Abstract
It has been suggested that plasma-derived factor VIII products induce fewer inhibitors than recombinant factor VIII products. We investigated the relationship of factor VIII product type and switching between factor VIII products with the risk to develop inhibitors. This multicenter retrospective cohort study included 316 patients with severe hemophilia A born between 1990 and 2000. The outcome was clinically relevant inhibitor development, defined as the occurrence of at least 2 positive inhibitor titers with decreased recovery. The risk of inhibitor development was not clearly lower in plasma-derived compared with recombinant factor VIII products (relative risk [RR], 0.8; 95% confidence interval [CI], 0.5-1.3). Among high-titer inhibitors, the possible reduction in risk was even less pronounced (RR, 0.9; CI, 0.5-1.5). Plasma-derived products with considerable quantities of von Willebrand factor (VWF) carried the same risk for inhibitor development as recombinant factor VIII products (RR, 1.0; CI, 0.6-1.6). Switching between factor VIII products did not increase the risk for inhibitors (RR, 1.1; CI, 0.6-1.8). In conclusion, our findings support neither the notion that plasma-derived factor VIII products with considerable concentrations of VWF confer a lower risk to develop inhibitory antibodies than recombinant factor VIII products, nor that switching between factor VIII product brands increases inhibitor risks in previously untreated patients with severe hemophilia A.
Introduction
At present, the main concern in the treatment of children with severe hemophilia A is the development of inhibitory antibodies that neutralize infused factor VIII, occurring in 10% to 30% of patients.1 Several patient-related factors have been associated with the risk of developing inhibitors, such as factor VIII gene mutation,2–4 other genetic factors,5–8 family history of inhibitors,9–11 and ethnicity.11,12 In addition to these determinants, a number of treatment-related factors have been suggested to affect the risk of inhibitor development, such as prophylaxis,13,14 age at first exposure,15,16 and intensity of treatment.17 One of the most intensively discussed potential risk factors is the factor VIII product type.
Indications for a potential role of the factor VIII product type in inhibitor development arose when prospective registration studies of recombinant factor VIII products among previously untreated hemophilia A patients reported inhibitor incidences between 29% and 32%.18–23 These incidences were considerably higher than the previously observed 0% to 12% in patients treated with single plasma-derived products.1,24 However, methodologic differences between studies rendered comparisons inconclusive.25 More convincing were the findings of a recent comparative study in which the inhibitor risk in patients treated with full-length recombinant factor VIII was again higher than the risk in patients treated with a high-purity plasma-derived factor VIII product containing von Willebrand factor (VWF).26 Yet, it remains unclear whether it is just the latter high-purity plasma-derived factor VIII product that carries a lower risk for inhibitors, or whether other plasma-derived products have the same effect. And, it is also unclear whether the VWF content of a factor VIII product brand indeed decreases the risk for inhibitors.
In addition, it has been suggested that switching between factor VIII products might trigger the development of inhibitors, because of observations of low inhibitor risks in patients treated with only one brand of plasma-derived factor VIII.24
We, therefore, set out to describe inhibitor risks according to different factor VIII product types and to examine whether switching between factor VIII products affected the risk of inhibitor development in a multicenter cohort of previously untreated patients with severe hemophilia A.
Patients and methods
Eligible for inclusion were all 376 patients with severe hemophilia A (residual factor VIII activity of < 0.02 IU/mL) born between 1990 and 2000, who were treated in one of the participating hemophilia treatment centers (13 European and 1 Canadian center) from their first clotting factor administration onwards. Ten patients were excluded for various reasons; 1 because of an unknown baseline factor VIII activity level, 1 because he was treated with DDAVP (desmopressin), 6 because they were lost to follow-up before they received treatment with factor VIII on a total of 50 exposure days, and 2 because they were treated with a particular immunogenic factor VIII product.27 In 322 (87%) patients, data on treatment with factor VIII up to 50 exposure days were available. Of these patients, 6 were excluded because they were treated with an unknown type of factor VIII product on one or more exposure days. This left 316 (84%) patients in the present analyses.
We collected data from the medical records and patients' infusion logbooks using standardized case report forms. Data were collected on patient characteristics, including factor VIII gene mutation, and on all clotting factor infusions up to 50 “exposure days” or until inhibitor development, including dates of infusion, doses and brand name of factor VIII product, reasons of treatment, types of bleeds, and surgery. We also collected details on all performed inhibitor tests and recovery measurements, including dates, infused doses of clotting factor, preinfusion and postinfusion factor VIII activity levels, and time between infusion of clotting factor and blood sampling for postinfusion factor VIII activity level. An “exposure day” was defined as a calendar day during which one or more infusions of factor VIII were given.
Definition of inhibitor development
We defined inhibitor development in 2 ways. First, the development of “all clinically relevant inhibitors” was defined as the occurrence of at least 2 positive inhibitor titers combined with a decreased factor VIII recovery within the first 50 exposure days. Second, the development of “high-titer inhibitors” occurred when the peak inhibitor titer was at least 5 Bethesda units per milliliter. A positive inhibitor titer was defined according to the used inhibitor assay and cut-off level of each center's laboratory. We considered the factor VIII recovery to be decreased when it was less than 66% of expected. The expected level of factor VIII activity was calculated according to Lee et al.28 Fifteen patients with marginally positive inhibitor titers (median titer, 0.7 BU/mL; range, 0.2 to 1.5 BU/mL) on only one occasion with normal factor VIII recoveries did not meet the definition of a clinically relevant inhibitor.
Data analyses
For each patient, follow-up time accrued from the first exposure day and ended at the 50th exposure day, or at the time of inhibitor development. To examine whether the factor VIII product type and switching between factor VIII products were associated with inhibitor development, we used pooled logistic regression29 with inhibitor development as the dependent variable and exposure day as time increment. Observations over all exposure days of all patients were pooled into a single sample, and a logistic regression model was used to relate the risk factors to the occurrence of inhibitors. The odds ratios can be interpreted as incidence rate ratios, given the low incidence of inhibitors within strata of exposure days. This method accounts for varying risks according to the cumulative number of exposure days, and is equivalent to Cox regression with exposure days as time-variable and time-dependent covariates.29
First, we compared the inhibitor risk between recombinant and plasma-derived factor VIII products.
Second, in order to investigate whether the von Willebrand factor (VWF) content was associated with the risk of inhibitor development, we categorized factor VIII products into those containing no VWF (all 4 recombinant factor VIII products), products containing less than 0.01 IU VWF antigen per IU factor VIII antigen (monoclonal antibody–purified plasma-derived products), and products containing more than 0.01 IU VWF per IU factor VIII antigen (other plasma-derived products).30
Third, we compared the risks of inhibitor development according to the different brands of recombinant factor VIII products, with the risk in the recombinant factor VIII product that was used most frequently in this study population as the reference.
Fourth, we investigated the effect of switching of factor VIII products, occurring at infusion of any new brand of factor VIII product, by comparing the risk of inhibitor development after switching to a new brand of factor VIII to the risk of inhibitor development among patients who had not (yet) switched from one product to another.
In the multivariate analyses, we adjusted all associations for baseline factor VIII activity level, ethnicity, factor VIII gene mutation type, age at first exposure, duration between exposure days, dose of factor VIII (as a measure of treatment intensity), and regular prophylaxis. Factor VIII gene mutation type was categorized as high-risk (large deletions, nonsense mutations, intron 1 and 22 inversions) and low-risk (small deletions/insertions, missense mutations, splice site mutations) factor VIII gene mutation types. The period of 5 exposure days prior to the day at which the inhibitor occurred was felt to be the most important period for treatment intensity. Therefore, “duration between exposure days” was defined as the time period between the current exposure day and the fifth exposure day prior to this exposure day; this was extrapolated to 5 exposure days in cases with fewer than 5 exposures. This “duration between exposure days” was calculated for all exposure days of all patients. Similarly, dose of factor VIII product was defined as the mean dose of factor VIII product per kilogram body weight of the last 5 exposure days prior to each exposure day (or in the first 4 exposure days, mean dose of all previous days). We defined regular prophylaxis as regular factor VIII infusions at least once a week aimed at preventing bleeds.
Missing values were imputed using multiple linear regression methods.31 Family history of inhibitors was not imputed because 57% of the patients did not have relatives with hemophilia, providing insufficient data to impute this variable.
Results
Patient characteristics
Table 1 presents the characteristics of the 316 patients included in the study according to the first used product type. Patients receiving plasma-derived products tended to be born and treated in an earlier calendar time period and switched to another product type more often than patients on recombinant factor VIII products. Since patients from many different countries were included, a wide variety of plasma-derived factor VIII concentrates was used (Supplemental Appendix). Patients received factor VIII products on a total of 12 918 exposure days, of which 8493 (66%) exposure days were on recombinant factor VIII products. Eighty-two patients (26%) developed clinically relevant inhibitors. High-titer inhibitors developed in 66 patients and low-titer inhibitors, in 16 patients. Patients developed inhibitors after a median of 14 exposure days (interquartile range [IQR], 8-19 days) and at a median age of 15 months (IQR, 10-22 months).
Factor VIII product type and inhibitor development
Table 2 presents crude and adjusted relative risks of inhibitor development (all clinically relevant inhibitors and high-titer inhibitors) according to recombinant and plasma-derived factor VIII product types.
The risk of inhibitor development was not clearly lower in plasma-derived compared with recombinant factor VIII products (relative risk [RR], 0.8; 95% confidence interval [CI], 0.5-1.3). Among high-titer inhibitors, the possible reduction in risk was even less pronounced (RR, 0.9; CI, 0.5-1.5).
Compared to recombinant factor VIII products, the inhibitor risk was similar in patients on plasma-derived products containing considerable quantities of VWF (RR, 1.0; CI, 0.6-1.6), and it was 70% decreased in patients receiving plasma-derived products containing small quantities of VWF (RR, 0.3; CI, 0.1-1.1). These findings did not change after adjustment for potentially confounding factors (Table 2).
Additionally, we compared the risks of inhibitor development in patients receiving B-domain–deleted recombinant factor VIII products with the risk in patients receiving a full-length recombinant factor VIII product (Kogenate). The risk to develop inhibitors in patients receiving B-domain–deleted recombinant factor VIII (Refacto) appeared to be somewhat, but not statistically significantly, higher (RR, 1.4; CI, 0.8-2.6). The risks of inhibitor development in patients receiving the other recombinant products were similar (Table 2).
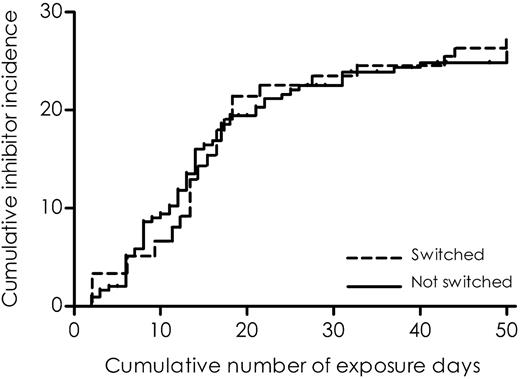
A total of 104 (33%) patients switched to another factor VIII product brand. They changed products for the first time after a median of 5 exposure days (IQR, 2-15 days; range, 2-48 days). Of the patients who switched products, 66% switched to another product only once. The reasons for switching were not known. To illustrate the association of switching between factor VIII products and the risk of inhibitor development, we plotted the cumulative incidences of inhibitor development according to switching from one product brand to any other brand (Figure 1), using a method described by Kurtzke.32 The risk of developing inhibitors was not increased after switching to another factor VIII product (adjusted RR, 0.9; CI, 0.6-1.6) (Table 3).
Cumulative incidence of clinically relevant inhibitor development according to switching products.
Cumulative incidence of clinically relevant inhibitor development according to switching products.
Discussion
We performed a retrospective multicenter cohort study among 316 previously untreated patients with severe hemophilia A treated on at least 50 exposure days. Patients who had received plasma-derived factor VIII products had a not statistically significant slightly lower risk to develop inhibitors than the patients who had received recombinant factor VIII products. Patients who had received product brands containing substantial amounts of von Willebrand factor did not have fewer inhibitors than patients who had received recombinant factor VIII products (containing no von Willebrand factor). Patients who had received monoclonal antibody–purified plasma-derived products containing very small amounts of von Willebrand factor developed fewer inhibitors. However, the number of patients receiving these products was low. Switching between factor VIII product brands was not associated with an increase in the risk for inhibitor development.
The absence of an evident difference in inhibitor incidences between plasma-derived and recombinant factor VIII products was at variance with a recent study by Goudemand et al.26 The latter study found a more than doubled risk of inhibitors in a historic cohort of patients treated with a full-length recombinant factor VIII product, Recombinate or Kogenate (inhibitor risk, 31%; adjusted relative risk for all inhibitors, 2.4 [CI, 1.0-5.8]) relative to a similar cohort of patients treated with a single plasma-derived factor VIII product containing large amounts of von Willebrand factor (inhibitor risk, 11%). A possible explanation for the difference with our findings is that the plasma-derived product studied by Goudemand et al26 is less immunogenic than the plasma-derived products used in our study. The patients in our study were treated with 23 different kinds of plasma-derived products. Monoclonal antibody–purified plasma-derived products containing very small amounts of von Willebrand factor induced fewer inhibitors in our patients. Yet, the number of patients receiving these products was low. This finding needs to be confirmed in future studies, in order to exclude a chance finding.
In accordance with our findings of no difference between the risk of developing inhibitors between plasma-derived and recombinant products were previous observations that multitransfused patients who switched from plasma-derived to recombinant factor VIII products did not appear to develop more inhibitors than similar patients receiving plasma-derived products, suggesting no obvious immunogenicity of recombinant products.33–36
The present observations on the association of von Willebrand factor and inhibitor development are at variance with several earlier reports, in which lower inhibitor risks in plasma-derived products have been ascribed to von Willebrand factor. Von Willebrand factor has been hypothesized to modulate the immunogenicity of factor VIII by masking B- and T-cell epitopes37 or by altering the whole tertiary structure of factor VIII.38 Several clinical observations and in vitro studies suggested that von Willebrand factor in factor VIII concentrates protects factor VIII from circulating inhibitory antibodies by masking antibody epitopes on the C2 domain.37,39,40 Additionally, hemophilic mice treated with factor VIII products without von Willebrand factor developed higher peak inhibitor titers than those treated with factor VIII products that contained von Willebrand factor.41 We showed that there is no decreasing trend in inhibitor development with an increasing von Willebrand factor content.
The risk of developing inhibitors was not increased after switching to another factor VIII product. This was in accordance with the findings of Baglin and Beacham.42 In 73 patients with hemophilia, the observed odds ratio for inhibitor development following a change in product as opposed to no change was 0.4 (CI, 0.1-2.1). It should be noted, however, that the effect of switching products may differ between previously untransfused patients and multitransfused patients. As we did not study multitransfused patients, our results cannot be generalized to this group.
A major strength of this study was that details on all first 50 exposure days were available, which enabled us to adjust associations for confounding factors. A well-known threat to the validity of nonrandomized comparison studies is confounding by indication. In the present comparison, we do not expect such confounding, because the choice of a factor VIII product for a previously untreated patient does not depend on the patient characteristics. Nevertheless, time trends in the choice of products may have confounded our findings. Patients treated with plasma-derived products are predominantly treated earlier in the time period of our study. In these earlier years, the use of prophylactic treatment was less common. Therefore, we adjusted all our findings for prophylactic treatment and for other treatment-related factors.
We prevented selection bias by studying the association of factor VIII product type and inhibitor development in an unselected cohort of patients. Selection of patients treated with only one type of factor VIII product would have biased the findings, because patients first treated with plasma-derived products more frequently switched product type than those who were first treated with recombinant products. Moreover, inhibitor patients were less likely to switch products because they developed an inhibitor after a few exposure days. As a consequence, selection of patients who never switched product would lead to an excess of inhibitor patients in the group of patients treated with plasma-derived products. Therefore, we analyzed factor VIII product type as a variable that could change on any exposure day, and we adjusted our findings accordingly.
When categorizing the plasma-derived products according to the von Willebrand factor content, we assumed that the stoichiometry of the factor VIII–von Willebrand factor complex was one factor VIII molecule per one von Willebrand factor monomer. However, it was reported to vary from 1:1 up to 1:50 according to the technique used.43 Since we aimed to classify patients according to high and low VWF, this classification seemed justified. Although Recombinate was reported to contain trace amounts of von Willebrand factor,44 it was grouped in the products containing no von Willebrand factor because other investigators did not detect any measurable von Willebrand factor.41,45
In conclusion, some, but not all, plasma-derived factor VIII products may confer a lower risk to develop inhibitors than recombinant factor VIII products in previously untreated patients with severe hemophilia. According to our data, it seems unlikely that the von Willebrand factor content of a product decreases inhibitor risks. Switching between products does not seem to affect inhibitor risk.
The online version of this manuscript contains a data supplement. that lists the members of the CANAL Study group, available on the Blood website; see the Supplemental Appendix link at the top of the online article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by an unrestricted educational grant of Novo Nordisk to the CANAL Study Group members, who are listed in Document S1 (available on the Blood website; see the Supplemental Document link at the top of the online article).
The authors would like to thank J. Over, PhD, Department of Product and Process Development, CLB, Sanquin Blood Supply Foundation, the Netherlands, for his expert advice on factor VIII products and S. le Cessie, PhD, department of medical statistics and clinical epidemiology, Leiden University Medical Center, the Netherlands, for her statistical advice.
Authorship
Contribution: All authors participated in designing the research; S.C.G., G.A., C.E.E., U.T., and all CANAL Study participants collected patient data; S.C.G. and J.G.B. analyzed data; S.C.G., J.G.B., and H.M.B. wrote the paper; all authors critically reviewed the final version of the paper.
Conflict-of-interest disclosure: S.C.G., H.M.v.d.B., and J.B.v.d.B. have received unrestricted research/educational funding for various projects at the Van Creveldkliniek from the following companies: Bayer, Baxter, ZLB Behring, Novo Nordisk, and Wyeth.
A complete list of the members of the CANAL study group is available as an online supplement on the Blood website; see the Supplemental Materials link at the top of the online article.
Correspondence: Johanna G. van der Bom, Department of Clinical Epidemiology, PO Box 9600, 2300 RC Leiden, the Netherlands; e-mail: j.g.vanderbom@lumc.nl.