes loaded with the HIV-1 gag p17 epitope (77-85, SL9, SLYNTVATL), which was from ProImmune (Oxford, United Kingdom). In addition, anti–PD-L1 blocking Ab, 5H1, was a kind gift from Prof Lieping Chen (Johns Hopkins University School of Medicine) and Dr Shengdian Wang (Center of Infection and Immunity, Institute of Biophysics, The Chinese Academy of Sciences).
Phenotypic analysis
PBMCs were stained with anti–CD3-PerCP, anti–CD4-FITC, anti–CD8-allophycocyanin and anti–PD-1-PE or the corresponding isotype control anti–IgG1-PE to measure PD-1 expression on total T-cell subsets. PBMCs were simultaneously stained with anti–CD3-PerCP, anti–CD8-allophycocyanin, pentamer-PE, and anti–PD-1-FITC or the corresponding isotype control anti–IgG1-FITC, to measure PD-1 expression on virus-specific CD8+ T cells. Flow cytometry was used to analyze the phenotypically distinct memory CD8+ T-cell subsets characterized by the expression of CD45RA and CCR7.16 In brief, PBMCs or purified CD8+ T cells were incubated with 2 cocktails of antibodies: CD45RA-allophycocyanin or CD45RA–PE-Cy5, CCR7-FITC, or CCR7-allophycocyanin PC; PD-1-PE or PD-1-FITC; and CD8-PerCP or pentamer-PE. Cells were also stained with the corresponding isotype antibodies as controls. To characterize the phenotype of PD-1+CD8+ T cells, PBMCs were stained with anti–CD8-PerCP, anti–PD-1-PE, and FITC-conjugated anti-CD38, anti–HLA-DR, anti-CD95, anti–granzyme A, anti–granzyme B, antiperforin, and the corresponding isotype antibodies.
For intracellular staining, these cells were further treated with the permeable agent Cytofix/Cytoperm (BD PharMingen, San Diego, CA) according to the manufacturer's instructions and were intracellularly stained with anti–IFN-γ, anti–granzyme A, anti–granzyme B, and antiperforin antibodies at room temperature for 20 minutes in the dark. After washing twice with Cytoperm/Wash (BD PharMingen), cells were fixed in 1% paraformaldehyde, and 4-color flow cytometric analysis was performed using FACSCalibur and CELLQuest software (Becton Dickinson, San Jose, CA).
Intracellular IFN-γ staining assay
Freshly isolated PBMCs were stimulated with anti-CD3 and anti-CD28 antibodies (each 1 μg/mL) or SL9 peptide (10 μg/mL) in RPMI 1640 containing 10% human AB serum and either purified 5H1 (anti–PD-L1, 10 μg/mL) or isotype control antibodies for 6 hours. GolgiStop (BD PharMingen) was added to the cells after 2 hours of stimulation. Unstimulated cells were used as controls. Cells were then stained with surface antibodies and intracellular anti–IFN-γ–FITC antibodies according to the above-mentioned intracellular staining protocols.
CFSE proliferation assay
Division of PBMCs was analyzed by CFSE labeling as described previously.10 In brief, PBMCs were incubated with 5 μM CFSE for 10 minutes at 37°C in PBS containing 0.1% BSA. Labeling was quenched with RPMI1640 containing 10% FCS on ice for 5 minutes, and cells were washed twice with PBS. The CFSE-labeled cells were seeded at 1 × 106 cells/mL in 48-well plates. PBMCs were stimulated with anti-CD3 and anti-CD28 (1 μg/mL) plus purified 5H1 (anti–PD-L1, 10 μg/mL) or isotype control antibodies. Proliferation was analyzed using FACSCalibur (Becton Dickinson) prior to and following 24, 60, 96, and 144 hours of in vitro cell culture. For some experiments, freshly isolated PBMCs were incubated in RPMI 1640 medium containing 10% human AB serum in the presence of HIV SL9 peptide (10 μg/mL) and either anti–PD-L1 (10 μg/mL) or isotype control antibodies for 6 days. The frequency of expanded pentamer+CD8+ T cells specific for MHC class I–restricted epitopes was analyzed using a FACSCalibur (Becton Dickinson).
Plasma HIV-1 RNA monitoring
The 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) was used to quantify HIV-1 RNA levels in plasma samples as previously described.9 The cut-off value was no more than 500 copies/mL.
Statistical analysis
All data were analyzed using SPSS software (SPSS Science, Chicago, IL). Comparison between healthy controls, LTNPs, CR carriers, and TPs was performed using the Mann-Whitney U test. For the blockade assay, statistical comparison was analyzed using the Wilcoxon matched pairs t test. Correlations between variables were evaluated using the Spearman rank correlation test. For all tests, 2-sided P less than .05 was considered significant.
Results
LTNPs have lower PD-1 expression on HIV-specific CD8+ T cells than do TPs
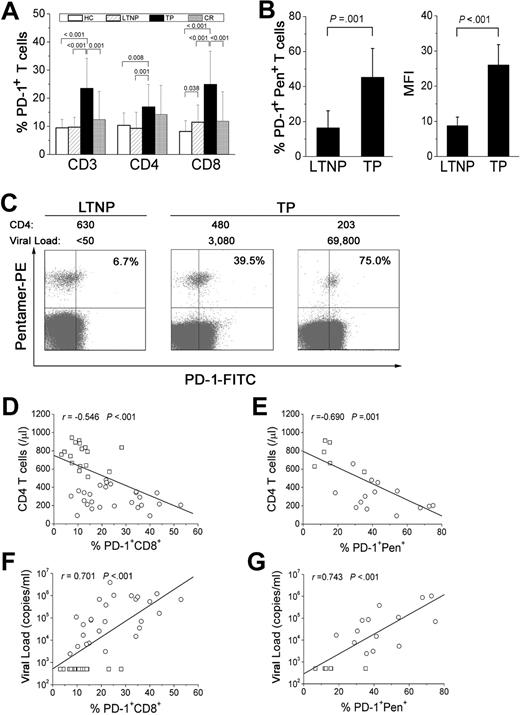
To determine whether PD-1 expression differs on the CD8+ T-cell population of TPs and LTNPs, we analyzed the frequency of PD-1–expressing T-cell subsets from all 4 human groups: HCs, LTNPs, TPs, and CRs and found that TPs expressed higher levels of PD-1 on total CD3+, CD4+, and CD8+ T cells than people from the other 3 groups (all P < .05; Figure 1A). No significant differences were observed between HC, LTNP, and CR carriers (Figure 1A). We also compared the levels of PD-1 expression on virus-specific CD8+ T cells from LTNPs and TPs. We found that LTNPs exhibited a smaller percentage of PD-1+pentamer+CD8+ T cells (mean, 16.3% ± 9.86%) than TP patients (mean, 45.2% ± 16.6%; P < .05; Figure 1B). Notably, the mean fluorescence intensity (MFI) of PD-1 on HIV-specific CD8+ T cells was significantly lower in LTNPs (mean, 8.7 ± 2.5) than in TP patients (mean, 26.0 ± 5.8), indicating that expression of the PD-1 receptor was also lower on a per cell basis in LTNPs. As shown in Figure 1C, the representative PD-1 expression using HIV pentamer specific for SL9 epitope was frequently targeted in HIV-infected TP patients with significantly lower CD4 T-cell numbers and a higher viral load but not in LTNPs, suggesting that PD-1 up-regulation on HIV-specific CD8+ T cells is probably associated with disease progression. Furthermore, correlation analysis of LTNPs and TP patients showed that PD-1 up-regulation on total and HIV-specific CD8+ T cells correlated significantly with reduced CD4 T-cell numbers (r = −0.546 and −0.690, respectively; both P < .05; Figure 1D-E) and increased plasma HIV-1 load (r = 0.701 and 0.743, respectively; both P < .05; Figure 1F-G). These data suggest that PD-1 up-regulation on HIV-specific CD8+ T cells is associated with disease progression in HIV-infected patients.
PD-1 expression on HIV-specific CD8+ T cells is higher in TP patients than in LTNPs. (A) Frequencies of PD-1–expressing CD3+, CD4+, and CD8+ T cells in the 4 groups, including healthy controls (HCs), long-term nonprogressors (LTNPs), typical progressors (TPs), and complete responders to HAART (CRs). Case numbers for the 4 groups are the same as shown in Table 1. (B) The frequency and mean fluorescence intensity (MFI) of PD-1 molecules expressed on pentamer+ HIV-specific CD8+ T cells in TPs and LTNPs. Error bars indicate standard deviation. (C) Representative flow cytometric plots of PD-1–expressing virus-specific CD8+ T cells from LTNPs and TP patients showing different CD4 T-cell numbers (cells/μL) and viral loads (copies/mL). Values in the upper right quadrant represent the percentage of pentamer+CD8+ T cells that express PD-1. (D-E) Total and virus-specific CD8+ T cells that up-regulate PD-1 molecules are inversely correlated with CD4 T-cell numbers. (F-G) Total and virus-specific CD8+ T cells that express PD-1 are positively correlated with viral load. □ indicates LTNPs; ○, TP patients; solid line, linear growth trend; r, correlative coefficient. P values are shown.
PD-1 expression on HIV-specific CD8+ T cells is higher in TP patients than in LTNPs. (A) Frequencies of PD-1–expressing CD3+, CD4+, and CD8+ T cells in the 4 groups, including healthy controls (HCs), long-term nonprogressors (LTNPs), typical progressors (TPs), and complete responders to HAART (CRs). Case numbers for the 4 groups are the same as shown in Table 1. (B) The frequency and mean fluorescence intensity (MFI) of PD-1 molecules expressed on pentamer+ HIV-specific CD8+ T cells in TPs and LTNPs. Error bars indicate standard deviation. (C) Representative flow cytometric plots of PD-1–expressing virus-specific CD8+ T cells from LTNPs and TP patients showing different CD4 T-cell numbers (cells/μL) and viral loads (copies/mL). Values in the upper right quadrant represent the percentage of pentamer+CD8+ T cells that express PD-1. (D-E) Total and virus-specific CD8+ T cells that up-regulate PD-1 molecules are inversely correlated with CD4 T-cell numbers. (F-G) Total and virus-specific CD8+ T cells that express PD-1 are positively correlated with viral load. □ indicates LTNPs; ○, TP patients; solid line, linear growth trend; r, correlative coefficient. P values are shown.
TP patients exhibit a dominant PD-1–expressing memory HIV-specific CD8+ T-cell population
We next compared the distribution of different populations within the total memory CD8+ T-cell pool of TP, LTNP, CR, and HC individuals. As shown in Figure 2A, TP patients retained the highest percentage of CD45RA−CCR7−CD8+ TEM cells (80.7%) and the lowest percentage of CD45RA+CCR7−CD8+ TEMRA cells (17.1%), whereas LTNPs had a moderate percentage of TEM cells (45.5%) and the highest frequency of TEMRA cells (46.3%), as compared with healthy subjects versus CR individuals (20.6% versus 37.9% and 31.5% versus 40.9% for TEM and TEMRA cells, respectively). Notably, we found that PD-1 was more dominantly expressed on TEM cells from HIV-infected TP, LTNP, and CR individuals than from HC subjects (Figure 2B). A significantly higher frequency of PD-1+ TEM and PD-1+ TEMRA subsets was found in TP patients than in individuals from the other 3 groups (all P < .05; Figure 2B). Thus, differentiation and maturation of memory CD8+ T-cell subsets appears to be significantly skewed in HIV-infected individuals.
PD-1 up-regulation occurs on effector memory CD8+ T-cell subsets from TP patients. (A) Distribution of memory CD8+ T-cell subsets in representative individuals from each group. The percentage of each memory subset within the total memory CD8+ T-cell pool is shown in each quadrant. (B) Pooled data show the frequencies of memory PD-1+CD8+ T-cell subsets in each group. P values are shown. Error bars indicate standard deviation. (C) Distribution of HIV-specific memory CD8+ T-cell subsets from LTNPs (n = 5) and TP patients (n = 7). The percentage of memory subsets of HIV-specific CD8+ T cells is shown in the upper right quadrant. (D) Distribution of HIV-specific memory CD8+ T-cell subsets that express PD-1 in representative individuals from the LTNP (n = 5) and TP group (n = 7). The percentage of PD-1+ cells within the pentamer+CD8+ memory T-cell subset is shown in the upper right quadrant.
PD-1 up-regulation occurs on effector memory CD8+ T-cell subsets from TP patients. (A) Distribution of memory CD8+ T-cell subsets in representative individuals from each group. The percentage of each memory subset within the total memory CD8+ T-cell pool is shown in each quadrant. (B) Pooled data show the frequencies of memory PD-1+CD8+ T-cell subsets in each group. P values are shown. Error bars indicate standard deviation. (C) Distribution of HIV-specific memory CD8+ T-cell subsets from LTNPs (n = 5) and TP patients (n = 7). The percentage of memory subsets of HIV-specific CD8+ T cells is shown in the upper right quadrant. (D) Distribution of HIV-specific memory CD8+ T-cell subsets that express PD-1 in representative individuals from the LTNP (n = 5) and TP group (n = 7). The percentage of PD-1+ cells within the pentamer+CD8+ memory T-cell subset is shown in the upper right quadrant.
We also identified PD-1 expression on HIV-specific memory CD8+ T-cell subsets from LTNP and TP patients. As shown in Figure 2C, the majority of HIV-specific CD8+ T cells were retained in the HIV-specific TEM subset (92.4%), and only a small number differentiated into HIV-specific TEMRA cells (5.8%) in TPs as compared with LTNPs (57.1% versus 18.6% for HIV-specific TEM versus TEMRA populations), which is similar to the population distribution within the total memory CD8+ T-cell pool. Interestingly, a significantly higher frequency of HIV-specific PD-1+pentamer+CD8+ TEM cells was observed in TPs than in LTNPs (Figure 2D). Our findings demonstrate a close correlation between PD-1 up-regulation on TEM cell subsets and the skewed maturation of memory CD8+ T cells in patients with chronic HIV infection.
PD-1+CD8+ T cells express an exhausted phenotype in TP patients
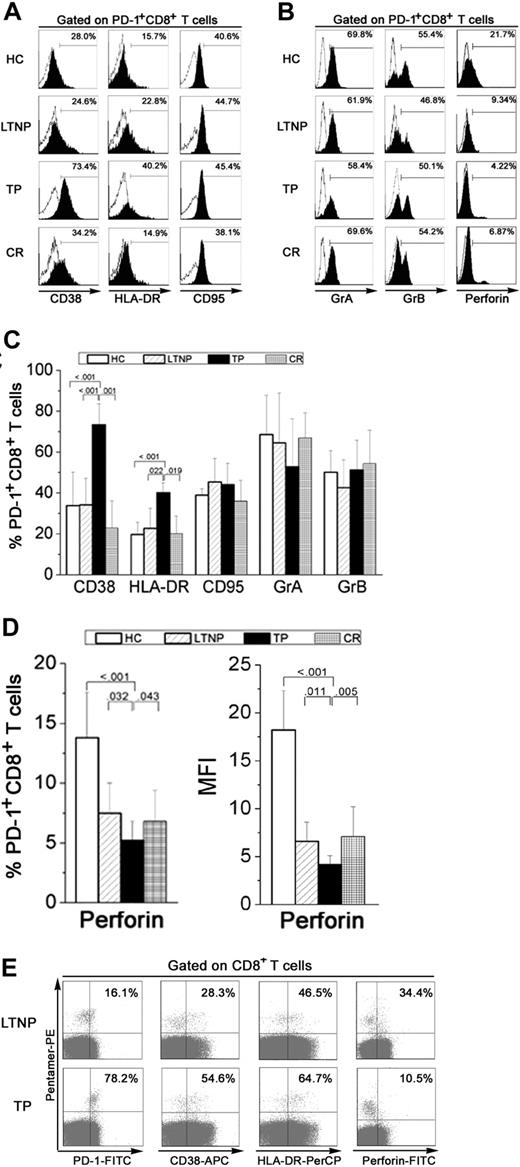
To determine whether PD-1 up-regulation induced functional exhaustion of HIV-specific CD8+ T cells by decreasing the expression of phenotypic markers and cytotoxic molecules, we measured the expression of CD38, HLA-DR, CD95, perforin, and granzyme in PD-1+CD8+ T cells from individuals in the 4 groups. As shown in Figure 3A to C, PD-1+CD8+ T cells from TP patients expressed the highest levels of activation markers such as CD38 (mean, 73.4% ± 10.4%) and HLA-DR (mean, 40.2% ± 4.9%) than LTNPs (mean, 34.2% ± 13.0% for CD38; 22.8% ± 9.8% for HLA-DR) and healthy subjects (mean, 33.8% ± 16.5% for CD38; 19.7% ± 6.0% for HLA-DR). However, TP patients expressed lower levels of perforin (mean, 5.2% ± 1.6%) than LTNPs and healthy subjects (mean, 7.5% ± 2.4% and 13.8% ± 3.86%, respectively). These results were consistent with analysis of the MFI of PD-1 expression on HIV-specific CD8+ T cells (Figure 3D). CR individuals showed similar levels of CD38 (mean, 22.9% ± 13.2%) and HLA-DR (mean, 20.0% ± 8.8%) expression as healthy controls but had markedly lower perforin expression (mean, 6.7% ± 3.2%). No significant differences were observed in CD95, GrA, and GrB expression on PD-1+CD8+ T cells from people in the 4 groups (Figure 3C-D).
PD-1+CD8+ T cells from TP patients express an exhausted phenotype. (A) Histogram plots of CD38, HLA-DR, and CD95 expression on PD-1+CD8+ T cells from representative individuals in each group. (B) Representative histogram plots of GrA, GrB, and perforin expression on PD-1+CD8+ T cells in subjects from each group. Open histograms indicate the staining of isotype controls; filled histograms, the staining of selected markers. (C) Percentage of CD38, HLA-DR, CD95, granzyme A, and granzyme B expressed on PD-1+CD8+ T cells from subjects in each group. Error bars indicate standard deviation. (D) Percentage and mean fluorescence intensity (MFI) of perforin expressed on PD-1+CD8+ T cells in subjects from each group. P values are shown. Error bars indicate standard deviation. (E) Representative expression of CD38, HLA-DR, and perforin on HIV-specific CD8+ T cells from LTNP (n = 5) and TP individuals (n = 6). Values in the upper right quadrant represent the percentage of CD8+ T cells that express each marker.
PD-1+CD8+ T cells from TP patients express an exhausted phenotype. (A) Histogram plots of CD38, HLA-DR, and CD95 expression on PD-1+CD8+ T cells from representative individuals in each group. (B) Representative histogram plots of GrA, GrB, and perforin expression on PD-1+CD8+ T cells in subjects from each group. Open histograms indicate the staining of isotype controls; filled histograms, the staining of selected markers. (C) Percentage of CD38, HLA-DR, CD95, granzyme A, and granzyme B expressed on PD-1+CD8+ T cells from subjects in each group. Error bars indicate standard deviation. (D) Percentage and mean fluorescence intensity (MFI) of perforin expressed on PD-1+CD8+ T cells in subjects from each group. P values are shown. Error bars indicate standard deviation. (E) Representative expression of CD38, HLA-DR, and perforin on HIV-specific CD8+ T cells from LTNP (n = 5) and TP individuals (n = 6). Values in the upper right quadrant represent the percentage of CD8+ T cells that express each marker.
We further compared expression of these phenotypes on HIV-specific CD8+ T cells from LTNPs and TPs and found that LTNPs expressed lower levels of HLA-DR and CD38 and higher levels of perforin than did TP patients (Figure 3E). We also compared expression of these markers on Flu-specific CD8+ T cells and CMV-specific CD8+ T cells (both had lower levels of PD-1 expression than HIV-specific CD8+ T cells) between TPs and LTNPs. We found that HIV-specific CD8+ T cells from TP patients expressed higher levels of HLA-DR and CD38 and lower levels of perforin than Flu- and CMV-specific CD8+ T cells (data not shown). Thus, our findings confirmed that PD-1+CD8+ T cells from TP patients express high levels of activation markers (CD38 and HLA-DR) and low levels of effector molecules (perforin) and represent a functionally exhausted phenotype.
Total and HIV-specific PD-1+CD8+ T cells from TP patients are less able to proliferate than their counterparts in LTNPs
To investigate the effect of PD-1 expression on the proliferation capability of CD8+ T cells, we compared the proliferation profiles of CD8+ T cells from LTNPs and TP patients in response to TCR stimulation. Our results showed that total CD8+ T cells from LTNPs divided twice after 60 hours of initial stimulation and maintained a strong proliferative capacity during the entire period of in vitro incubation. Total CD8+ T cells from TP patients, however, began to proliferate after 60 hours of stimulation and exhibited poor proliferative capacity (Figure 4A-D). We also compared the proliferation between PD-1+ and PD-1−CD8+ T cells from TP patients and found that PD-1 expression inversely correlated with in vitro cell division. PD-1+CD8+ T cells were maintained in a resting state, whereas PD-1−CD8+ T cells proliferated successfully on TCR stimulation, indicating that PD-1+CD8+ T cells were less able to proliferate than PD-1−CD8+ T cells (Figure 4B). To further confirm the results, we sorted PD-1+ and PD-1−CD8+ T cells from TP patients (> 95% purity) at the start of culture, because PD-1 is transiently up-regulated by activation on most but not all T cells. Sorted PD-1+CD8+ T cells were maintained in a resting state, whereas sorted PD-1−CD8+ T cells were able to proliferate effectively on TCR stimulation (data not shown).
HIV-specific PD-1+CD8+ T cells from TP patients are less able to proliferate than their counterparts in LTNPs. (A) Comparison of proliferation profiles of total CD8+ T cells from representative LTNPs and TPs. CFSE-labeled PBMCs were harvested at different time points, and CD8+ T-cell proliferation was analyzed using flow cytometry. (B) Proliferation profiles for PD-1+CD8+ and PD-1−CD8+ T cells in a representative TP patient. Cells were gated on PD-1+ (top) and PD-1−CD8+ T cells (bottom) at different incubation times. (C) PD-1/PD-L1 blockade restores expansion of CD8+ T cells from TP patients in the presence of either anti–PD-L1 antibodies (bottom) or the corresponding isotype control antibodies (top). (D) Summary data for the frequency of CFSElowCD8+ T cells from LTNPs (n = 5) and TPs (n = 7) that were incubated in vitro in the presence of anti–PD-L1 antibodies or isotype control antibodies for different incubation times. P values were shown for the differences of CFSElowCD8+ T-cell percentages between TP patients and LTNPs. Error bars indicate standard deviation. (E) Representative expansion of pentamer+CD8+ T cells in the presence of anti–PD-L1 antibody in TP patients (n = 4). Values in the upper right quadrant represent the percentage of pentamer+CD8+ T cells.
HIV-specific PD-1+CD8+ T cells from TP patients are less able to proliferate than their counterparts in LTNPs. (A) Comparison of proliferation profiles of total CD8+ T cells from representative LTNPs and TPs. CFSE-labeled PBMCs were harvested at different time points, and CD8+ T-cell proliferation was analyzed using flow cytometry. (B) Proliferation profiles for PD-1+CD8+ and PD-1−CD8+ T cells in a representative TP patient. Cells were gated on PD-1+ (top) and PD-1−CD8+ T cells (bottom) at different incubation times. (C) PD-1/PD-L1 blockade restores expansion of CD8+ T cells from TP patients in the presence of either anti–PD-L1 antibodies (bottom) or the corresponding isotype control antibodies (top). (D) Summary data for the frequency of CFSElowCD8+ T cells from LTNPs (n = 5) and TPs (n = 7) that were incubated in vitro in the presence of anti–PD-L1 antibodies or isotype control antibodies for different incubation times. P values were shown for the differences of CFSElowCD8+ T-cell percentages between TP patients and LTNPs. Error bars indicate standard deviation. (E) Representative expansion of pentamer+CD8+ T cells in the presence of anti–PD-L1 antibody in TP patients (n = 4). Values in the upper right quadrant represent the percentage of pentamer+CD8+ T cells.
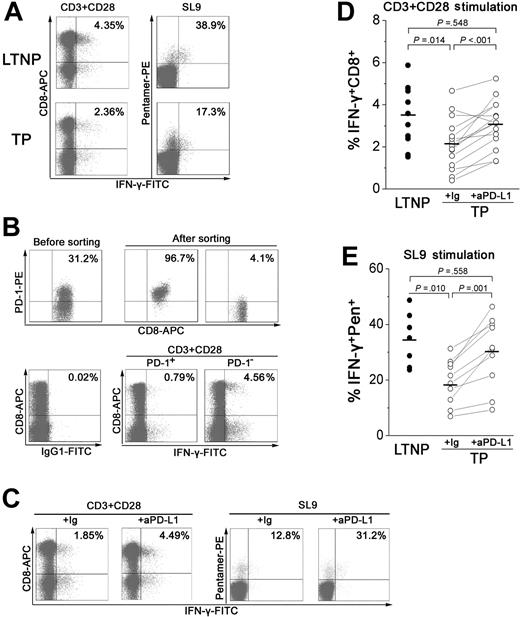
To test the effect of blocking PD-1/PD-L1 interactions on T-cell proliferation, we measured anti-CD3– and anti-CD28–induced CD8+ T-cell proliferation after blocking one of the PD-1 ligands, PD-L1 (B7H1). CD8+ T cells from TP patients began to proliferate 24 hours after blockade of the PD-L1 pathway, whereas those not receiving the blocking antibody did not proliferate until 60 hours after TCR stimulation (Figure 4C). Thus, blocking PD-1/PD-L1 interactions significantly enhanced proliferation of CD8+ T cells from TP patients (all P < .05 at 60, 96, and 144 hours after TCR stimulation; Figure 4D). We further analyzed the proliferative ability of HIV-specific CD8+ T cells from both TPs and LTNPs. These data showed a significant increase in the expansion of HIV-specific CD8+ T cells in the presence of SL9 peptide plus anti–PD-L1 antibodies, as compared with expansion following stimulation with peptide alone (Figure 4E). Thus, our findings confirm previous reports that PD-1/PD-L1 signaling inhibits HIV-specific CD8+ T-cell proliferation.
Blocking PD-L1 antibodies reversed the decrease in IFN-γ production by PD-1+CD8+ T cells from TP patients
To further investigate whether PD-1 up-regulation could weaken the IFN-γ–releasing capacity of total and HIV-specific CD8+ T cells during chronic HIV infection, PBMCs from TPs and LTNPs were stimulated with anti-CD3 and anti-CD28 antibodies or their corresponding cognate peptides plus blocking antibodies against PD-L1 or an isotype control for 6 hours, and the percentage of IFN-γ–producing cells was assessed by flow cytometry (Figure 5A-C). Both total and HIV-specific CD8+ T cells from LTNPs produced higher levels of IFN-γ than those from TP patients (mean, 3.45% ± 1.40% versus 2.14% ± 1.2% for TCR stimulation; Figure 5A,D; 34.7% ± 9.6% versus 19.4% ± 8.1% for peptide stimulation; Figure 5A,E). To determine whether PD-1+CD8+ T cells produced less IFN-γ than PD-1−CD8+ T cells from TP patients, we further sorted PD-1+CD8+ and PD-1−CD8+ T cells, added them back individually to CD8+ T-cell deleted PBMCs at a ratio of 1:3 (PD-1+CD8+ or PD-1−CD8+ T cells versus CD8-deleted PBMCs), and stimulated them with a TCR mimic. PD-1 expression was inversely correlated with in vitro IFN-γ production. Only a minority of PD-1+CD8+ T cells produced IFN-γ, whereas there was a 5.8-fold increase in IFN-γ production by PD-1−CD8+ T cells from TP patients, indicating that PD-1+CD8+ T cells were less able to produce cytokines than PD-1−CD8+ T cells (Figure 5B).
PD-1 up-regulation on total and virus-specific CD8+ T cells inhibits IFN-γ production in TP patients. (A) Representative dot plots of IFN-γ intracellular staining gated on CD3+ T cells and CD8+ T cells from LTNPs and TP subjects. (B) PD-1−CD8+ but not PD-1+CD8+ T cells produced a large amount of IFN-γ in response to TCR stimulation. Dot plots show the intracellular IFN-γ staining profile of PD-1+CD8+ and PD-1−CD8+ T cells from a representative TP patient. CD8+ T cells that were isolated from PBMCs using the MiniMACS system were further sorted into PD-1+CD8+ and PD-1−CD8+ T cells using FACSVantage. The sorted cells were added back to CD8-deleted PBMCs at a ratio of 1:3. Cells were further stimulated with a TCR mimic for 6 hours and intracellular IFN-γ production was assessed. Values in the upper right quadrant indicate the percentage of sorted CD8+ T cells that express PD-1 (top) and the percentage of PD-1+CD8+ and PD-1−CD8+ T cells that express IFN-γ. (C) Blocking PD-1/PD-L1 restores the percentage of IFN-γ+CD8+ T cells. IFN-γ intracellular staining of total and HIV-specific CD8+ T cells is shown in dot plots from a representative TP patient. (D-E) Summary data for the percentage of IFN-γ+CD8+ T cells (D) or IFN-γ+pentamer+CD8+ T cells (E) from LTNPs and TPs. Horizontal bars indicate mean values.
PD-1 up-regulation on total and virus-specific CD8+ T cells inhibits IFN-γ production in TP patients. (A) Representative dot plots of IFN-γ intracellular staining gated on CD3+ T cells and CD8+ T cells from LTNPs and TP subjects. (B) PD-1−CD8+ but not PD-1+CD8+ T cells produced a large amount of IFN-γ in response to TCR stimulation. Dot plots show the intracellular IFN-γ staining profile of PD-1+CD8+ and PD-1−CD8+ T cells from a representative TP patient. CD8+ T cells that were isolated from PBMCs using the MiniMACS system were further sorted into PD-1+CD8+ and PD-1−CD8+ T cells using FACSVantage. The sorted cells were added back to CD8-deleted PBMCs at a ratio of 1:3. Cells were further stimulated with a TCR mimic for 6 hours and intracellular IFN-γ production was assessed. Values in the upper right quadrant indicate the percentage of sorted CD8+ T cells that express PD-1 (top) and the percentage of PD-1+CD8+ and PD-1−CD8+ T cells that express IFN-γ. (C) Blocking PD-1/PD-L1 restores the percentage of IFN-γ+CD8+ T cells. IFN-γ intracellular staining of total and HIV-specific CD8+ T cells is shown in dot plots from a representative TP patient. (D-E) Summary data for the percentage of IFN-γ+CD8+ T cells (D) or IFN-γ+pentamer+CD8+ T cells (E) from LTNPs and TPs. Horizontal bars indicate mean values.
We also analyzed the effect of blocking PD-1/PD-L1 interactions on IFN-γ production from CD8+ T cells. Both total and HIV-specific CD8+ T cells showed a significant enhancement in IFN-γ production (mean, 3.08% ± 1.1% for TCR stimulation; 30.0% ± 12.5% for peptide stimulation) after PD-L1 blockade, as compared with cells without blockade (both P < .05; Figure 5C-E). Thus, anti–PD-L1 antibodies significantly enhanced IFN-γ production by virus-specific memory CD8+ T cells from TP patients, further indicating that PD-1 up-regulation inhibited IFN-γ production by HIV-specific CD8+ T cells. Blocking the PD-1/PD-L1 pathway was able to restore the function of virus-specific CD8+ T cells.
Discussion
Increasing evidence demonstrates that up-regulation of the inhibitory receptor, PD-1, mediates HIV-specific CD8+ T-cell functional exhaustion and is more sensitive to apoptosis, resulting in immune deficiency of CD8+ T cells to control virus replication.29–31 To our knowledge, our findings are the first to demonstrate that HIV-specific CD8+ T cells from LTNPs express significantly less PD-1 than TP patients, providing one explanation for why they are able to maintain control of HIV infection. Our findings support previous studies,29–31 in particular the finding that PD-1/PD-L1–dependent inhibition is operating in TP patients.
The immunosuppressive effects of PD-1 on virus-specific memory CD8+ T cells may contribute to disease progression during chronic HIV infection. Previous studies show that PD-1+CD8+ T cells from TP patients have a range of dysfunctions, including defective perforin and IFN-γ expression, and impaired proliferative capability.2–10,16–18 Thus, accumulation of PD-1+CD8+ T cells in TPs, but not in LTNPs, may prevent renewal of a functionally competent CD8+ T cell repertoire. The close association between PD-1 up-regulation and CD8+ T-cell suppression also suggests that PD-1 is involved in negative feedback regulation of the host immune response that contributes to progression of chronic HIV infection. However, previous studies show a correlation between PD-1 expression and viral load both cross-sectionally and longitudinally,29,30 suggesting that PD-1 expression is induced by antigen stimulation. Thus, an alternative possibility is that PD-1 is only a marker of high viral load or disease state and is not a direct cause of increased viral replication. This cause-and-effect relationship requires further investigation.
PD-1 up-regulation is closely associated with the skewed maturation of virus-specific CD8+ T cells in TPs but not LTNPs. In this study, we found that PD-1 up-regulation occurred predominantly on HIV-specific CD45RA−CCR7−CD8+ TEM cells from HIV-infected patients. It is likely that the PD-1 signaling pathway can arrest cells in the G0/G1 stage and prevent further cell division,24 potentially promoting accumulation of the CD45RA−CCR7−TEM subset in TP patients.16–18 LTNPs, in contrast, maintain a moderate percentage of CD45RA−CCR7−TEM and CD45RA+CCR7−CD8+ TEMRA cells. This skewed maturation or differentiation is associated with the impaired function of CD8+ T cells from TP patients.16–18 One of the key features of memory HIV-specific CD8+ T cells is the ability to rapidly reactivate multiple effector functions and proliferate vigorously on antigen restimulation. In our study, HIV-specific CD8+ T cells from TP patients possessed a memory phenotype but did not demonstrate functionality, suggesting that these cells may not represent true memory CD8+ T cells. Taken together, our data suggest that PD-1 up-regulation on TEM cells may be associated with the skewed maturation of virus-specific CD8+ T cells in TP but not LTNP patients. It remains unknown how the intracellular mechanisms by which the inhibitory PD-1/PD-L1 pathway affects disease severity in TPs.
T-cell activation is a necessary component of an effective acquired immune response against HIV infection, although hyperactivation of T cells can cause autoimmune destruction in HIV-infected patients.24 The CD28 family, in particular the PD-1/PD-L1 pathway, plays a prominent role in controlling T-cell activation.23–25 We found that PD-1 expression correlated with elevated CD38 and HLA-DR expression in HIV-infected patients. In particular, PD-1 up-regulation was associated with high levels of CD38 and HLA-DR expression in TP patients, whereas lower PD-1 expression correlated with reduced levels of CD38 and HLA-DR in LTNPs. Thus, PD-1 may act as a surrogate activation marker during HIV infection. It is also likely that high levels of both CD38 and PD-1 expression may act synergistically to induce HIV-specific CD8+ T-cell exhaustion and promote increased sensitivity to apoptosis in TP patients.31 However, the regulation of T-cell activation and expansion is a complex process that requires the coordinated interaction of multiple signaling pathways. One report illustrates that PD-L1 is up-regulated and B7-2 (CD86) is down-regulated on APCs during chronic HIV infection, providing increased inhibitory and reduced activating signals for T cells.33 In addition, a gene expression study has recently shown that in addition to PD-1, a large number of other genes are altered in exhausted CD8+ T cells during viral infection (Dr E. J. Wherry, Wistar Institute, personal e-mail communication, August 20–27, 2006). Furthermore, other immunoinhibitory receptors, including PD-1, BTLA, and FcγRIIB, may also cooperate to induce HIV-specific CD8+ T-cell exhaustion.20,32 Future studies will be required to clarify these findings.
Consistent with recent studies in both mice and humans,28–31 our data confirmed that blocking the PD-1/PD-L1 inhibitory pathway could be exploited therapeutically, particularly for HIV-infected patients with low CD4 T-cell numbers. Importantly, HAART therapy is found to down-regulate PD-1 expression in TP patients,29,30 suggesting that this treatment may partially restore the innate and adaptive immune responses of the host to HIV-1.9,34–36 Future longitudinal studies should address the correlation between PD-1 down-regulation and the recovery of host immune responses in patients with AIDS undergoing HAART therapy.
Overall, we found that PD-1 expression was significantly lower on HIV-specific memory CD8+ T cells from LTNPs than TPs. Furthermore, our study indicated that blocking PD-1/PD-L1 interactions in vitro could, at least in part, revitalize some HIV-specific CD8+ T-cell functions in TP patients. These findings not only extend our understanding of how the PD-1/PD-L1 inhibitory pathway functions in LTNPs but also support the notion that blocking PD-1/PD-L1 interactions may represent a potential therapeutic strategy for HIV-infected patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thanks all HIV-infected individuals and healthy participants in this study.
This work was supported by grants from the National Outstanding Youth Foundation of China (30525042) and the National Key Basic Research Program of China (2006CB504205).
Authorship
Contribution: J.-Y.Z. designed, performed research, analyzed data; Z.Z. designed, performed research, analyzed data, wrote paper; X.W. collected samples; J.-L.F. performed research; J.Y. performed research; Y.J. performed research; L.C. designed research; H.Z. performed flow cytometry; J.W. took charge of clinical observation; L.J. performed research; M.S. designed research; G.F.G. analyzed data; H.W. took charge of clinical observation; F.-S.W. coordinated, designed, and performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
J.-Y.Z. and Z.Z. contributed equally to this study.
Correspondence: Fu-Sheng Wang, Research Center of Biological Therapy, Beijing 302 Hospital, 100039, China; e-mail: fswang@public.bta.net.cn