Abstract
Eltrombopag (SB-497 115) is a first-in-class, oral, small-molecule, nonpeptide agonist of the thrombopoietin receptor (TpoR), being developed as a treatment for thrombocytopenia of various etiologies. In this phase 1 placebo-controlled clinical trial in 73 healthy male subjects, eltrombopag was administered as once-daily oral capsules for 10 days at doses of 5, 10, 25, 30, 50, and 75 mg. The pharmacokinetics of eltrombopag were dose dependent and linear, and eltrombopag increased platelet counts in a dose-dependent manner. There were no apparent differences in the incidence or severity of adverse events in subjects receiving active or placebo study medication. These observations indicate that eltrombopag is a once-daily, oral TpoR agonist with demonstrated thrombopoietic activity in human subjects, encouraging further studies in patients with thrombocytopenia.
Introduction
Thrombocytopenia, a reduction in platelet count, is a frequent finding in several medical disorders, such as aplastic anemia, some infections, myelodysplasia, idiopathic thrombocytopenic purpura (ITP), and chronic liver disease. Another etiology of clinically significant thrombocytopenia is the use of myelosuppressive chemotherapy or interferon antiviral treatment. Currently, there is an unmet need for thrombopoietic agents for the treatment of thrombocytopenia.
Thrombopoietin (Tpo) is the key cytokine involved in thrombopoiesis, and is the endogenous ligand for the thrombopoietin receptor (TpoR) that is expressed on the surface of megakaryocytes, and megakaryocytic precursors.1,2 Binding of Tpo to its receptor triggers the activation of the JAK-STAT pathway, leading to changes in gene expression that promote progression along the megakaryocytic pathway, ultimately leading to the release of platelets into the peripheral circulation.3–5
Administration of recombinant Tpo to rodents, primates, and humans leads to significant increases in circulating platelet levels.6–11 However, due to immunogenicity issues, forms of recombinant Tpo are no longer in clinical trials. Other molecules with Tpo-like activities are in clinical trials to treat thrombocytopenic patients.13 Peptidyl thrombopoietic agents, such as AMG-531, have been shown to increase platelet counts in healthy volunteers12 and patients with ITP13 ; however, they are not orally bioavailable and they have the potential to be immunogenic. Nonpeptide, small-molecule, TpoR agonists can be orally bioavailable and are less likely to induce an immune or injection site response.14–16
In this report we describe a first-in-class, small-molecule, nonpeptide, orally bioavailable thrombopoietin receptor agonist, eltrombopag (SB-497 115). Preclinical studies have shown that eltrombopag interacts selectively with TpoR, thereby activating intracellular signal transduction pathways, leading to increased proliferation and differentiation of human bone marrow progenitor cells.17 Platelet counts increased when eltrombopag was administered as an oral suspension to chimpanzees.18 Therefore, eltrombopag may be considered an oral platelet growth factor.
This phase 1 clinical trial in healthy human subjects was conducted to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of eltrombopag, when administered as a once-daily oral dose.
Patients, materials, and methods
Seventy-three healthy male subjects were randomized into 6 groups of 12 subjects to receive active eltrombopag (9 patients) or placebo (3 patients) as oral capsules once daily for 10 days at doses of 5, 10, 20, 30, 50, or 75 mg. The criteria for enrollment included no previous history of deep vein thrombosis (DVT), thrombocytopenia, abnormal platelet function, heart attack, stroke or heart murmur, and all clinical laboratory tests should be within the normal range at screening. Subjects were blinded to study medication; however, the investigator and sponsor were not blinded. One subject in the 50-mg cohort withdrew for personal reasons and was replaced.
All subjects provided written, informed consent to participate in the study, in accordance with the Declaration of Helsinki. The Hammersmith Medicine Research at the Central Middlesex Hospital Institutional Review Board provided formal approval for the study, which was conducted in accordance with good clinical practice, all known regulatory requirements.
Intensive safety, tolerability, pharmacokinetic, and pharmacodynamic assessments were made throughout the study. Safety assessments included electrocardiography, vital signs, serum chemistry, hematology, urinalysis, and adverse event reporting. Plasma samples for eltrombopag concentration-time analysis were collected after the administration of a single dose and at several time points during the 10-day repeat dose phase. Eltrombopag plasma concentration-time data were analyzed using actual collection times recorded during the study. Pharmacodynamic parameters included platelet count, platelet activation, and aggregation. Platelet activation was assessed using a Becton Dickinson flow cytometer to measure the percentage of PAC-1– and P-selectin–positive platelets, and a Helena Labs (Beaumont, TX) PACKS-4 system was used to measure platelet aggregation in the presence of ADP as an agonist.
Results and discussion
In this phase 1 placebo-controlled clinical trial, eltrombopag was administered as oral capsules to healthy male subjects at doses of 5 mg to 75 mg once daily for 10 days. There was a similar pattern of age (mean 27.5 years), height (1.8 m), and weight (79.8 kg) across the 7 dose cohorts. The mean baseline platelet count in the 73 subjects was 239 × 109/L (range, 134 × 109/L-347 × 109/L).
Pharmacodynamic assessments
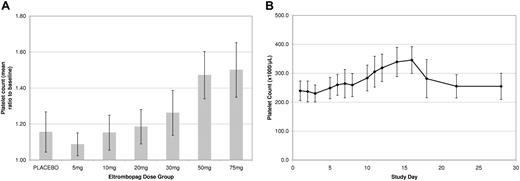
None of the 9 subjects in the 5-mg or 10-mg dose groups, 1 of 9 subjects in the 20-mg dose group, 6 of 9 subjects in the 30-mg dose group, and 9 of 9 in the 50-mg and 75-mg dose groups achieved a platelet count more than 20% above baseline. In the 5-mg, 10-mg, and 20-mg dose groups the 95% confidence interval (CI) for each active versus placebo comparison encompassed unity. The mean increases in platelet count for the 30-, 50-, and 75-mg dose levels were 24.1% (257.6 × 109/L to 319.6 × 109/L), 42.9% (254.3 × 109/L to 363.4 × 109/L), and 50.4% (235.9 × 109/L to 354.9 × 109/L), respectively, shown as a mean (SD) ratio to baseline in Figure 1A. The highest individual platelet count recorded was 457 × 109/L, an increase of 175 × 109/L from baseline on day 16 at the 50-mg dose.
Pharmacodynamic data. (A) Platelet response in healthy male subjects following oral dosing with eltrombopag (once per day) for 10 days. Increases are apparent at 30, 50 and 75 mg. Values in graph indicate mean and 1 SD. (B) Kinetics of platelet response in healthy male subjects following 10 days oral dosing of 75 mg eltrombopag. The platelet number began rising at 5 days and peaked at day 15. Values in graph indicate mean and 1 SD.
Pharmacodynamic data. (A) Platelet response in healthy male subjects following oral dosing with eltrombopag (once per day) for 10 days. Increases are apparent at 30, 50 and 75 mg. Values in graph indicate mean and 1 SD. (B) Kinetics of platelet response in healthy male subjects following 10 days oral dosing of 75 mg eltrombopag. The platelet number began rising at 5 days and peaked at day 15. Values in graph indicate mean and 1 SD.
A consistent increase in platelet count started after 8 days of repeat dosing with eltrombopag, and the time from first dose to peak platelet count was 16 days. By day 22 (12 days after the last dose of eltrombopag), platelet count had returned to baseline values (Figure 1B). These data are consistent with published platelet count profiles observed after the administration of recombinant MGDF in humans,14 therefore, the pharmacodynamic effects of eltrombopag likely reflect the normal kinetics of thrombopoiesis. Furthermore, there was no evidence of rebound thrombocytopenia, whereby platelet counts did not go below baseline levels following discontinuation of treatment.
Platelet function, as measured by platelet aggregation and activation, was not affected by the administration of eltrombopag, with similar results observed in subjects administered placebo and active study medication. For example, the mean percentage of PAC-1–positive cells in the placebo-treated subjects was 7.5% on day 1 and 11.7% on day 12, and in the 75-mg–treated subjects was 4.3% on day 1 and 4.8% on day 12. Platelet aggregation was reported as normal in all 73 subjects after receiving active or placebo treatment.
Pharmacokinetic assessments
Confirming preclinical data, eltrombopag was orally bioavailable when administered as oral capsules to healthy male subjects. The pharmacokinetics of eltrombopag were dose dependent and linear. In the 75-mg cohort, the mean Cmax was 7.3 μg/mL (15% coefficient of variability [CV]) at approximately 2.5 hours to 5 hours after dosing, with a mean area under the curve (AUC) of 79.0 μg.hour/mL (23% CV). T1/2 averaged more than 12 hours on day 10 across all doses, with the exception of the 5-mg dose (9 hours). There was no accumulation of eltrombopag after once-daily dosing at 5 mg and 10 mg, but there was approximately 40% to 50% accumulation at the 20-mg, 30-mg, 50-mg, and 75-mg dose levels.
Safety and tolerability assessments
Adverse events (AEs) did not differ among the healthy human subject treatment groups, including placebo, and were not dose related (Table 1). There were no changes in vital signs, laboratory tests, or ECGs, that were clinically significant or that could be reasonably attributed to study medication. Overall, 10-day administration of 5 mg to 75 mg eltrombopag was safe and well-tolerated, as judged by laboratory safety tests, blood pressure, heart rate, ECG, and AE. There were no serious AEs reported during the study.
In summary, eltrombopag represents the first in a class of orally bioavailable, small-molecule, nonpeptide thrombopoietin receptor agonists. The pharmacodynamic, pharmacokinetic, and safety profile demonstrated in this study supports further clinical studies in thrombocytopenic patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from GlaxoSmithKline.
Authorship
Contribution: J.M.J. and C.E.-M. designed research, analyzed data, and wrote the paper; D.W., Y.D., and D.C. collected and analyzed data; J.U. designed research; and V.K. designed research and acted as medical advisor.
Conflict-of-interest disclosure: All of the authors have declared a financial interest in a company whose (potential) product was studied in the present work. Two of the authors (C.E.-M., D.C.) have declared a financial interest in a competitor of a company whose (potential) product was studied in the present work. All of the authors are employed by a company or a competitor of a company (GSK) whose potential product was studied in the present work. Two of the authors (J.M.J., C.E.-M.) hold a patent related to the work that is described in the present study.
Correspondence: Julian Jenkins, GlaxoSmithKline, 1250 South Collegeville Rd, Collegeville, PA 19426; e-mail: julian.m.jenkins@gsk.com.