Abstract
Although interactions with bone marrow stromal cells are essential for multiple myeloma (MM) cell survival, the specific molecular and cellular elements involved are largely unknown, due in large part to the complexity of the bone marrow microenvironment itself. The T-cell costimulatory receptor CD28 is also expressed on normal and malignant plasma cells, and CD28 expression in MM correlates significantly with poor prognosis and disease progression. In contrast to T cells, activation and function of CD28 in myeloma cells is largely undefined. We have found that direct activation of myeloma cell CD28 by anti-CD28 mAb alone induces activation of PI3K and NFκB, suppresses MM cell proliferation, and protects against serum starvation and dexamethasone (dex)–induced cell death. Coculture with dendritic cells (DCs) expressing the CD28 ligands CD80 and CD86 also elicits CD28-mediated effects on MM survival and proliferation, and DCs appear to preferentially localize within myeloma infiltrates in primary patient samples. Our findings suggest a previously undescribed myeloma/DC cell-cell interaction involving CD28 that may play an important role in myeloma cell survival within the bone marrow stroma. These data also point to CD28 as a potential therapeutic target in the treatment of MM.
Introduction
Multiple myeloma (MM) remains an incurable clonal B lymphoid neoplasm of plasma cells, second only to non-Hodgkin lymphoma in incidence.1 Despite significant initial responses to chemotherapy, more than 90% of patients with MM relapse with resistant disease,2 underscoring the need to identify novel therapeutic targets that affect myeloma survival and resistance pathways. Given that MM cells are critically dependent on normal elements of the bone marrow stroma for cell growth and survival, these interactions are attractive targets. One such interaction is stromal production of soluble growth factors, such as IL-6 and TRANCE.1,3 Another important set of interactions involves direct myeloma cell contact with extracellular matrix (ECM) and/or stromal cells. Such direct contact up-regulates stromal cell IL-6 and VEGF production, induces NFκB signaling, drops myeloma cells out of cell cycle, and enhances resistance to chemotherapy.4–6 However, the specific molecular (eg, integrins4,7 ) and cellular components (eg, osteoclasts8 ) of these direct interactions within the complex bone marrow microenvironment are only beginning to be described. As important, the characteristic progression of myeloma to stromal independence marks a clinically worse disease,9 yet the mechanisms that underlie this transition are also poorly understood.
Identification of prosurvival receptors typically expressed on myeloma cells may point to the stromal cells expressing the receptor ligands. One potential receptor is CD28. CD28 has a restricted lineage expression, found predominantly on T cells but also on normal plasma cells, primary myeloma isolates, and myeloma cell lines at levels comparable with T cells.10–13 In T cells, CD28 receptor activation occurs following binding to its ligands, CD80 (B7-1) and CD86 (B7-2), which are expressed predominantly on professional antigen-presenting cells (APCs), and in particular on dendritic cells (DCs).14 The signaling pathways downstream of the CD28 receptor in T cells include PI-3 kinase → PDK1 → Akt and Vav → Rac1/Cdc42 → MEKK (both of which regulate NFκB activation),15 and, importantly, in myeloma cells, PI3K/Akt signaling transduces the antiapoptotic effects of IL-6 and insulin-like growth factor 1 (IGF-1),16 and for IGF-1, involves sustained activation of NFκB.17 Functionally, CD28 delivers the costimulatory signal that in conjunction with T-cell receptor (TCR) signaling results in augmented T-cell proliferation, effector function,18,19 and enhanced survival via up-regulation of the antiapoptotic gene bcl-xL20 and more efficient glucose metabolism.21
In contrast to T cells, little is known about CD28 function in myeloma cells. Clinically, however, CD28 expression highly correlates with myeloma disease progression, such that high CD28 expression is seen in 26% of newly diagnosed myelomas, 59% of medullary recurrences, 93% of extramedullary relapses, and 100% of secondary plasma cell leukemias (including nearly all the human and murine MM cell lines).12,22 Moreover, myeloma cell expression of CD28 in newly diagnosed patients is a major prognostic predictor of poor clinical outcome following high-dose chemotherapy.23,24 These clinical findings suggest that CD28 expression helps these MM cells better survive treatment and result in their selective outgrowth. In addition, the possibility that CD28 is involved in the progression to stroma-independent MM is supported by observations that primary CD28+ myelomas coexpress CD86 (10 of 10 patient samples in Robillard et al12 ) and that CD86+ myelomas have a significantly poorer prognosis.25 The possibility of autocrine CD28-CD86 activation is supported by some,13 but not all,26 in vitro studies. Other functional studies of CD28 activation in MM cell lines have been equivocal.10,13 CD28 activation does not induce IL-6 secretion in MM cells22,26 like it does in T cells,27 but does up-regulate expression of the proangiogenic chemokine IL-8.22 Direct evidence of a prosurvival role for CD28 in myeloma cells has not been reported, although such a role in normal plasma cells is indirectly suggested by our previous observations that CD28 knockout mice have markedly diminished serum immunoglobulin levels,18,28 including T-independent antibody responses.29 Conversely, an anti-CD28 mAb recently developed by Qiu et al inhibits MM cell line proliferation and induces some morphologic aspects of apoptosis; whether this is due to an activating or blocking effect of the antibody was not determined.30
If CD28 is supporting myeloma cell survival, its activation in vivo is likely to be via direct cell contact with a B7+ cell within the microenvironment. These include other CD86+ myeloma cells and/or professional antigen-presenting cells (APCs) expressing CD80/CD86 (B cells, monocyte/macrophages, DCs). Consistent with this, DCs and other myeloid APCs actively infiltrate implanted plasmacytomas in murine models,31 and DCs are readily found throughout myeloma infiltrates in patient bone marrow biopsies.32 Altogether, these has led us to examine whether CD28 can transduce survival signals to myeloma cells, and whether MM CD28 is activated through cell-cell contact with other B7+ cells.
Materials and methods
The University of Miami institutional review board (IRB) approval has been obtained for the sample collection protocols for obtaining the primary myeloma samples.
Cells, reagents, and culture
RPMI 8226, U266, K562, and KG1 cell lines were obtained from American Type Culture Collection (Manassas, VA). The MM.1S cell line was the gift of Dr S. Rosen (Robert H. Lurie Cancer Center, Chicago, IL). Early passage cells (ie, in continuous culture for less than 2 months) were used for all experiments, and all cell lines were more than 85% viable at the beginning of all experiments. Primary myeloma cells were obtained from bone marrow aspirates from patients with recurring myeloma (under IRB-approved protocols) and purified by CD138 immunomagnetic selection (Miltenyi Biotec, Auburn, CA). The agonistic anti-CD28 mAb 9.333 was either used as soluble antibody (1 μg/mL) or immobilized to Dynal beads (Lake Success, NY) and used at the indicated beads per cell ratio.34 For the serum-starvation experiments, myeloma cell lines were cultured in media (RPMI 1640) without serum with or without anti-CD28 mAb, and viability was determined by PI/annexin V staining and fluorescence-activated cell sorter (FACS) analysis. For dex treatment experiments, myeloma cell lines were cultured in 0.1% FBS with or without anti-CD28 mAb plus 100 μM dex, and viability was assessed after 72 hours by PI/annexin V staining. For the primary myeloma cell survival, total viable cell numbers were enumerated by quadruplicate cell counts using trypan blue.
Cell proliferation assays were done in 0.1% FCS. [methyl-3H] thymidine (0.5 μCi [0.0185 MBq]/well) was added for the final 18 hours of culture, and incorporation was measured using the Beta Plate scintillation counting system (Wallac Inc, Gaithersburg, MD).35 All conditions were performed in triplicates, and data are represented as the mean counts ± 1 SD.
Myeloma-DC coculture
K562 and KG1 were differentiated into dendritic cells/myeloid APCs as previously described.35,36 Briefly, cells were cultured in media alone or differentiated for 5 to 7 days with PMA (10 ng/mL; Sigma, St Louis, MO) with or without TNF-α (10 ng/mL; R&D Systems, Minneapolis, MN). Primary monocytes were enriched from the peripheral blood mononuclear cells (MNCs) of healthy donors (in IRB-approved protocols) by plastic adherence and differentiated into DCs using GM-CSF (1000 U/mL; Immunex, Seattle, WA) and IL-4 (1000 U/mL; R&D Systems) for 8 days with or without TNF-α (20 ng/mL) for the last 4 days of culture.37 DCs were then washed, irradiated at 3000 R (30 Gy) (KG1; monocyte-derived DCs) or 12000 R (120 Gy) (K562; 137Cs), and seeded into round-bottom wells alone or with myeloma cells at the cell numbers indicated. Cocultures were done in media plus 10% FCS for the proliferation assays. After 24 hours, wells were pulsed with [3H] TdR, and incorporation was measured for the next 18 hours of culture using the Beta Plate (Wallac, Gaithersburg, MD) scintillation counting system and expressed as means ± standard deviation of triplicate wells. Where indicated, hCD28-Ig (R&D Systems) was added to the DCs 1 hour before addition of MM cells.
The coculture viability experiments were done in 0.1% FCS. Myeloma cells were cultured alone, or 1:1 with irradiated, undifferentiated DC precursors or differentiated DCs, treated for 72 hours with 100 μM dex, and analyzed by FACS for 7AAD (dead cells; Beckman Coulter, Fullerton, CA) and CD28 expression (MM cells).
Flow cytometry
Cells were stained as previously reported35 with anti-CD28, CD80, CD86 mAb, or isotype control (Immunotech, Westbrook, ME). Cells were also stained with CTLA4-Ig (or isotype-matched human Ig) and goat anti–human Ig PE. A total of 10 000 live cells were analyzed by flow cytometry on a Coulter XL flow cytometer (Beckman Coulter) using software supplied by the manufacturer.
For cell-cycle analysis, 8226 were labeled with CSFE and cocultured at a 1:1 ratio (2 × 104 cells) with either undifferentiated K562 or K562 differentiated with PMA for 24 hours, permeabilized, and stained with propidium iodide (PI) in the presence of RNase A.38 Cell-cycle analysis of CSFE+ cells was conducted on a BD LSR1 (Becton Dickinson, Franklin Lakes, NJ) collection of viable cells through the G0/G1 (M2), S (M3), and G2/M (M4) gates. Data are representative of 3 independent experiments.
Western blot
Western blot analysis was performed as previously described.35 Briefly, cell lysates were made from 8226 or U266 cells that were cultured with or without anti-CD28 mAb for 24 hours. Cell lysates were made, protein levels were quantitated by using the Micro BCA reagent kit (Pierce, Rockford, IL), and equal amounts of protein were separated by SDS-PAGE (4% stacking/10% resolving), electroblotted to nitrocellulose, and probed with antibodies specific for Bcl-xL,20 IκBα, or Rel B actin (Santa Cruz Biotechnology, Santa Cruz, CA). The proteins were visualized by chemoluminescent detection (ECL; Amersham Life Sciences, Aylesbury, United Kingdom).
PI3K p85 ELISA assay
PI3K activation was assayed by Fast Activated cell-based enzyme-linked immunosorbent assay (ELISA) (FACE; Active Motif, Carlsbad, CA). Briefly, 17 000 cells were seeded into poly-L-lysine–coated 96-well culture plates and serum-starved for 16 hours. Cells were then treated with 1 μg/mL anti-CD28 mAb 9.3 for the indicated times, fixed, and incubated with anti–phospho-p85 antibodies. The ELISA assay was developed and read as per the manufacturer's instructions.
EMSA
Electromobility shift assays (EMSAs) were done for NFκB family members as previously described.36 Briefly, 8226 or U266 cells were cultured with or without anti-CD28 mAb for 24 hours. Nuclear extracts were made, and equal amounts of protein were incubated with 32P-labeled primer containing consensus NFκB-binding sites (GAT CCA ACG GCA GGG GAA TTC CCC TCT CCT TA) and separated on 4% polyacrylamide gels. For supershift assays, samples were first incubated with anti-Rel B, anti-p50, anti-p65, and anti–c-Rel (all from Santa Cruz Biotechnology). Samples were visualized by autoradiography.
Immunohistochemistry
Biopsies of bone marrow and extramedullary plasmacytomas were obtained from patients with refractory/recurring MM as part of IRB-approved protocols and following informed consent. Consecutive sections were then stained with anti-CD138 (myeloma; R&D Systems), antifascin (D; Research Diagnostics, Flanders, NJ), anti-CD28 (R&D Systems), or hematoxylin/eosin. Photomicrographs were taken on a Leica DMIRB microscope (Leica Microsystems, Wetzlar, Germany) with a Coolsnap camera (Roper Scientific Ottobrun, Germany) controlled by MetaMorph v4.0 software (Molecular Devices, Downington, PA).
Statistical analysis
Pairwise comparisons were conducted using the Student t test.
Results
Myeloma cells express CD28 and CD86
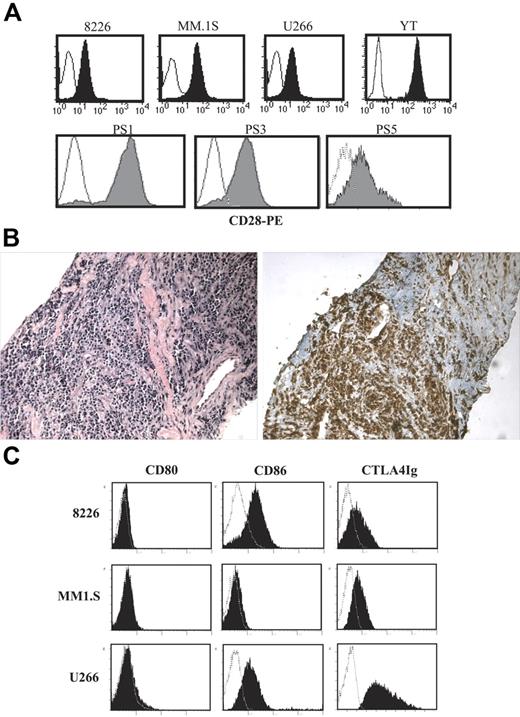
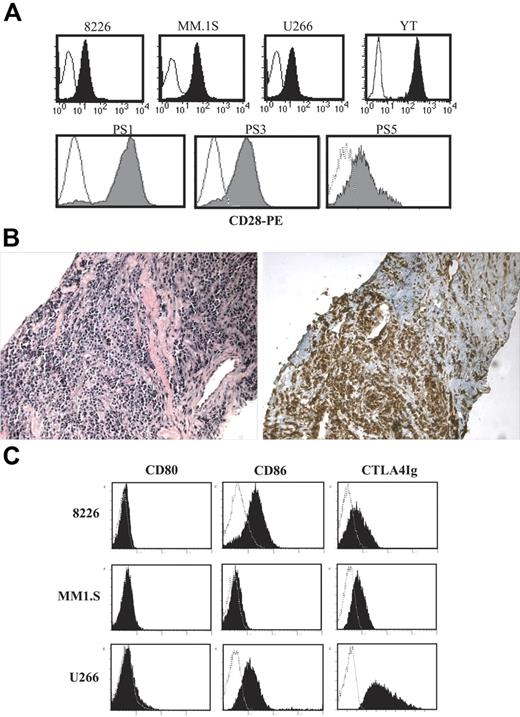
As seen in Figure 1A (top panels), CD28 is expressed on 3 human myeloma cell lines (RPMI 8226 [8226], MM.1S, and U266) at levels slightly lower than the cytotoxic T-cell line YT. CD28 is also expressed on primary MM cells purified from bone marrow aspirates of patients with recurring disease (Figure 1A; bottom panels), and also on the infiltrating myeloma cells from patients with extramedullary intramuscular plasmacytomas (Figure 1B). As suggested by previous studies, all 3 MM cell lines also coexpressed CD86 but not CD80 (Figure 1C), which is more clearly seen using indirect staining with CTLA4-Ig.
CD28 and CD86 expression. (A) CD28. The indicated cells were stained with anti-CD28 PE (filled histogram) or isotype control (open histogram) and analyzed by FACS. Top panels show cell lines. Bottom panels show primary myeloma isolates. Primary myeloma cells were purifed from 3 different patient samples (PS; bone marrow aspirates, recurring MM) by CD138 immunomagnetic selection. (B) Extramedullary plasmacytoma. Serial sections from an extramedullary plasmacytoma were stained with hematoxylin/eosin (left panel) or anti–human CD28 (brown staining, right panel). Magnification, 20×/0.40 NA N Plan objective. Representative sections from 1 of 2 patients with intramuscular extramedullary plasmacytomas. (C) CD80 and CD86. The cell lines indicated were stained with isotype control (open histograms), anti-CD80, CD86 mAb, or CTLA4-Ig (closed histograms), and analyzed by FACS. Data are representative of 2 independent experiments.
CD28 and CD86 expression. (A) CD28. The indicated cells were stained with anti-CD28 PE (filled histogram) or isotype control (open histogram) and analyzed by FACS. Top panels show cell lines. Bottom panels show primary myeloma isolates. Primary myeloma cells were purifed from 3 different patient samples (PS; bone marrow aspirates, recurring MM) by CD138 immunomagnetic selection. (B) Extramedullary plasmacytoma. Serial sections from an extramedullary plasmacytoma were stained with hematoxylin/eosin (left panel) or anti–human CD28 (brown staining, right panel). Magnification, 20×/0.40 NA N Plan objective. Representative sections from 1 of 2 patients with intramuscular extramedullary plasmacytomas. (C) CD80 and CD86. The cell lines indicated were stained with isotype control (open histograms), anti-CD80, CD86 mAb, or CTLA4-Ig (closed histograms), and analyzed by FACS. Data are representative of 2 independent experiments.
CD28 triggers PI3K signaling in myeloma cells
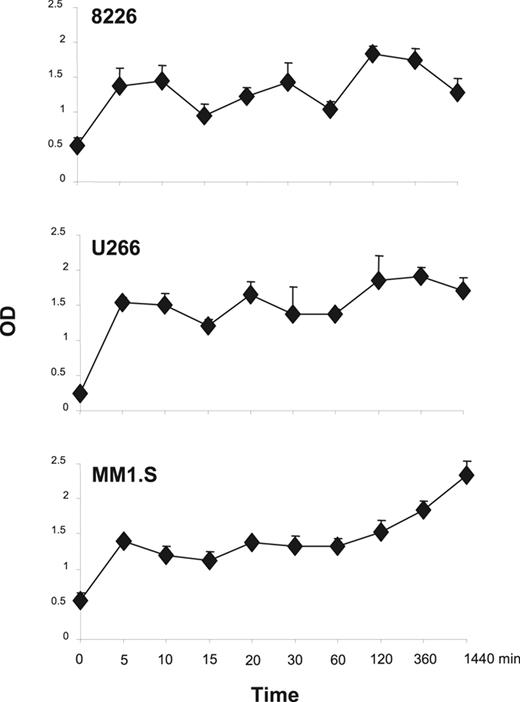
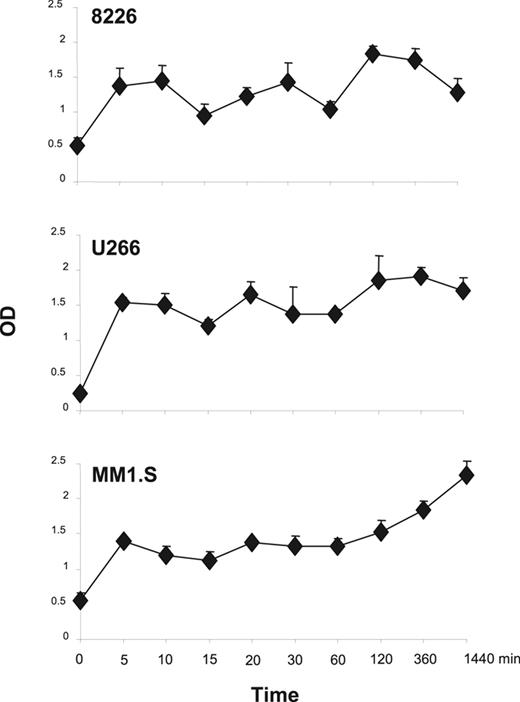
In T cells, 1 pathway downstream of the CD28 receptor is PI3K/Akt activation,39 and in myeloma cells the prosurvival effects of IGF-1 are transduced through PI3K/Akt signaling and downstream NFκB activation.16,17 To assess whether CD28 activation can also trigger this pathway in MM cells, PI3K activation was characterized via ELISA-based measurement of the phosphorylated (activated) p85 subunit of PI3K following activation with the anti-CD28 mAb 9.3. As can be seen in Figure 2, CD28 activation results in rapid (by 5 minutes) detection of phospho-p85 in all 3 MM cell lines, with sustained levels out to 24 hours. This is consistent with previous studies demonstrating association of p85 to the CD28 receptor in myeloma cells.26
CD28 signaling induced PI3K activation. The indicated MM cells were treated with 1 μg/mL of agonistic anti-CD28 mAb 9.3 for different time course and fixed. Levels of phosphorylated PI3K were assayed by FACE PI3 Kinase p85 ELISA kit (Active Motif) per the manufacturer's instructions. Mean + SEM is shown. Data are performed in triplicate for each time point and is representative of 2 independent experiments. The x-axis (time) is nonlinear. Compared with time 0, at 5 minutes P = .11 for 8226, P = .006 for U266, and P = .02 for MM1.S. Compared with time 0, at 1440 minutes P = .13 for 8226, P = .017 for U266, and P = .005 for MM1.S.
CD28 signaling induced PI3K activation. The indicated MM cells were treated with 1 μg/mL of agonistic anti-CD28 mAb 9.3 for different time course and fixed. Levels of phosphorylated PI3K were assayed by FACE PI3 Kinase p85 ELISA kit (Active Motif) per the manufacturer's instructions. Mean + SEM is shown. Data are performed in triplicate for each time point and is representative of 2 independent experiments. The x-axis (time) is nonlinear. Compared with time 0, at 5 minutes P = .11 for 8226, P = .006 for U266, and P = .02 for MM1.S. Compared with time 0, at 1440 minutes P = .13 for 8226, P = .017 for U266, and P = .005 for MM1.S.
Activation of downstream NFκB signaling
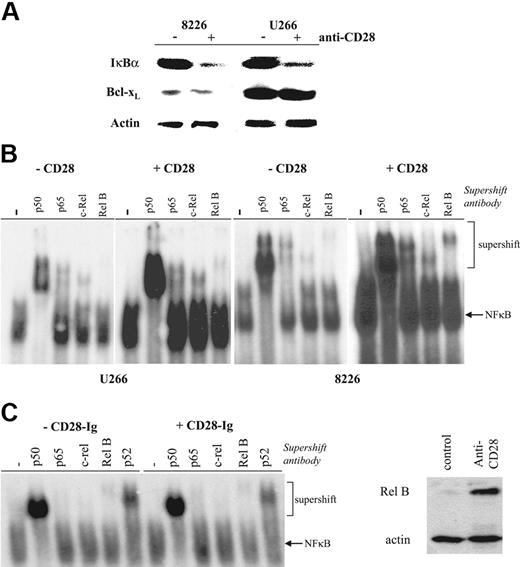
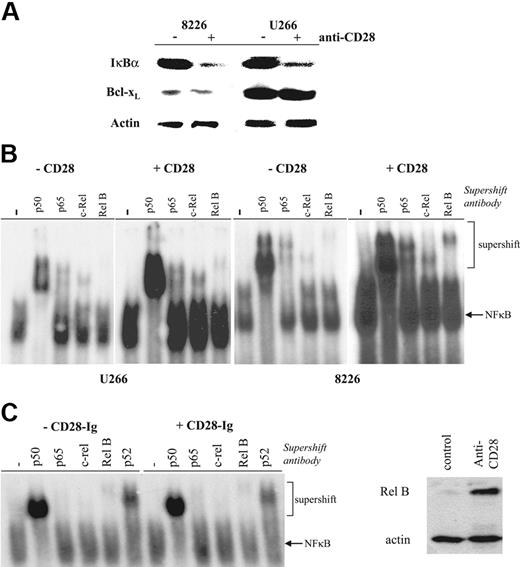
To determinewhether CD28-induced PI3K triggered downstream NFκB signaling, we initially sought evidence for IκBα degradation. As seen in Figure 3A, antibody-mediated activation of CD28 alone results in reduction of cellular IκBα in both 8226 and U266. The antiapoptotic protein Bcl-xL is not up-regulated, consistent with previous reports that CD28 activation alone does not up-regulate this expression in T cells.20 To more directly assess CD28-induced NFκB signaling, levels of free nuclear NFκB dimers were measured by electromobility gel shift assay. CD28 activation (using either bead bound or soluble mAb [not shown]) increases total nuclear NFκB binding in both U266 and 8226 (Figure 3B). Supershift assays for NFκB family members demonstrate increased levels of p50, p65, Rel B, and c-Rel, suggesting that both the canonical (p65, p50/p52 homodimers) and noncanonical (RelB) pathways are being activated. In contrast, binding of CD86 with CD28-Ig has no effect on NFκB signaling (Figure 3C), indicating that 9.3's effects are not paradoxically due to the blocking of a myeloma CD28–myeloma CD86 interaction. Finally, we and others have previously shown that NFκB signaling plays the primary role in up-regulating expression of the NFκB family member Rel B,40 and similarly find that CD28 activation induces Rel B expression in myeloma cell lines (Figure 3D). Together, these findings indicate that CD28 activation by itself results in NFκB signaling in MM cells.
Effect of CD28 activation on NFκB activation. (A) IκBα and Bcl-xL expression. 8226 or U266 cells were cultured with or without anti-CD28 mAb beads as indicated, and analyzed by Western blot using Abs specific for IκBα, Bcl-xL, or actin as indicated. (B) Nuclear NFκB binding activity. 8226 or U266 cells were cultured with or without immobilized anti-CD28 mAb as indicated, and nuclear extracts analyzed by EMSA for binding to 32P-labeled primers containing consensus NFκB binding sites. For supershift assays, samples were first incubated with no antibody (−), anti-p50, anti–Rel B, anti–c-Rel, or anti-p65. Data are representative of 3 independent experiments. (C) Binding to/blocking CD86 does not induce NFκB signaling. U266 were treated as in panel B except with or without CD28-Ig (100 μg/mL), and analyzed after 24 hours for nuclear NFκB binding activity. Data are representative of 2 experiments. (D) Up-regulation of Rel B expression by CD28 activation. U266 were treated with control or anti-CD28 mAb (soluble; 1 μg/mL) for 24 hours and analyzed for Rel B and actin expression by Western blot. Data are representative of 2 experiments.
Effect of CD28 activation on NFκB activation. (A) IκBα and Bcl-xL expression. 8226 or U266 cells were cultured with or without anti-CD28 mAb beads as indicated, and analyzed by Western blot using Abs specific for IκBα, Bcl-xL, or actin as indicated. (B) Nuclear NFκB binding activity. 8226 or U266 cells were cultured with or without immobilized anti-CD28 mAb as indicated, and nuclear extracts analyzed by EMSA for binding to 32P-labeled primers containing consensus NFκB binding sites. For supershift assays, samples were first incubated with no antibody (−), anti-p50, anti–Rel B, anti–c-Rel, or anti-p65. Data are representative of 3 independent experiments. (C) Binding to/blocking CD86 does not induce NFκB signaling. U266 were treated as in panel B except with or without CD28-Ig (100 μg/mL), and analyzed after 24 hours for nuclear NFκB binding activity. Data are representative of 2 experiments. (D) Up-regulation of Rel B expression by CD28 activation. U266 were treated with control or anti-CD28 mAb (soluble; 1 μg/mL) for 24 hours and analyzed for Rel B and actin expression by Western blot. Data are representative of 2 experiments.
CD28 downmodulates myeloma cell proliferation
The ability of CD28 to costimulate T-cell proliferation and survival can be segregated into 2 independent downstream signaling pathways, with PI3K being essential for survival while factors binding the C-terminal proline motifs for proliferation and cytokine responses.41 We next assessed the effect CD28 activation on myeloma cell proliferation, which were done in low FCS conditions (0.1%) as normal serum contains IGF-1 that may mask a CD28 signal. In comparison with 8226, U266, and MM.1S cultured with control (uncoated) beads, cell proliferation was significantly suppressed by culture with 9.3-coated beads. In comparison, the 9.3-coated beads had no effect on the CD28− cell line K562 (Figure 4).
CD28 activation inhibits myeloma cell proliferation. 8226, U266, and MM.1S (2 × 105) were cocultured with uncoated beads (beads alone) or 9.3-coated beads. K562 (CD28− control; 2 × 104) was cultured in media alone or 9.3-coated beads. After 24 hours, cells were pulsed with 3H TdR, and incorporation was measured 18 hours later and expressed as means ± SD of triplicate wells. Data shown are 1 experiment representative of 6 independent experiments. Comparing the proliferation between the control versus 9.3-coated beads: P = .025 for 8226, P = .003 for U266, P = .001 for U266, and P = .82 for K562.
CD28 activation inhibits myeloma cell proliferation. 8226, U266, and MM.1S (2 × 105) were cocultured with uncoated beads (beads alone) or 9.3-coated beads. K562 (CD28− control; 2 × 104) was cultured in media alone or 9.3-coated beads. After 24 hours, cells were pulsed with 3H TdR, and incorporation was measured 18 hours later and expressed as means ± SD of triplicate wells. Data shown are 1 experiment representative of 6 independent experiments. Comparing the proliferation between the control versus 9.3-coated beads: P = .025 for 8226, P = .003 for U266, P = .001 for U266, and P = .82 for K562.
CD28 activation enhances myeloma cell survival
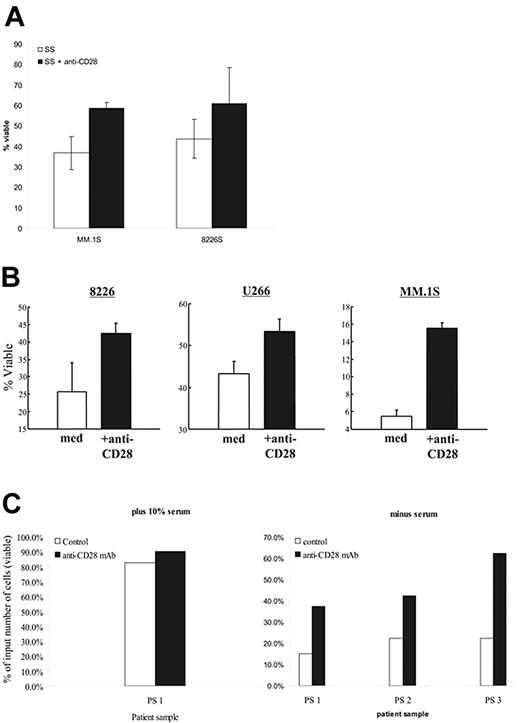
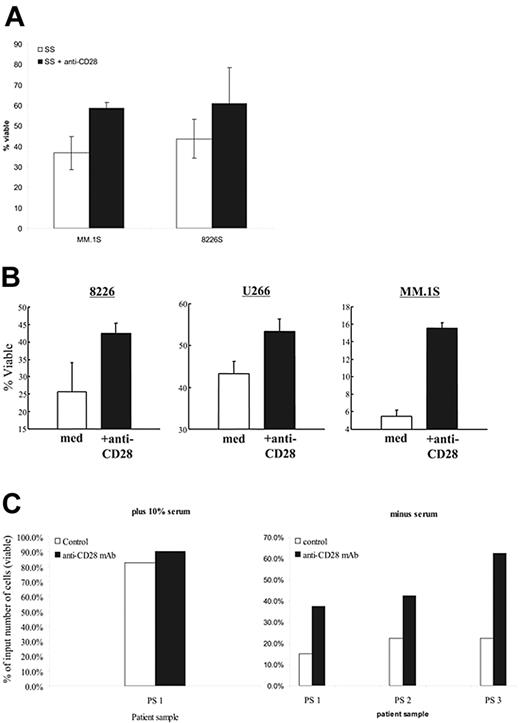
Prosurvival factors IGF-1 and IL-6 activate NFκB,42 suggesting that CD28 may also support myeloma cell survival (we [data not shown] and others22 have found that CD28 does not induce IL-6 secretion in MM cells). As serum contains sufficient IGF-1 to prevent apoptosis,43 we asked whether withdrawal of serum induces cell death in 8226 and MM.1S, and whether CD28 activation could provide substitute survival signals. As seen in Figure 5A, serum starvation results in substantial cell death in both 8226 and MM.1S by 48 hours that could be abrogated in part by the addition of anti-CD28 mAb. The data does not quite reach statistical significance, but is clearly trending toward improved survival with CD28 activation.
CD28 activation protects against induced cell death. (A) Serum starvation. The indicated myeloma cells were cultured in media (RPMI 1640) without serum (SS), or medium without serum plus soluble anti-CD28 mAb (SS+CD28). After 48 hours, viability was determined by annexin V/PI staining. Data are the aggregate mean ± SD of 3 independent experiments. P = .07 for MM.1S serum starvation versus SS + anti-CD28; P = .12 for 8226S. (B) Dex-mediated cell death. The indicated myeloma cell lines were cultured in 0.1% FBS with or without soluble anti-CD28 mAb and 100 μM dex. After 72 hours, viability was determined by Annexin V/PI staining. Data are the aggregate mean ± SD of 3 independent experiments. In comparing the survival in dex versus dex plus anti-CD28: P = .02 for MM.1S, P = .03 for 8226S, and P = .002 for U266. (C) Primary myeloma cells. Primary myeloma cells were purified from 3 different patient samples (bone marrow aspirates; recurring MM) by CD138 immunomagnetic selection (Miltenyi Biotech). The samples were more than 90% CD138+ and were all CD28+. Input cells (2 × 105) were cultured in 10% FCS (PS 1; left panel) or no serum (PS 1-3; right panel) with or without anti-CD28 mAb (9.3; 1 μg/mL) for 24 hours. Total viable cell numbers were then enumerated in trypan blue and expressed as the percentage of the starting input number of cells.
CD28 activation protects against induced cell death. (A) Serum starvation. The indicated myeloma cells were cultured in media (RPMI 1640) without serum (SS), or medium without serum plus soluble anti-CD28 mAb (SS+CD28). After 48 hours, viability was determined by annexin V/PI staining. Data are the aggregate mean ± SD of 3 independent experiments. P = .07 for MM.1S serum starvation versus SS + anti-CD28; P = .12 for 8226S. (B) Dex-mediated cell death. The indicated myeloma cell lines were cultured in 0.1% FBS with or without soluble anti-CD28 mAb and 100 μM dex. After 72 hours, viability was determined by Annexin V/PI staining. Data are the aggregate mean ± SD of 3 independent experiments. In comparing the survival in dex versus dex plus anti-CD28: P = .02 for MM.1S, P = .03 for 8226S, and P = .002 for U266. (C) Primary myeloma cells. Primary myeloma cells were purified from 3 different patient samples (bone marrow aspirates; recurring MM) by CD138 immunomagnetic selection (Miltenyi Biotech). The samples were more than 90% CD138+ and were all CD28+. Input cells (2 × 105) were cultured in 10% FCS (PS 1; left panel) or no serum (PS 1-3; right panel) with or without anti-CD28 mAb (9.3; 1 μg/mL) for 24 hours. Total viable cell numbers were then enumerated in trypan blue and expressed as the percentage of the starting input number of cells.
We next examined whether CD28 activation could protect against death induced by dex, a more defined death signal and clinically relevant agent. To minimize the possibility of serum IGF-1 masking a CD28 prosurvival signal, we conducted these assays in low-serum conditions (0.1%, which alone did not affect the viability of our cell lines). As seen in Figure 5B, dex effectively killed all 3 MM cell lines, and anti-CD28 mAb–mediated activation resulted in significant protection against this dex-induced death.
Whether CD28 activation also had prosurvival effects in primary myeloma cells was examined in cells purified from 3 patient samples (recurring disease), with the resulting cell populations more than 90% positive for the plasma cell marker CD138 (all 3 samples were CD28+). The purified MM cells were then cultured in 10% serum (Figure 5C; left, for patient sample 1) or without serum (Figure 5C; right) with or without anti-CD28 mAb for 24 hours. Serum starvation was used, as all the patients were clinically dex resistant. While primary myeloma cells fared reasonably well in 10% FCS (and were largely unaffected by anti-CD28 mAb, which also demonstrates that the mAb was not inducing proliferation), there was a considerable loss of viable cells when serum was withdrawn. Consistent with our cell-line findings, activation of CD28 substantially improved myeloma cell survival.
DCs associate with myeloma cells in vitro and in vivo
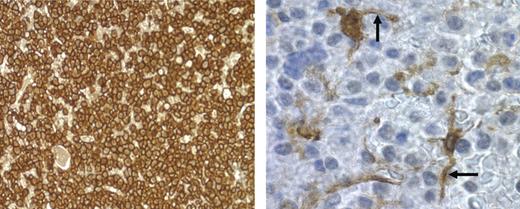
Biologically relevant activation of CD28 in myeloma cells likely occurs the same way it does in T cells, namely by direct cell-cell contact with CD80/CD86 cells (especially APCs). Given that previous studies have found DCs in myeloma/plasmacytoma infiltrates,32,44 we first examined whether DCs could be preferentially found within myeloma infiltrates in patient bone marrow biopsies. CD138 staining was used to identify myeloma cells, and fascin staining plus morphology was used to identify DCs.32 Previous studies have found that the expression of the actin-bundling protein fascin is very specific for DCs in paraffin-embedded tissue,45–47 and superior to HLA-DR or S100 staining for distinguishing DCs from tissue macrophages.48 We found that 4 of 5 patients with recurring myeloma had numerous fascin+ cells with DC morphology within the CD138+ myeloma cell infiltrates, while the fifth patient (no. 90) had fewer but detectable numbers of DCs (Table 1). These fascin+ cells were predominantly localized within the CD138+ cell infiltrates, as noninfiltrated areas of bone marrow within the same marrow section had significantly fewer fascin+ cells (except for patient no. 90, who had the same low number in both areas). Figure 6 demonstrates the numerous fascin+ cell processes and less frequent cell bodies (right panel) that could be readily found interdigitating between CD138+ myeloma cells (left panel). Because fascin expression positively correlates with DC maturation,48 these findings suggest that myeloma-infiltrating DCs have the high CD80/CD86 expression typical of mature DCs. Consistent with this, CD83 staining yields similar findings (not shown).
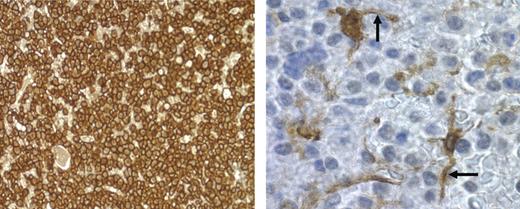
DCs physically interact with myeloma cells. Bone marrow biopsy from patient 88 with recurring MM was stained for CD138 for myeloma cells (brown-staining cells; left panel; magnification, 40×/0.55 NA N PLAN objective) or fascin for DCs (brown-staining cells; right panel; magnification, 100×/1.4 NA oil objective). Photomicrograph of fascin+ cells was taken from a region in the center of the field shown in the left panel. Arrows point to interdigitating DC dendrites.
DCs physically interact with myeloma cells. Bone marrow biopsy from patient 88 with recurring MM was stained for CD138 for myeloma cells (brown-staining cells; left panel; magnification, 40×/0.55 NA N PLAN objective) or fascin for DCs (brown-staining cells; right panel; magnification, 100×/1.4 NA oil objective). Photomicrograph of fascin+ cells was taken from a region in the center of the field shown in the left panel. Arrows point to interdigitating DC dendrites.
Coculture with DCs modulates myeloma cell proliferation and survival
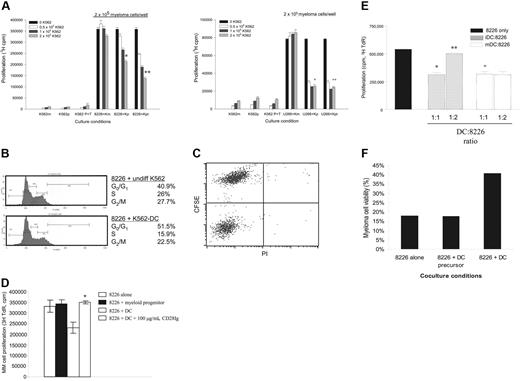
To characterize the possible effects of DC interaction on myeloma cells, we initially used myeloid DCs derived from human CD34+ leukemia cell lines (KG136 and K56235 ). Cell-line–derived DCs are a more homogenous population than primary monocyte-derived DCs, with less variability due to progenitor purity, maturation differences, etc. We and others have also shown that these myeloid blasts are not immunostimulatory when undifferentiated, but when differentiated with cytokines or phorbol esters (PMA), result in APCs that have characteristic DC markers (MHC I and II, CD40, CD80, CD86, and CD83), expression of DC-specific molecular markers (eg, DC-CK1, DC-STAMP), and unique DC function (eg, the ability to cross-present antigen, potently activate T cells to a significantly greater degree compared with normal monocytes, etc).35,36,36,49–55 We first characterized the effect of DCs on myeloma cell proliferation. Proliferation of 8226 (Figure 7A; left panel) and U266 (Figure 7A; right panel) was not affected when cocultured with irradiated, undifferentiated K562. However, similar to activation with anti-CD28 mAb, coculture with K562 differentiated to DCs by PMA or PMA plus TNF-α (TNF addition drives further DC maturation) significantly downmodulated the proliferation of 8226 and U266 proliferation. KG1 yielded the same results (Figure 7D). It is unlikely that this decrease is due to residual PMA carryover, as PMA is not detected in other sensitive assays (eg, T-cell proliferation), and that PMA alone has no effect on 8226 or U266 proliferation (data not shown; Zhang et al26 ). Initial cell-cycle analysis of 8226 suggests the inhibition of proliferation is via a G0/G1 arrest when cultured with K562-derived DCs, but not with undifferentiated K562 (Figure 7B). To formally exclude the induction of MM cell death as the cause of decreased proliferation, CSFE-labeled 8226 was assayed for cell viability (by PI staining) after coculture with K562-derived DCs. After 24 hours of coculture there are very few PI+ dead cells in either the CFSE+ 8226 or CFSE− K562 DC populations (Figure 7C).
Coculture with DCs downmodulates MM proliferation and enhances survival. (A) Proliferation. K562 were cultured in media alone (K562m; Km), or differentiated with PMA (K562p or Kp) or PMA plus TNF-α (K562 P+T or Kpt), irradiated, and cocultured with 8226 (right panel) or U266 (left panel). Proliferation was measured by thymidine incorporation and expressed as means ± SD of triplicate wells. Data are representative of 4 independent experiments. Compared with coculture with undifferentiated K562 (K562m): *P = .001; **P < .001; +P = .004; ++P = .001. (B) Cell-cycle analysis. CSFE-labeled 8226 were cocultured at a 1:1 ratio (2 × 104 cells) with either undifferentiated K562 (top) or K562 differentiated with PMA (K562-DC; bottom) for 24 hours, permeabilized, and stained with PI. Cell-cycle analysis of CSFE+ cells was conducted on a BD LSR1; percentage of cells in G0/G1, S, and G2/M are shown to the right. Data are representative of 3 independent experiments. (C) MM viability following coculture with DCs. CFSE-labeled 8226 were cocultured with irradiated PMA-differentiated K562 at a 1:1 ratio. After 24 hours, the cultures were stained with PI and analyzed by flow cytometry. Data are representative of 3 independent experiments. (D) CD28-Ig blockade. DCs were differentiated from KG1 using PMA, irradiated, and cocultured with 8226. CD28-Ig was added to the DCs 1 hour before addition of MM cells. Proliferation was measured by thymidine incorporation. Data are representative of 2 experiments. *P = .01 compared with 8226 plus DCs. (E) iDCs versus mDCs derived from normal monocytes. To generate iDCs, monocytes were cultured in GM-CSF and IL-4 for 8 days. To generate mDCs, TNF-α was added for the last 4 days of culture. These cells were then irradiated and cocultured with myeloma cells at the ratios indicated. Proliferation was measured by thymidine incorporation. Data are representative of 2 independent experiments. Compared with 8226 alone: *P = .006; **P = .007; +P = .006. (F) Protection against dex-induced cell death. 8226 were cultured alone, or 1:1 with irradiated, undifferentiated K562 (DC precursors) or K562-derived DCs (DC), treated for 72 hours with 100 μM dex, and analyzed by FACS for 7AAD (dead cells) and CD28 expression (MM cells). Percentage viable refers to myeloma cell viability. Data are representative of 2 independent experiments.
Coculture with DCs downmodulates MM proliferation and enhances survival. (A) Proliferation. K562 were cultured in media alone (K562m; Km), or differentiated with PMA (K562p or Kp) or PMA plus TNF-α (K562 P+T or Kpt), irradiated, and cocultured with 8226 (right panel) or U266 (left panel). Proliferation was measured by thymidine incorporation and expressed as means ± SD of triplicate wells. Data are representative of 4 independent experiments. Compared with coculture with undifferentiated K562 (K562m): *P = .001; **P < .001; +P = .004; ++P = .001. (B) Cell-cycle analysis. CSFE-labeled 8226 were cocultured at a 1:1 ratio (2 × 104 cells) with either undifferentiated K562 (top) or K562 differentiated with PMA (K562-DC; bottom) for 24 hours, permeabilized, and stained with PI. Cell-cycle analysis of CSFE+ cells was conducted on a BD LSR1; percentage of cells in G0/G1, S, and G2/M are shown to the right. Data are representative of 3 independent experiments. (C) MM viability following coculture with DCs. CFSE-labeled 8226 were cocultured with irradiated PMA-differentiated K562 at a 1:1 ratio. After 24 hours, the cultures were stained with PI and analyzed by flow cytometry. Data are representative of 3 independent experiments. (D) CD28-Ig blockade. DCs were differentiated from KG1 using PMA, irradiated, and cocultured with 8226. CD28-Ig was added to the DCs 1 hour before addition of MM cells. Proliferation was measured by thymidine incorporation. Data are representative of 2 experiments. *P = .01 compared with 8226 plus DCs. (E) iDCs versus mDCs derived from normal monocytes. To generate iDCs, monocytes were cultured in GM-CSF and IL-4 for 8 days. To generate mDCs, TNF-α was added for the last 4 days of culture. These cells were then irradiated and cocultured with myeloma cells at the ratios indicated. Proliferation was measured by thymidine incorporation. Data are representative of 2 independent experiments. Compared with 8226 alone: *P = .006; **P = .007; +P = .006. (F) Protection against dex-induced cell death. 8226 were cultured alone, or 1:1 with irradiated, undifferentiated K562 (DC precursors) or K562-derived DCs (DC), treated for 72 hours with 100 μM dex, and analyzed by FACS for 7AAD (dead cells) and CD28 expression (MM cells). Percentage viable refers to myeloma cell viability. Data are representative of 2 independent experiments.
Given that the DC-MM interaction involves multiple receptor-ligand bindings that could have cellular effects, the specific contribution of CD28 on the modulation of proliferation was assessed by blocking CD80/CD86 with the chimeric CD28 receptor–Ig Fc molecule CD28-Ig. As seen in Figure 7D, CD28-Ig reversed the inhibition of 8226 proliferation induced by coculture with DCs derived from KG1. Similar results were obtained with U266 and MM.1S (not shown).
Although we have previously shown that cell line–derived DCs are very similar to DCs derived from normal progenitors, it is formally possible that these DCs have aberrant properties in their interaction with myeloma cells. To address this, 8226 were cocultured with normal monocyte-derived DCs (mo-DCs).37 In addition, we asked whether immature DCs (iDCs; differentiated with GM-CSF plus IL-4) affected myeloma cell proliferation differently than mature DCs (mDCs; GM-CSF + IL-4 + TNF-α). iDCs have lower expression of costimulatory ligands than mDCs and are less effective at activating T cells,56 and would be predicted to be less effective in suppressing myeloma proliferation. As seen in Figure 7E, both iDCs and mDCs significantly downmodulate 8226 proliferation at cell ratios of 1:1. mDCs appear to be more potent as they can do this at lower DC/myeloma cell ratios than iDCs, which would be consistent with greater CD80/CD86 expression.
To assess whether DCs can also transduce a survival signal similar to anti-CD28 mAb, 8226 was cultured alone, with undifferentiated K562 or K562-derived DCs in 100 μM dex (Figure 7F). Similar to our results with antibody-mediated activation, coculture with DCs doubles the viability of 8226 versus myeloma cells alone or plus DC precursors.
Discussion
Consistent with the clinical observation that CD28 expression on myeloma cells correlates with poor prognosis and disease progression, we have found that CD28 activation induces PI3K and NFκB signaling in myeloma cells, and delivers both antiproliferative and prosurvival signals. Although the intracellular signaling pathways downstream of CD28 have not been well described in myeloma cells, CD28 signaling in T cells induces PI3K activation15 and significantly augments NFκB activation57 in combination with mitogen, while superagonistic anti-CD28 antibodies can induce NFκB signaling without a concurrent TCR signal.58 Our findings and those of others26 thus suggest that at least 1 CD28 signaling pathway is the same in myeloma cells as it is in T cells, namely CD28 → PI3K → PDK-1 → Akt →IκB.
The observation that PI3K activation of Akt and NFκB is induced by IGF-1 (an established survival factor for myeloma)16,17 further supports a similar survival signal transduced by CD28. Interestingly, the mammalian target of rapamycin (mTOR) is a central downstream component of Akt signaling, and inhibition by rapamycin both inhibits CD28-mediated mTOR activation in T cells59 and sensitizes cells to dex-induced apoptosis in myeloma.60 Finally, NFκB signaling itself (separate from any upstream signaling pathway) has been clearly shown to enhance myeloma survival,42,61 and all these data together support a prosurvival function of CD28 in myeloma.
Despite the similarities in intracellular signaling, we find significant differences in CD28 activation in myeloma versus T cells. First, a synchronous antigen receptor “signal 1” that is required for CD28 costimulation in T cells appears unnecessary in myeloma cells. Myeloma does not express an antigen receptor, and attempts to define an alternative signal 1 (eg, IL-6, PKC agonists22,26 ) that is costimulated by CD28 have been equivocal. However, even in T cells, CD28 can signal in the absence of a concurrent TCR signal,15,62 and superagonistic anti-CD28 antibodies can activate T cells without a signal 1.58 Thus, it is possible that CD28 activation alone induces cellular responses in myeloma because it is triggered at a lower threshold. Alternatively, there may be less negative regulation of downstream signaling (eg, PI3K by PTEN), resulting in transduction of a comparatively larger signal in myeloma versus T cells.
A second difference is the effect of CD28 on proliferation, with augmentation in T cells and downmodulation in myeloma cells. Our findings are consistent with previous studies demonstrating that soluble anti-CD28 mAb 9.3 (the same as used in our studies) could suppress the proliferation of the MER myeloma cell line by 50%,13 as well as a recent study using another anti-CD28 mAb.30 Although the reasons for this MM/T difference are unclear, it has been shown in T cells that the ability of CD28 to augment proliferation and survival can be segregated into 2 downstream signaling pathways.41 It is possible that myeloma cells lack the downstream pathways involved in proliferation while retaining the prosurvival pathway, and prosurvival signals by themselves can negatively regulate cell-cycle progression.5,6,63 Another possibility is that CD28 in myeloma cells does not elicit autocrine secretion of proliferative cytokines, whereas CD28-induced autocrine secretion of IL-2 is a major factor driving T-cell proliferation.
Interestingly, the G1 arrest we find for CD28 has also been reported in myeloma cells following integrin-mediated adhesion (involving p27kip1), which similarly results in induction of NFκB, decreased proliferation, and enhanced survival/resistance to chemotherapeutic agents.4–6 These latter studies also suggest that the decreased proliferation plays a significant role in cell adhesion–mediated drug resistance (CAM-DR), protecting MM cells from chemotherapies that target cycling cells.
In addition to defining differences between myeloma and T cells, our findings also stand in contrast to some previous studies in myeloma that have found variable effects of CD28 activation on proliferation and survival, including a recent report of the induction of MM cell apoptosis by anti-CD28 mAb.30 There are potentially several technical variables that may underlie these differences. First, the different anti-CD28 antibodies may differ in their agonistic or antagonistic/blocking effects. Second, our survival studies were done in no/low serum conditions, whereas previous studies were done in 10% serum. It is possible that in the higher-serum conditions, the level of exogenous prosurvival factors (especially IGF-1) were sufficient to mask any effect of CD28 activation on survival.
If CD28 is supporting myeloma cell survival, activation in vivo must be occurring through direct contact with CD80/CD86+ cells. There are 2 nonexclusive possibilities: binding to CD86 on other myeloma cells, and myeloma cell interaction with normal professional APCs. Although we do not have clear evidence for a myeloma-myeloma interaction, the aggregate findings of several studies demonstrates that more than 50% of primary relapsed myelomas are CD86+, 100% of CD28+ myelomas are CD86+, and that CD86+ myelomas have a significantly worse prognosis.12,25 Similarly, we and others13,26 have found that myeloma cell lines typically coexpress CD28 and CD86, although these latter 2 reports are contradictory as to whether autocrine CD28 activation is occurring. The second possibility is that CD28 activation in myeloma occurs the same way it does on T cells, namely by direct contact with CD80/CD86+ APCs. In addition to CD80/CD86 expression, professional APCs have specialized ability to directly interact with other immune cells that includes the expression of appropriate adhesion molecules and chemoattractant chemokines. This is particularly true for DCs.56 There is considerable evidence that DCs are directly involved in the survival, proliferation, and differentiation of normal B cells and plasma cells. These include DC expression of IL-6,64,65 that DCs and B cells form clusters in vitro and in vivo,66 and that this direct interaction provides B cells with proliferation and survival signals67 and drives their differentiation to plasma cells.68–70 Recent studies have found that DCs enhance plasmablast survival and differentiation, in part through secretion of APRIL and/or BAFF.71 And very recently it has been shown that DCs support the clonogenicity of human MM cells.72 It seems likely that this advantageous interaction is maintained by transformed plasma cells, and are consistent with: (1) substantial numbers of host DCs (and other APCs) rapidly infiltrate implanted plasmacytomas;31 (2) in primary patient isolates, bone marrow DCs are selectively and intimately associated with myeloma cells (our findings and Rettig et al32 ); and (3) exogenously added APRIL and BAFF protect myeloma cells against apoptosis caused by IL-6 withdrawal and dex.73 Our results indicate that 1 molecular component of a DC/MM interaction is activation of CD28, but given the molecular complexity of DC interactions with other immune cells, it seems likely that other important signals (eg, integrin-mediated signals) are also transduced to myeloma cells by this contact.
Finally, identifying CD28 and bone marrow APCs as potential contributors to the pathogenesis of MM raises the possibility of targeting them therapeutically. Such strategies would include direct targeting/blocking CD28 by mAbs and agents that block CD28 signaling (such as rapamycin, which is being clinically developed for organ transplantation). Finally, a large number of factors (drugs, cytokines, microbial products) are known to modulate DC activation/function in the context of eliciting immune responses, and may also have effects on any myeloma/DC interaction. Along these lines, it is interesting to note that both thalidomide and bortezomib, which have significant activity in myeloma, have been shown to modulate DC function74,75 and induce DC apoptosis.76
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Supported by the Senior Investigator Research Award from the Multiple Myeloma Research Foundation, and National Institutes of Health grants CA85208, CA097243, and CA95829.
National Institutes of Health
Authorship
Author contributions: N.J.B., A.M.K., D.K., L.M.C., H.Y.L., and G.E.B. Jr all performed vital research for this paper. M.H., H.T., and B.L.L. provided vital reagents. L.H.B. analyzed the data. K.L. designed the research and wrote the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Kelvin P. Lee, Roswell Park Cancer Institute, Elm and Carlton St., Buffalo, NY 14263; e-mail: kelvin.lee@roswellpark.org.