Abstract
Malignancies arising from retrovirally transduced hematopoietic stem cells have been reported in animal models and human gene therapy trials. Whether mature lymphocytes are susceptible to insertional mutagenesis is unknown. We have characterized a primary human CD8+ T-cell clone, which exhibited logarithmic ex vivo growth in the absence of exogenous cytokine support for more than 1 year after transduction with a murine leukemia virus–based vector encoding the T-cell growth factor IL-15. Phenotypically, the clone was CD28−, CD45RA−, CD45RO+, and CD62L−, a profile consistent with effector memory T lymphocytes. After gene transfer with tumor-antigen–specific T-cell receptors, the clone secreted IFN-γ upon encountering tumor targets, providing further evidence that they derived from mature lymphocytes. Gene-expression analyses revealed no evidence of insertional activation of genes flanking the retroviral insertion sites. The clone exhibited constitutive telomerase activity, and the presence of autocrine loop was suggested by impaired cell proliferation following knockdown of IL-15Rα expression. The generation of this cell line suggests that nonphysiologic expression of IL-15 can result in the long-term in vitro growth of mature human T lymphocytes. The cytokine-independent growth of this line was a rare event that has not been observed in other IL-15 vector transduction experiments or with any other integrating vector system. It does not appear that the retroviral vector integration sites played a role in the continuous growth of this cell clone, but this remains under investigation.
Introduction
Human gene therapy strategies using replication-incompetent retroviral vectors were implemented cautiously because of many concerns, including the potential for adverse events resulting from vector insertions in the human genome.1 Theoretical concerns about insertional mutagenesis became reality with the report that 3 of 11 patients with severe combined immunodeficiency-X1 (SCID-X1) developed T-cell leukemia after treatment with hematopoietic stem cells (HSCs) engineered with the common γ-chain cytokine receptor gene. These leukemias resulted from integration events which activated the LMO2 proto-oncogene.2 Nevertheless, after more than 17 years of clinical gene therapy trials this remains the only report of overt cancer development resulting from gene-modified cell therapy. Our understanding of the pathogenesis of human malignancy resulting from insertional mutagenesis remains limited to this specific clinical scenario.
Several observations from the SCID-X1 gene therapy trial fall in line with general principles established through animal models and other human clinical trials investigating retroviral gene transfer. The well-characterized murine leukemia virus (MLV)–based gene transfer vectors integrate preferentially in the vicinity of cellular promoters,3,4 potentially increasing the probability of activating or inactivating vector insertions which may dysregulate gene expression.5,6 The target cells most at risk of insertional mutagenesis are believed to be primitive progenitor cells.7 Potentially, therapeutic transgenes may have leukemogenic properties in the appropriate setting.6 The interactions between retroviral gene transfer vectors and hematopoietic cells are being investigated intensely.
In this report, we have characterized a primary human T-cell clone, which exhibited continuous growth after transduction with an MLV-based retroviral vector encoding the T-cell growth factor, IL-15. This was an unexpected and intriguing finding for several reasons. First, human cells are inherently difficult to immortalize, and the process of transformation in human cells is believed to be considerably more complex than that of experimental animals. Second, the cells that were transduced consisted of mature peripheral blood lymphocytes (PBLs), whereas the current literature on insertional mutagenesis consists entirely of reports in which primitive progenitor cells were transformed by retroviral vectors. Third, the transgene used was IL-15, which signals via the common γ-chain cytokine receptor. This raises the issue of whether IL-15 is leukemogenic in human T cells. Potentially, this transformed cell line may reveal new insights on the regulation of human lymphocyte growth, survival, and transformation by retroviral vectors.
Materials and methods
Any patient-derived material used was approved by the National Cancer Institute Institutional Review Board in accordance with the Declaration of Helsinki. This study involved no direct contact with human patients.
Generation of the LC15 cell line, vector transduction, and cell culture conditions
PBLs were obtained by leukapheresis from a patient with a history of melanoma prior to treatment on an adjuvant peptide vaccine protocol at the National Cancer Institute, Bethesda, MD. Lymphocytes were purified by centrifugation using Ficoll/Hypaque, washed in HBSS, and cultured in AIM-V (Invitrogen, Carlsbad, CA), 5% human AB serum (Valley Biomedical, Winchester, VA), penicillin-streptomycin (Invitrogen), L-glutamine (Invitrogen), 2-mercaptoethanol, and HEPES (Invitrogen). Cells were activated by adding 50 ng OKT3/mL and 300 IU IL-2/mL on culture day 0 and cultured at 37°C in a 5% CO2 humidified incubator.
IL-15 retroviral vector supernatants were collected from a high-titer PG13 packaging cell clone transduced with the retroviral vector pMSGV1 PPL CO IL-15.8 This retroviral vector contains the murine stem cell virus LTR and RNA processing signals similar to the MFG-class of retroviral vectors. The anti-MART-1 AIB TCR retroviral vector was used for control transductions.9 Retroviral transduction plates were prepared by sequentially coating nontissue culture-treated 6-well plates with RetroNectin (Takara, Otsu, Japan) and then 6 mL/well of retroviral vector supernatants. Plates were incubated at 32°C for 2 to 4 hours prior to use.
Lymphocytes were retrovirally transduced a total of 4 times on culture days 2 and 3. Retroviral supernatant was removed from transduction plates. Cells (106) were added to each well in 2 mL lymphocyte culture medium. Cells were cultured at 32°C during transduction and 37°C thereafter. On culture day 7, the cells were washed thoroughly and returned to culture in lymphocyte culture medium without added IL-2. Viable cells were enumerated and passaged every 4 to 7 days by replacing half of the conditioned media with fresh lymphocyte culture medium. IL-15 concentrations were measured by enzyme-linked immunoabsorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Retroviral vectors containing shRNAs directed to either the human IL-15Rα (clone ID V2HS_133348) or endogenously expressed human IL-15 (clone ID V2HS_111551) were obtained from Open Biosystems (Huntsville, AL). Retroviral vector DNA and a GaLV envelope-expressing plasmid (generously provided by Maribeth Eiden, NIH, Bethesda, MD) were transfected into 293GP cells using Lipofectamine 2000 (Invitrogen), and vector-containing media were collected 48 hours after transfection. LC15 cells were transduced as described, and engineered cells were selected for by growth in the presence of 0.5 μg puromycin/mL (Sigma, St Louis, MO) for 2 weeks.
Control CD8+ T cells were isolated from activated PBLs using the CD8+ T-cell Isolation Kit II (Miltenyi Biotec, Auburn, CA). Culture of tumor-infiltrating lymphocyte cultures was described previously.10 Briefly, these lymphocytes were grown from excised tumor fragments and expanded for approximately 2 weeks prior to analysis, using allogeneic feeder cells, anti-CD3 Ab (Ortho Biotech, Bridgewater, NJ), and IL-2.
The cell lines used include the PG13 gibbon ape leukemia virus-packaging cell line (ATCC CRL-10686), the human lymphoid cell line Sup T1 (ATCC CRL-1942), the human breast cancer line MDA-MB-231 (ATCC HTB-26), the non–small-cell lung cancer line H2087 (ATCC CRL-5922), the osteosarcoma line Saos-2 (ATCC HTB-85), 293GP (Invitrogen), and 4 melanoma lines: 526mel, 624mel, 888mel, and 938mel (generated at the Surgery Branch from resected tumors). With the exception of Saos-2, cell lines were cultured in RPMI 1640 (Invitrogen), 10% FCS (Invitrogen), penicillin-streptomycin (Invitrogen), L-glutamine (Invitrogen), and HEPES (Invitrogen). Saos-2 was cultured in McCoy 5A (Invitrogen), 15% FCS (Invitrogen), and gentamicin (180 μg/mL).
Thymidine incorporation assay
Cells were plated at 1 × 105 cells/well in a 96-well microplate in the presence or absence of indicated concentrations of recombinant human IL-15 (R&D Systems) and/or monoclonal anti–IL-15 antibody (R&D Systems). The cells were cultured for a total of 24 hours and in the final 16 hours of culture, 1 μCi (0.037 MBq) [methyl-3H] thymidine/well (PerkinElmer Life Sciences, Boston, MA) was added to each well. In shRNA knockdown experiments, transduced and selected cells were cultured in medium without IL-2 for 3 days before addition of [methyl-3H] thymidine. Cellular DNA was harvested and counted by liquid scintillation counting.
Flow cytometry
Cell-surface expression of CD2, CD4, CD5, CD7, CD8, CD25, CD28, CD38, CD45RA, CD45RO, and CD62L was measured using FITC-, PE-, or APC-conjugated antibodies and corresponding isotype controls (BD Pharmingen, San Diego, CA). PE-conjugated MART-1 tetramer was used to detect MART-1–specific T-cell receptor (TCR; Beckman Coulter, Fullerton, CA). Immunofluorescence was measured using a fluorescence-activated cell scanner (FACscan) flow cytometer and analyzed using CellQuest Pro software (BD Biosciences, San Jose, CA).
Telomerase length and telomerase activity assays
Telomere length was measured using a flow–fluorescence in situ hybridization (FISH) technique described previously.10,11 Telomerase activity was assayed using the TRAPeze telomerase detection kit (Chemicon, Temecula, CA). Briefly, cell pellets were lysed in CHAPS at a concentration of 107 cells/mL. Telomerase products generated by serial dilutions of each sample were visualized as 6-bp (base pair) ladders by gel electrophoresis. Telomerase activity is expressed as the intensity of the total telomerase product divided by a competitive internal polymerase chain reaction (PCR) standard. Telomerase activity was normalized to the activity of EL4 (ATCC TIB-39).
TCR gene transfer
Cloning of the p53264-272 murine TCR and construction of a bicistronic retroviral vector were described previously.12 The MART-127-35 TCR used in this study was cloned from a culture of tumor-infiltrating lymphocytes designated DMF5, derived from a tumor explant from a patient with HLA-A2–positive melanoma. To promote specific TCR chain pairing, the human TCR constant regions were replaced with murine constant regions.13
In vitro–transcribed mRNA for p53 and MART-1 TCR chains was generated using mMESSAGE mMACHINE (Ambion, Austin, TX) and purified using the RNeasy mini kits (Qiagen, Valencia, CA) according to the manufacturer's protocols. LC15 cells were cotransfected with TCRα and β chains via mRNA electroporation as described previously.14 Cells were suspended in Opti-MEM (Invitrogen) at 2.5 × 107 cells/mL. mRNA (5 μg) from each TCR chain was mixed with 200 μL of the cell suspension and transferred to a 2-mm electroporation cuvette (BTX, Holliston, MA). Samples were electroporated at 300 V for 0.5 ms using an ElectroSquare Porator ECM 830 (BTX). Electroporated cells were used in tumor recognition assays 2 hours after electroporation. FACS was performed 24 hours after electroporation.
Tumor recognition assay
Effector cells (1 × 105) were cocultured with 1 × 105 tumor targets in a final volume of 0.2 mL in each well of a 96-well microplate. Cell culture supernatants were harvested after 24 hours and assayed for IFN-γ or GM-CSF by ELISA (R&D Systems, Minneapolis, MN; Endogen, Rockford, IL).
Spectral karyotyping
Using techniques detailed previously,15,16 metaphase preparations derived from 7-month cultured LC15 cells were hybridized with 24 differentially labeled chromosome-specific painting probes. Image acquisition was performed using the SpectraCube SD200 (Applied Spectral Imaging,Carlsbad, CA) connected to an epifluorescence microscope (DMRXA; Leica Microsystems, Wetzlar, Germany). SkyView software (Skylark Technology, Glasgow, United Kingdom) was used for image analysis.
5′-RACE analysis of TCR β-chain gene expression
TCR β-chain sequences were obtained using the SMART RACE (rapid amplification of cDNA ends) cDNA Amplification kit (BD Biosciences). First-strand cDNA was synthesized from 0.5 μg total RNA from LC15 cells using a TCR β-chain C region primer: 5′-CTCTTGACCATGGCCATC-3′ in conjunction with the SMART II A oligo according to the manufacturer's instructions. The cDNA was amplified by 5′-RACE and cloned for sequencing using the TOPO TA cloning kit (Invitrogen).
Analysis of retroviral vector integration sites
Genomic vector junctions were cloned by linker-mediated (LM)–PCR and linear amplification-mediated (LAM)–PCR. LM-PCR was performed using a modification of a previously described technique in which genomic DNA was digested with either NsiI or NcoI in an attempt to obtain the maximal number of bands.17 Extension primers (SL-AP1, SL-AP2) and blunt-end adaptors were those described in Laufs et al.18 The following primers were designed specifically for the pMSGV1 PPL CO IL-15 vector, LTRa-5′Biotin-TGCTTACCACAGATATCCTG-3′; first-round LTR primer, 5′-CCTTGATCTGAACTTCTCTATTC-3′; and second-round LTR primer, 5′-TTCCATGCCTTGCAAAATGGC-3′. LAM-PCR was described previously.3,17,19 Briefly, linear amplification of the genomic vector junctions was done using a 5′ biotinylated primer, LTR-linear2 (5′-GAGAAGCGAACTGATTGG-3′). The products were then digested using Tas1 and ligated to the previously described linker cassette.17 The outer exponential PCR was done using a vector LTR-specific primer, LTR-LCI (5′-GGCAGGAACTGCTTACCA-3′) and a linker cassette specific primer, LCI (5′-GACCCGGGAGATCTGAAT-3′). The inner exponential PCR was done using LTR primer, LTR-R2 (5′-GCTAGCTTGCCAAACCTAC-3′) and linker cassette primer, LCIII (5′-AGTGGCACAGCAGTTAGG-3′). The amplified genomic-vector junctions were run on a spreadex gel (Elchrom Scientific, Cham, Switzerland) and purified using Qiaquick PCR purification kit (Qiagen). LM- and LAM-PCR products were TA cloned for sequencing.
Gene-expression analysis
RNA was extracted from samples using RNeasy mini kits (Qiagen) and subsequently treated with TURBO DNA-free (Ambion) to remove contaminating genomic DNA. For reverse transcription (RT)–PCR, cDNA was prepared using the ThermoScript RT-PCR system, using the random hexamers (Invitrogen). PCR was performed using Platinum PCR Supermix (Invitrogen). The PCR primers were 5′-GCAACAACATAACACTGCACCTT-3′ and 5′-CAATTGTCCTTTGAACCAACAGA-3′ for ACVR1C, 5′-CCTTACGATGGACGATAATAGAA-3′ and 5′-ATATTTGCAAGGACATCACCAGC-3′ for PSCDBP, 5′-GAAATGGAAAGAAAACTAAAAGGCC-3′ and 5′-GAAATGTCTTCCAGACAGACGAA-3′ for PGBD4, 5′-AAGTGGGGGCTGTGACATGGCAA-3′ and 5′-TGGTGAGCACAGGAAGGCAATCT-3′ for C15orf29, 5′-CACCCCCATAGCCATCCTCTCTA-3′ and 5′-GGTGGTCTAACTTCTGCGTTCTT-3′ for FOXP1. QuantumRNA Universal 18S rRNA primers (Ambion) were used as internal standards. ACVR1C and PSCDBP were amplified using 40 cycles of PCR. FOXP1, C15orf29, and 18S rRNA were amplified using 35 cycles of PCR. After a 2-minute denaturing step at 94°C, cycling parameters were as follows: 30 seconds at 94°C, 30 seconds at 55°C, and 60 seconds at 68°C. PCR products were run on a 2% agarose gel, digitized, and then quantitated with an LAS-1000 luminescent image analyzer system (Fujifilm Medical Systems, Stamford, CT).
For microarray analysis, RNA samples were indirectly labeled via a single round of linear amplification with Amino Allyl MessageAmp II reagents (Ambion). The labeled samples were combined and hybridized overnight to 38k gene long-oligo Operon V3.0 arrays supplied by the Advanced Technology Center (NCI, Gaithersburg, MD). Data image files were obtained using a GenePix 4000B scanner (Axon, Union City, CA) and imported into Genespring v.7.2 (Silicon Genetics, Redwood City, CA) for data analysis. Each array was normalized using intensity-dependent (LOWESS) normalization and analyzed independently for determination of changes in gene expression.
Results
Dysregulated growth of a single culture of human T cells transduced with IL-15
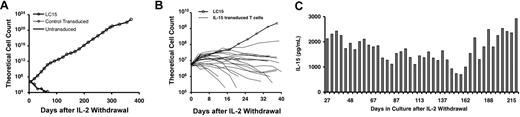
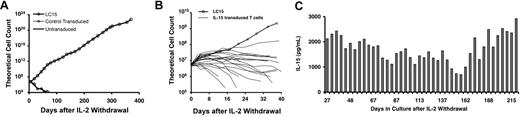
An IL-15 retroviral vector was developed for the transduction of human T lymphocytes with the aim of enhancing the function and/or survival of tumor-infiltrating lymphocytes in adoptive cell transfer therapies for cancer.20 T lymphocytes transduced with IL-15 demonstrated prolonged survival and resistance to apoptosis in the absence of exogenous IL-2 support.8 A total of 23 T-lymphocyte cultures, all from different patients, were transduced during the course of these studies. Unexpectedly, one of the cultures, designated LC15, exhibited logarithmic growth in the absence of exogenous cytokine support for longer than 1 year, with a 1016-fold expansion (Figure 1A). In 22 other experiments in which T-cell cultures were transduced with the same IL-15 vector, the logarithmic expansion of IL-15–transduced T cells typically occurred for fewer than 2 weeks after IL-2 withdrawal (Figure 1B), and all of these cultures stopped expanding within 6 weeks. LC15 cell culture medium was periodically sampled during long-term culture and found to contain between 700 and 2900 pg IL-15/mL (Figure 1C).
LC15 growth profile and IL-15 expression after withdrawal from exogenous cytokine support. PBLs were activated with OKT3 and IL-2. Cells were transduced 4 times with IL-15 retroviral supernatants on culture days 2 and 3. Control cells were not transduced or were transduced with a retroviral vector encoding irrelevant proteins. On day 7 of the culture, the PBLs were washed extensively, and 5 × 106 cells from each culture were plated in fresh media, in the absence of exogenous cytokine. (A) Viable cells were enumerated every 4 to 7 days by trypan blue exclusion; concurrently, the cell culture media were refreshed by replacing half of the spent media with fresh media. Cells were maintained at a density of 1 × 106 cells/mL. (B) Twenty-two other experiments were performed in which OKT3-activated PBLs were transduced with the IL-15 retroviral vector and subsequently withdrawn from IL-2. The growth of these cultures, and the growth of LC15, was plotted over the first 40 days after IL-2 withdrawal. (C) LC15 cell culture media were periodically assayed for IL-15 content by ELISA.
LC15 growth profile and IL-15 expression after withdrawal from exogenous cytokine support. PBLs were activated with OKT3 and IL-2. Cells were transduced 4 times with IL-15 retroviral supernatants on culture days 2 and 3. Control cells were not transduced or were transduced with a retroviral vector encoding irrelevant proteins. On day 7 of the culture, the PBLs were washed extensively, and 5 × 106 cells from each culture were plated in fresh media, in the absence of exogenous cytokine. (A) Viable cells were enumerated every 4 to 7 days by trypan blue exclusion; concurrently, the cell culture media were refreshed by replacing half of the spent media with fresh media. Cells were maintained at a density of 1 × 106 cells/mL. (B) Twenty-two other experiments were performed in which OKT3-activated PBLs were transduced with the IL-15 retroviral vector and subsequently withdrawn from IL-2. The growth of these cultures, and the growth of LC15, was plotted over the first 40 days after IL-2 withdrawal. (C) LC15 cell culture media were periodically assayed for IL-15 content by ELISA.
Phenotype of LC15 cells
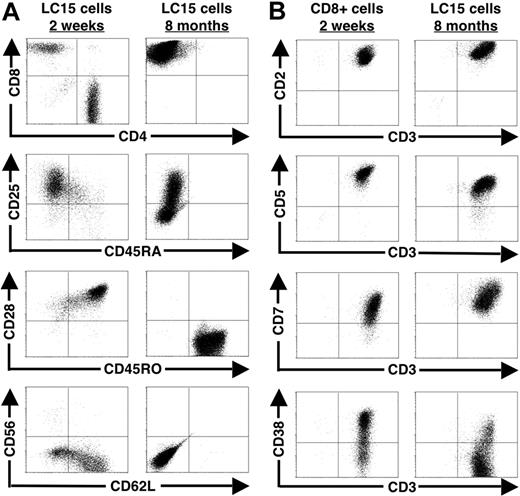
We observed a shift in the cell-surface marker profile of LC15 cells as they were cultured long term (Figure 2A). Initially, we examined the cells after 2 weeks in culture by flow cytometry and found that they were a heterogeneous population: 31% were CD8+, 65% CD4+, 91% CD25+, 98% CD28+, 19% CD45RA+, 91% CD45RO+, and 67% CD62L+. After 8 months in culture, LC15 cells had become homogeneous with an effector memory phenotype: CD8+, CD28−, CD45RA−, CD45RO+, and CD62L−. Eight-month cultured LC15 cells exhibited multiple activation states, with approximately half of the cells expressing the IL-2Rα (CD25).
Cell-surface molecule profile of LC15 cells. (A) LC15 cells were examined for common T-lymphocyte markers at 2 weeks and 8 months after IL-2 withdrawal. Cells were stained with fluorescent-labeled antibodies against CD4, CD8, CD25, CD28, CD45RA, CD45RO, CD56, and CD62L. Lymphocytes were subsequently analyzed by FACS. (B) Activation marker profiles of OKT3-stimulated CD8+ T cells cultured with IL-15 (10 ng/mL) and 8-month cultured LC15 cells. OKT3-stimulated CD8+ T cells were cultured for 2 weeks in media containing IL-2 prior to analysis. LC15 cells were cultured for 8 months after IL-2 withdrawal. Cells were double stained with CD3 and either CD2, CD5, CD7, or CD38 prior to analysis by FACS.
Cell-surface molecule profile of LC15 cells. (A) LC15 cells were examined for common T-lymphocyte markers at 2 weeks and 8 months after IL-2 withdrawal. Cells were stained with fluorescent-labeled antibodies against CD4, CD8, CD25, CD28, CD45RA, CD45RO, CD56, and CD62L. Lymphocytes were subsequently analyzed by FACS. (B) Activation marker profiles of OKT3-stimulated CD8+ T cells cultured with IL-15 (10 ng/mL) and 8-month cultured LC15 cells. OKT3-stimulated CD8+ T cells were cultured for 2 weeks in media containing IL-2 prior to analysis. LC15 cells were cultured for 8 months after IL-2 withdrawal. Cells were double stained with CD3 and either CD2, CD5, CD7, or CD38 prior to analysis by FACS.
We examined a panel of markers associated with T-cell leukemia (CD2, CD5, CD7, and CD38). Using this panel of antibodies, transformed T lymphocytes would be expected to exhibit homogeneous staining. As a control, we stained OKT3-activated lymphocytes that were purified for the CD8+ subset and grown in IL-15 for 2 weeks prior to analysis. Control cells exhibited heterogeneous staining for CD7 and CD38, whereas 8-month cultured LC15 cells exhibited heterogeneous staining for CD5 and CD38 (Figure 2B).
LC15 demonstrates antigen-specific tumor recognition after TCR gene transfer
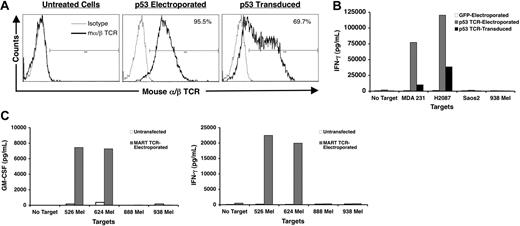
Transformed T-cell lines are typically dysregulated to an extent such that normal effector functions are lost. Because we are uncertain of the native antigen specificity of LC15 cells, we forced the cells to express a murine-derived TCR with specificity for the HLA-A2–restricted human p53264-272 epitope by both mRNA electroporation and retroviral transduction. TCR gene transfer resulted in 95% and 70% positive staining (in electroporated and transduced cells, respectively) with an antibody specific for murineα/β TCR (Figure 3A). The retrovirally transduced cells demonstrated stable gene expression, exhibiting approximately 68% murine α/β TCR positivity 4 weeks after transduction. p53 TCR electroporated and transduced LC15 cells secreted IFN-γ when cocultured with tumor targets expressing both p53 and HLA-A2 (MDA-MB-231 and H2087) but did not react with p53-negative tumor lines p53 (Saos-2 and 938). GFP-transfected LC15 cells did not recognize any of the tumor lines (Figure 3B).
TCR gene transfer and tumor recognition assays. (A) Efficiency of gene transfer. A mouse TCR with specificity for an HLA-A2–restricted epitope of p53 was transferred to LC15 cells by either RNA electroporation or retroviral transduction. The cells were stained with anti–mouse α/β TCR antibody (heavy lines) or the matched isotype control antibody (light lines) 24 hours after electroporation or 2 days after transduction. Cells were analyzed by flow cytometry. The number in the upper right corner denotes the percentage of cells positive for mouse α/β TCR. (B) p53+ tumor antigen recognition assay. LC15 effector cells were electroporated with GFP mRNA, electroporated with the p53 TCR mRNA, or transduced with the p53 TCR retroviral vector. Effector cells (1 × 105) were plated in 96-well plates with 1 × 105 tumor targets that were either p53 positive (MDA231 and H2087) or p53 negative (Saos2 and 938mel). After an 18-hour coculture, the cell culture media was collected and assayed for IFN-γ content by ELISA. (C) MART tumor antigen recognition assay. LC15 effector cells were untreated or electroporated with MART TCR mRNA. Tumor targets were melanoma lines either HLA-A2 positive (526mel and 624mel) or HLA-A2 negative (888mel and 938mel). After an 18-hour coculture, the cell culture medium was collected and assayed for IFN-γ and GM-CSF content by ELISA.
TCR gene transfer and tumor recognition assays. (A) Efficiency of gene transfer. A mouse TCR with specificity for an HLA-A2–restricted epitope of p53 was transferred to LC15 cells by either RNA electroporation or retroviral transduction. The cells were stained with anti–mouse α/β TCR antibody (heavy lines) or the matched isotype control antibody (light lines) 24 hours after electroporation or 2 days after transduction. Cells were analyzed by flow cytometry. The number in the upper right corner denotes the percentage of cells positive for mouse α/β TCR. (B) p53+ tumor antigen recognition assay. LC15 effector cells were electroporated with GFP mRNA, electroporated with the p53 TCR mRNA, or transduced with the p53 TCR retroviral vector. Effector cells (1 × 105) were plated in 96-well plates with 1 × 105 tumor targets that were either p53 positive (MDA231 and H2087) or p53 negative (Saos2 and 938mel). After an 18-hour coculture, the cell culture media was collected and assayed for IFN-γ content by ELISA. (C) MART tumor antigen recognition assay. LC15 effector cells were untreated or electroporated with MART TCR mRNA. Tumor targets were melanoma lines either HLA-A2 positive (526mel and 624mel) or HLA-A2 negative (888mel and 938mel). After an 18-hour coculture, the cell culture medium was collected and assayed for IFN-γ and GM-CSF content by ELISA.
Similarly, when we electroporated LC15 cells with genes encoding a TCR that conferred recognition of the HLA-A2–restricted melanoma antigen MART-127-35, electroporated cells exhibited 91% MART-1 tetramer binding (data not shown). These cells secreted IFN-γ as well as GM-CSF upon encounter with HLA-A2–positive melanoma lines (526mel and 624mel) but not HLA-A2–negative melanoma lines (888mel and 938mel). Untreated LC15 cells did not recognize any of the tumor lines (Figure 3C).
LC15 is a clonal population containing multiple sites of vector integration
Germline TCR genes are rearranged during T-cell development to yield highly variable, unique joining regions. We evaluated TCR β-chain usage to determine whether LC15 is a clonal population. Thirteen TRBV sequences were amplified from 9-month cultured LC15 cells using a 5′-RACE technique. LC15 expressed TRBV6-1; more importantly, all sequences contained identical TCRBD and TRBJ regions (Figure 4A), highly suggestive that LC15 derived from a single T-lymphocyte clone.
LC15 is a clonal population. (A) Alignment of VDJ junctional regions of TRBV6-1 sequences amplified from LC15 by 5′-RACE, demonstrating that LC15 expresses a single, unique TCR β-chain. (B) A spectral karyotype of LC15 demonstrates 4 translocations; all autosomal breaks occurred at centromeres. These were clonal abnormalities.
LC15 is a clonal population. (A) Alignment of VDJ junctional regions of TRBV6-1 sequences amplified from LC15 by 5′-RACE, demonstrating that LC15 expresses a single, unique TCR β-chain. (B) A spectral karyotype of LC15 demonstrates 4 translocations; all autosomal breaks occurred at centromeres. These were clonal abnormalities.
We performed spectral karyotyping (SKY) on 7-month cultured LC15 to address the issue of clonality and to evaluate for translocations with the potential for altering the growth characteristics of the cell line (Figure 4B). Chromosomal translocations most commonly observed in T-cell leukemia involve translocation involving the T-cell receptor gene locus, but other genes such as TAL1 and LMO2 can also be involved.21–23 SKY revealed a clonal abnormality with 4 translocations: 44, X, der(X)t(X;16)(p22.3;p10), der(13;16)(q10;p10), der(14;21)(q10;q10), −16, +22, i(22)(q10). All of the chromosomal breaks occurred at centromeres. The observed karyotype ruled out chromosomal translocations typically associated with leukemia or lymphoma as a mechanism for the dysregulated growth of LC15.
To investigate the possibility of insertional mutagenesis, we analyzed LC15 vector integration sites. Vector-genome junctions were cloned by 3 independent laboratories using LAM-PCR, LM-PCR, or both. A total of 3 vector insertion sites were identified; these were located at 2q24.1, 3p13, and 15q14 (Table 1). Genes flanking the insertion sites were as follows: PSCDBP, ACVR1C, FOXP1, EIF4E3, PGBD4, and C15orf29. None of the insertion sites corresponded with the recently reported database of 300 retroviral integrations in mature lymphocytes after transduction with herpes simplex virus thymidine kinase.24 Using the distance of 5 kb (kilobyte) to define a proximal integration site,4 LC15 integration sites were not in the proximity of transcription start sites.
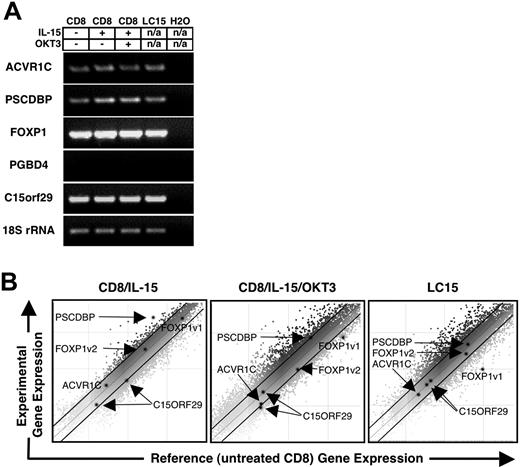
Recent evidence suggests that insertional gene activation is not simply a function of the distance or orientation of the retroviral vector relative to the transcription start site24 ; thus, we examined the expression of all genes within 200 kb of LC15 integration sites. Semiquantitative RT-PCR was performed using RNA extracted from LC15 as well as RNA extracted from the original donor's CD8+ T cells (Figure 5A). CD8+ T cells, subjected to a variety of culture conditions, and LC15 exhibited comparable expression of PSCDBP, ACVR1C, FOXP1, and C15orf29. PGBD4 was not expressed in any of the cells.
Expression profile of genes flanking the LC15 retroviral vector integration sites. (A) Semiquantitative RT-PCR was performed on RNA extracted from LC15 or control PBLs that were obtained from the same patient used to generate LC15. CD8 cells were isolated from PBLs by negative selection, and RNA was extracted immediately after purification or after an 8-hour exposure to 10 ng IL-15/mL. A fraction of the PBLs were stimulated with OKT3 and cultured with IL-15 (10 ng/mL) for 7 days prior to RNA extraction. Primers were designed to amplify ACVR1C, PSCDBP, FOXP1, PGBD4, and C15orf29 which are genes flanking the retroviral integration sites in LC15. 18S rRNA primers were used as an internal control. (B) Gene expression was also examined using a cDNA array. Untreated CD8+ T cells were used as a reference for all other samples. Gene expression in IL-15–exposed CD8+ cells (left), OKT3-stimulated CD8+ cells cultured in IL-15 (10 ng/mL) for 7 days (middle), and LC15 (right) is depicted. The abscissa represents the relative gene expression minus background in the CD8+ cDNA control. The ordinate represents the relative gene expression minus background of the experimental conditions listed above. The darkened lines on each plot delineate 2-fold differential gene expression. The darkened spots represent ACVR1C, PSCDPB, FOXP1 splice variant 1, FOXP1 splice variant 2, and 2 unique oligos corresponding to C15orf29. OKT3-stimulated CD8+ cells cultured in IL-15, and LC15 exhibited down-regulation of splice variant 1 of FOXP1.
Expression profile of genes flanking the LC15 retroviral vector integration sites. (A) Semiquantitative RT-PCR was performed on RNA extracted from LC15 or control PBLs that were obtained from the same patient used to generate LC15. CD8 cells were isolated from PBLs by negative selection, and RNA was extracted immediately after purification or after an 8-hour exposure to 10 ng IL-15/mL. A fraction of the PBLs were stimulated with OKT3 and cultured with IL-15 (10 ng/mL) for 7 days prior to RNA extraction. Primers were designed to amplify ACVR1C, PSCDBP, FOXP1, PGBD4, and C15orf29 which are genes flanking the retroviral integration sites in LC15. 18S rRNA primers were used as an internal control. (B) Gene expression was also examined using a cDNA array. Untreated CD8+ T cells were used as a reference for all other samples. Gene expression in IL-15–exposed CD8+ cells (left), OKT3-stimulated CD8+ cells cultured in IL-15 (10 ng/mL) for 7 days (middle), and LC15 (right) is depicted. The abscissa represents the relative gene expression minus background in the CD8+ cDNA control. The ordinate represents the relative gene expression minus background of the experimental conditions listed above. The darkened lines on each plot delineate 2-fold differential gene expression. The darkened spots represent ACVR1C, PSCDPB, FOXP1 splice variant 1, FOXP1 splice variant 2, and 2 unique oligos corresponding to C15orf29. OKT3-stimulated CD8+ cells cultured in IL-15, and LC15 exhibited down-regulation of splice variant 1 of FOXP1.
A cDNA array was used to further quantify gene expression, comparing untreated CD8+ T cells to CD8+ cells exposed to IL-15 for 8 hours, CD8+ cells exposed to IL-15 and OKT3 for 1 week, as well as LC15 cells (Figure 5B). This confirmed that PSCDBP, ACVR1C, FOXP1, and C15orf29 were expressed in LC15 as well as normal CD8+ T cells. PSCDBP (H200000072) was up-regulated (5.7-fold) in CD8+ T cells exposed to IL-15. ACVR1C (H300006047) and C15orf29 (H300012727 and H300011422) did not show differential expression in any of the experimental conditions. Down-regulation of FOXP1 (H300013912, representing the most 3′ region of variant 1 of FOXP1) was observed in both the LC15 cells (9.7-fold) and CD8+ cells exposed to IL-15 and OKT3 (3.1-fold).
Telomerase activity and telomere length analyses
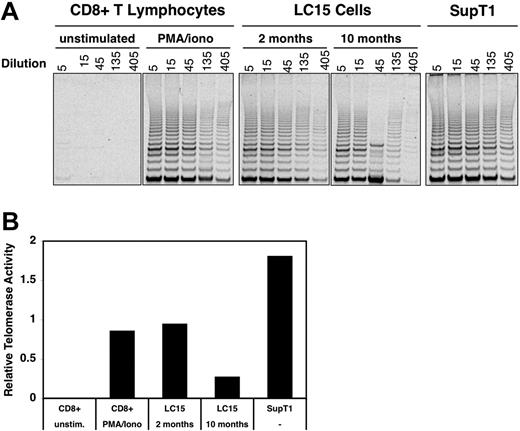
T lymphocytes activate telomerase upon stimulation, which allows significant expansion while minimizing loss of telomere length. Telomerase activity peaks in CD8+ T cells within 5 days of antigen stimulation and drops to undetectable levels by 3 weeks after stimulation.25 The ability to up-regulate telomerase decreases in parallel to telomere length as T cells differentiate from a naive to a memory phenotype.26,27 Using a PCR technique, we examined telomerase activity in LC15 cells (Figure 6A-B). Telomerase activity in resting CD8 T cells was undetectable. In contrast, 2-month cultured LC15 cells expressed telomerase at levels comparable to normal control CD8 cells that were stimulated with PMA/ionomycin; these levels were also comparable to telomerase activity seen in the human leukemia cell line SupT1. Ten-month cultured LC15 cells continued to express telomerase, albeit at decreased levels compared with 2-month cultured cells.
Comparison of telomerase activity in various cell types. (A) Telomerase activity was evaluated in CD8+ T cells, LC15 cells, and the SupT1 lymphoblast line. Naive CD8+ T cells were analyzed directly or after stimulation with 2 nM PMA and 100 nM ionomycin for 4 days. LC15 cells were analyzed 2 months and 10 months after IL-2 withdrawal. Nuclear extracts were obtained from all cell cultures and serially diluted; these samples were then subjected to the Telomeric Repeat Amplification Protocol (TRAP) assay, and the products were visualized by agarose gel electrophoresis. Extracts divided into aliquots from the EL-4 cell line were used as an interexperimental control (not shown). (B) For each set of reactions, telomerase activity was determined by measuring the image intensity with a phosphor imager and dividing this value by the intensity of an internal PCR standard (not shown).
Comparison of telomerase activity in various cell types. (A) Telomerase activity was evaluated in CD8+ T cells, LC15 cells, and the SupT1 lymphoblast line. Naive CD8+ T cells were analyzed directly or after stimulation with 2 nM PMA and 100 nM ionomycin for 4 days. LC15 cells were analyzed 2 months and 10 months after IL-2 withdrawal. Nuclear extracts were obtained from all cell cultures and serially diluted; these samples were then subjected to the Telomeric Repeat Amplification Protocol (TRAP) assay, and the products were visualized by agarose gel electrophoresis. Extracts divided into aliquots from the EL-4 cell line were used as an interexperimental control (not shown). (B) For each set of reactions, telomerase activity was determined by measuring the image intensity with a phosphor imager and dividing this value by the intensity of an internal PCR standard (not shown).
A flow-FISH technique was used to evaluate telomere lengths (Table 2). Unmanipulated PBLs have telomere lengths measuring approximately 8 to 9 kb, whereas activated tumor-infiltrating lymphocytes have telomeres averaging 5 to 6 kb in length.10 In this experiment, anti–CD3-stimulated, 2-week cultured tumor-infiltrating lymphocytes were obtained from 4 different patients; telomere lengths in these samples ranged between 3.5 and 5.6 kb. LC15 had telomeres measuring 4.0 kb after 2 months in culture and 2.3 kb after 12 months in culture. The SupT1 line had telomeres that were 91.9 kb in length. Despite having telomeres that were critically shortened at 12 months, LC15 cultures have been grown in culture for up to 14 months with no evidence of senescence.
Blocking of LC15 proliferation
Because common γ-chain cytokines, such as IL-2 and IL-15, stimulate the proliferation of T lymphocytes, we speculated that constitutive IL-15 expression by the LC15 cell line contributed to the aberrant growth demonstrated by these cells. Using a monoclonal anti–IL-15 blocking antibody, the proliferation of normal, activated T cells grown in media containing 10 ng IL-15/mL could be almost completely attenuated when the blocking antibody was present at a concentration of 1 μg/mL (Figure 7A). We found that the proliferation of LC15 was not impeded by the addition of up to 10 μg blocking antibody/mL (Figure 7B). Similarly, adding up to 100 μg anti–IL-15 antibody/mL to LC15 cultures did not limit in vitro expansion of the cells (data not shown). We subsequently tested the biologic activity of anti–IL-15Rα and IL-15Rβ antibodies and found that they only mildly inhibited the proliferation of normal T cells (exposed to IL-15) and would thus be unsuitable to repress LC15 cell growth.
LC15 proliferation is not affected by α–IL-15 blocking antibody. For each assay, 1 × 105 cells were plated in triplicate in 96-well plates. Cells were cultured for a total of 24 hours. [3H]-thymidine was added to cultures 18 hours prior to scintillation counting. (A) To confirm activity of the α–IL-15 antibody, normal, activated T cells were plated with indicated concentrations of either α–IL-15 antibody or matched isotype control antibody. Subsequently, 10 ng IL-15/mL was added to the culture media. (B) To evaluate the effect of α–IL-15 antibody on LC15 cells, LC15 cells were washed and plated with serial dilutions of either α–IL-15 antibody or matched isotype control antibody. No inhibition of LC15 cells was seen. (C) Retroviral vectors containing shRNAs directed to either the human IL-15Rα or endogenously expressed human IL-15 were used to transduce LC15 cells and cells cultured in medium containing IL-2 and puromycin. Selected cells were withdrawn from IL-2–containing medium for 3 days, and their proliferation was measured by [3H] thymidine incorporation. [3H]-thymidine was added to cultures 48 hours prior to scintillation counting. Standard deviations indicated by error bars. Significance was evaluated by Student t test.
LC15 proliferation is not affected by α–IL-15 blocking antibody. For each assay, 1 × 105 cells were plated in triplicate in 96-well plates. Cells were cultured for a total of 24 hours. [3H]-thymidine was added to cultures 18 hours prior to scintillation counting. (A) To confirm activity of the α–IL-15 antibody, normal, activated T cells were plated with indicated concentrations of either α–IL-15 antibody or matched isotype control antibody. Subsequently, 10 ng IL-15/mL was added to the culture media. (B) To evaluate the effect of α–IL-15 antibody on LC15 cells, LC15 cells were washed and plated with serial dilutions of either α–IL-15 antibody or matched isotype control antibody. No inhibition of LC15 cells was seen. (C) Retroviral vectors containing shRNAs directed to either the human IL-15Rα or endogenously expressed human IL-15 were used to transduce LC15 cells and cells cultured in medium containing IL-2 and puromycin. Selected cells were withdrawn from IL-2–containing medium for 3 days, and their proliferation was measured by [3H] thymidine incorporation. [3H]-thymidine was added to cultures 48 hours prior to scintillation counting. Standard deviations indicated by error bars. Significance was evaluated by Student t test.
We next attempted to inhibit LC15 proliferation using RNA interference-mediated knockdown of the IL-15Rα.28,29 Retroviral vectors containing shRNAs directed to either the human IL-15Rα or endogenously expressed human IL-15 were used to transduce LC15 cells. The shRNA against endogenous human IL-15 was used as a control because it does not impact on the expression of IL-15 in LC15 cells (the IL-15 genetic sequence in the vector was codon optimized and is not homologous to the shRNA IL-15 knockdown sequence). Transduced cell populations were selected for by growth in puromycin-containing medium with exogenously added IL-2 so that proliferation could continue in the absence of any potential IL-15/IL-15Rα autocrine loop. Once cells were selected, cultures were withdrawn from IL-2–containing medium, and proliferation was measured by 3H thymidine incorporation. The results of this experiment (Figure 7C) demonstrated that control IL-15 shRNA knockdown cultures continue to proliferate in the absence of IL-2, whereas cultures transduced with the IL-15Rα shRNA vector showed a significant reduction (P = .001) in proliferation. These results suggest that the autocrine loop created by the constitutive expression of IL-15 by vector-transduced LC15 cells permitted these cells to continue to grow in the absence of exogenous cytokine. The lack of complete inhibition of proliferation by the IL-15Rα shRNA may indicate that mechanisms in addition to the autocrine loop may also function in LC15.
Finally, several studies were performed to further define the degree of transformation of the LC15 line. We were unable to grow these cells after repeated attempts at limiting dilution cloning when 3, 1, or 0.3 cells/well were plated in 96-well tissue culture plates; this was attempted in the presence and absence of conditioned media from active LC15 cultures. LC15 cells did not manifest colony formation after 3-week culture in soft agar. In the same experiment, the mouse EL-4 line formed colonies on soft agar, whereas 938mel and SupT1 cells did not. Subcutaneous injection of several melanoma lines (including 526mel, 624mel, and 888mel) results in tumor formation in nonobese diabetic–severe combined immunodeficient (NOD-scid)–A2 mice (C.H., unpublished data, March 2006). LC15 cells (107) were injected into NOD-scid-A2 mice either intravenously (n = 10) or subcutaneously (n = 10). Four of the mice that received intravenous LC15 injections were killed 5 days later to evaluate the persistence of LC15 cells. LC15 cells were not detected in the blood, liver, lungs, or spleens by FACS (using anti–human CD8 antibody) in any of these animals. The remaining mice were followed for 3 months, and these animals remained healthy for the duration, with no evidence of subcutaneous tumor formation. When the animals were killed, no visceral tumors were noted.
Discussion
Complete malignant transformation is believed to require multiple, cooperating genetic alterations which perturb cell differentiation and stimulate proliferation.30,31 In the SCID-X1 gene therapy trial, 3 of 11 patients developed T-cell leukemias that were related to vector integrations activating the LMO2 proto-oncogene.2 Because LMO2 activation alone is insufficient for the development of leukemia, investigators speculate that a complex interplay between the gene transfer vector and host biology predisposed patients to these tragic consequences.32 MLV-based vectors, which were used in this study, increase the probability of integration near transcriptionally active cellular promoters.4,33 Because LMO2 is expressed in HSCs, this locus is susceptible to vector integration. The γ-chain gene has been implicated in leukemogenesis and may promote transformation in the context of nonphysiologic expression.32,34 In mouse retrovirally induced leukemias, synergy between the γ-chain and LMO2 has been postulated.35 In a mouse X-SCID model, retroviral vector-mediated γ-chain gene transfer caused T-cell lymphomas without evidence of insertional activation of LMO2.36 Furthermore, in the SCID-X1 host, gene-corrected cells have a marked survival advantage which may have facilitated the acquisition of secondary mutations required for malignant transformation.37
IL-15 is potentially leukemogenic and its expression is tightly regulated.38 Transgenic mice overexpressing IL-15 exhibit profound lymphocytosis and are prone to the development of T-cell leukemias.39 This is believed to be related to chronic T-cell stimulation in the setting of chronic inflammation secondary to a proinflammatory cytokine environment. In human studies, dysregulated IL-15 expression has been demonstrated in HTLV-1–mediated adult T-cell leukemia.38 IL-15 can also promote the growth and survival of other human leukemia lines.38
Several factors make LC15 unique in the context of the established literature on insertional mutagenesis. Groups reporting clonal expansion of hematopoietic cells after retroviral transduction uniformly targeted primitive progenitor cells.2,36,40–43 Accordingly, many reports identified activating integrations at EVI1 or LMO2, which are transcription factors only active in progenitor cells. Furthermore, in vivo clonal expansion of transduced hematopoietic clones after retroviral transduction was observed after a period of latency. Possibly, in accordance with multistep models of leukemogenesis,30,31 additional transforming mutations were acquired by transduced cells during this latent period.32 In contrast, LC15 was generated from a culture of mature, activated lymphocytes. The findings that LC15 expressed a single Vβ gene and exhibited antigen-specific cytokine secretion support the notion that the line derived from a mature T lymphocyte. Abnormal in vitro expansion of LC15 was evident within 3 weeks after transduction. This suggests that the transforming event or events in LC15 occurred during or immediately after transduction.
In a recent report describing the clinical use of mature lymphocytes retrovirally transduced with a suicide gene in 46 patients, activation of proliferation-related genes was detected in transduced lymphocyte samples prior to infusion but not after engraftment.24 Thus, the investigators suggest that vector integrations affecting proliferation-related genes in mature lymphocytes may actually have a negative effect on their in vivo survival. Notably, there was no evidence of insertional mutagenesis in this patient population. Potentially, the rules for transformation of mature lymphocytes differ significantly from HSCs, and it is possible that LC15 would have been ablated in vivo had the cells been returned to the donor. Clearly, the growth of LC15 has been dysregulated, yet the tumorigenicity of the line is questionable because it could not be grown under limiting dilution conditions, in soft agar, nor could the cell line be established in immunocompromised mice.
Three retroviral vector integration sites were identified in LC15, and none of these are in proximity to known oncogenes. LC15 vector integrations were 7 to 300 kb from flanking genes. FOXP1 was slightly down-regulated in both CD8+ T cells cultured with IL-15/OKT3 and LC15. Thus, it appears that down-regulation of FOXP1 is a consequence of CD3/IL-15 stimulation. Transgene insertion-mediated silencing of FOXP1 is unlikely, because this would require an independent mutation in the second allele. Nevertheless, this merits further investigation because FOXP1 has been implicated as a tumor suppressor.44 The other genes flanking LC15 integration sites were found to be expressed in control CD8+ T cells and LC15 cells at similar levels. These findings concur with the recent report that in mature T cells, MLV-derived vectors tend to integrate in proximity of genes that are already active in the target cells.24 We did not find evidence of retroviral insertion-mediated gene activation.
LC15 bears some similarities to the SCID-X1 scenario and may be a relevant model for the study of retrovirus-induced transformation of human T lymphocytes. LC15 was generated by transducing T lymphocytes with a MLV-derived vector carrying the IL-15 gene. LC15 constitutively expressed IL-15 and exhibited prolonged clonal expansion. The cell line arose following growth in the absence of exogenous cytokine support; seemingly, this would favor the survival of cells producing IL-15. Interestingly, LC15 growth and proliferation were not attenuated by an anti–IL-15 blocking antibody. This may be related to the observation that IL-15 binds stably to high-affinity α-receptors which can be internalized through endosomes and subsequently recycled and presented to neighboring cells.45 Studies on an IL-3–-dependent hematopoietic cell line transduced with a modified IL-3 gene displayed properties similar to LC15.46 Specifically, the modified cells were able to grow without added cytokine and could not be inhibited with neutralizing anti–IL-3 antibodies. Stimulation occurred intracellularly in an autocrine fashion.46 The relatively high intracellular concentration of cytokine in this report and in LC15 could explain the proliferative advantage of these clones over their neighbors, which are dependent on extracellular cytokine. Potentially, LC15 is not dependent on secreted IL-15 being available in the cell culture medium. In T cells, IL-15 signal transduction occurs through a receptor complex consisting of IL-2/15Rβ and γ-chain.45 As in the case of the SCID-X1 trial, but by a different mechanism, increased stimulation of the γ-chain would be expected in LC15 because of constitutive IL-15 expression.
There is the possibility that the individual lymphocyte, which gave rise to LC15, contained preleukemic alterations prior to transduction; however, the donor's history is unremarkable for medical problems other than melanoma. Furthermore, the karyotype of LC15 revealed centromeric translocations, which are not generally seen in leukemia.21–23 We have repeated the transduction of this patient's cells with the IL-15 vector and have not observed another incident of long-term cytokine independent growth. In addition, this patient's cells have been repeatedly used in other transduction experiments involving TCR-containing retroviral vectors and have been engineered with both lentiviral vectors and transposons, and we have never observed a cell culture with similar properties to LC15. The fact that LC15 is clonal (based on Vβ rearrangement analysis and SKY; Figure 4) suggests that this is a rare event because we estimate that 6 to 7 million cells were transduced during the initial gene transfer procedure. These results support the conclusion that this patient's cells are not predisposed to immortalization.
LC15 demonstrated abnormal, constitutive telomerase activity which is believed to be a requirement for the maintenance of transformed clones.31,34 Typically, T lymphocytes may be cultured in vitro for 20 to 30 population doublings before reaching senescence.47 It has been demonstrated that IL-15 stimulates modest telomerase activity in vitro in memory CD8+ T cells, but this activity declines after prolonged culture.48 Thus, abnormally regulated telomerase activity in LC15 is unlikely to be a direct result of constitutive expression of the IL-15 transgene alone. Whether LC15 telomerase expression is an intrinsic property of the clone or a consequence of retroviral vector integration remains uncertain. Our observations that knockdown of the IL-15Rα in LC15 lead to decreased cell proliferation, suggested that an autocrine loop was established leading to chronic IL-2/15Rβ + γ-c stimulation and continued cell growth. Constitutive telomerase activity would support continued cell division.
LC15 cells were isolated from the peripheral blood and have an unknown reactivity. The potential target of IL-15 vector transduction, tumor antigen-specific T cells, would be dependent on antigen stimulation in addition to cytokine for in vivo expansion. Although the requirement for antigen stimulation might limit sustained growth, the clinical application of IL-15 gene transfer using integrating vectors should be approached with caution. This cell line presents a unique opportunity to study the regulation of primary human T-cell growth and may reveal new insights on leukemogenesis secondary to retroviral vector integration.
In summary, we report the continued in vitro growth of a mature T lymphocyte after retroviral transduction with a vector carrying the γ-chain cytokine IL-15. Replication incompetent retroviral vectors have been used to deliver genes such as Bcl-2, Bcl-XL, IL-2, hTERT, and Tax that could prolong the survival of primary T cells.49–54 However, none of these studies report prolific, clonal, cell expansion in the absence of repeated stimulation and exogenous cytokine as demonstrated by LC15. The LC15 cell line appears to be the result of an infrequent vector integration event and possibly synergy between constitutive IL-15 transgene expression and telomerase activity. Whether some undetermined genes dysregulated by the process of retroviral transduction influence the phenotype of these cells is unknown. In the past 2 decades of research involving retroviral vector-mediated gene transduction of human lymphocytes, LC15 is, to our knowledge, a unique report of a vector-transduced primary cell line displaying the properties of cytokine independent growth. The rareness of this observation is, in of itself, significant and suggests that genetic engineering of mature human lymphocytes may be less prone to transformation than similar procedures targeting progenitor cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shawn Farid and Arnold Mixon (Surgery Branch, National Cancer Institute) for FACS analyses and Laura Johnson (Surgery Branch) for providing the DMF5 MART-specific TCR. We also thank Robert Getty (Department of Medical and Molecular Genetics, Indiana University School of Medicine) for assistance in integration site cloning.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
National Institutes of Health (NIH)
Authorship
Contribution: C.H. designed and performed research, analyzed data, and wrote the paper; S.A.J., C.J.C., Z.Z., K.K., J.Z., P.D.P., X.S., T.J.G., D.J.M., C.S., K.C., and D.W. performed research and analyzed data; P.F.R., C.E.D., and T.R. analyzed data; S.A.R. and R.A.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Morgan, 10 Center Dr, Bldg 10/Rm 3-5940, Bethesda, MD 20892; e-mail: rmorgan@mail.nih.gov.




![Figure 7. LC15 proliferation is not affected by α–IL-15 blocking antibody. For each assay, 1 × 105 cells were plated in triplicate in 96-well plates. Cells were cultured for a total of 24 hours. [3H]-thymidine was added to cultures 18 hours prior to scintillation counting. (A) To confirm activity of the α–IL-15 antibody, normal, activated T cells were plated with indicated concentrations of either α–IL-15 antibody or matched isotype control antibody. Subsequently, 10 ng IL-15/mL was added to the culture media. (B) To evaluate the effect of α–IL-15 antibody on LC15 cells, LC15 cells were washed and plated with serial dilutions of either α–IL-15 antibody or matched isotype control antibody. No inhibition of LC15 cells was seen. (C) Retroviral vectors containing shRNAs directed to either the human IL-15Rα or endogenously expressed human IL-15 were used to transduce LC15 cells and cells cultured in medium containing IL-2 and puromycin. Selected cells were withdrawn from IL-2–containing medium for 3 days, and their proliferation was measured by [3H] thymidine incorporation. [3H]-thymidine was added to cultures 48 hours prior to scintillation counting. Standard deviations indicated by error bars. Significance was evaluated by Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-06-029173/4/m_zh80120702050007.jpeg?Expires=1765037238&Signature=pREC-UsvoRwMLGFKn0hBomqZS7W5Wqi1P0et8qO4JLjJQpWs13rm-GGCCm88LhrHANKm31HP6EpRAxhfds6RW-uevqmxQdClqsSoG2fMXj8bvfl~beQ8agQeTy2dHGhzs6q3xzqZdbczilB2nMo51dN1xeVuLyoJzS1ipS8Z7c-VtGz1tjQNXuyCTToqrbzZrGlwrEqIWBkvmJaKTiA-qdzJWf9yCBJ7yQvXU3YjlHc41jQAm5~JjwTeeqEQSf0GtcGB2PW9vp9-x-QveRWbu3Vz65IOCMoV71VJOd7woSnpDKMtxpaPnIIS0OzMvBd7yWUBsMsp30aR8cqPBiWGWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 7. LC15 proliferation is not affected by α–IL-15 blocking antibody. For each assay, 1 × 105 cells were plated in triplicate in 96-well plates. Cells were cultured for a total of 24 hours. [3H]-thymidine was added to cultures 18 hours prior to scintillation counting. (A) To confirm activity of the α–IL-15 antibody, normal, activated T cells were plated with indicated concentrations of either α–IL-15 antibody or matched isotype control antibody. Subsequently, 10 ng IL-15/mL was added to the culture media. (B) To evaluate the effect of α–IL-15 antibody on LC15 cells, LC15 cells were washed and plated with serial dilutions of either α–IL-15 antibody or matched isotype control antibody. No inhibition of LC15 cells was seen. (C) Retroviral vectors containing shRNAs directed to either the human IL-15Rα or endogenously expressed human IL-15 were used to transduce LC15 cells and cells cultured in medium containing IL-2 and puromycin. Selected cells were withdrawn from IL-2–containing medium for 3 days, and their proliferation was measured by [3H] thymidine incorporation. [3H]-thymidine was added to cultures 48 hours prior to scintillation counting. Standard deviations indicated by error bars. Significance was evaluated by Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-06-029173/4/m_zh80120702050007.jpeg?Expires=1765183576&Signature=oApJeNgJpqDIVTSR0Yw~20wMv3naNlyF8k8dK~GY1xcn49nUm5FaKWdMsHYKOqNEBudhm0z5O~ieU16q6c1ubjBrPpegvS7jxqoQScAKaFXJUStyxzXnHmtcXC4I9sV~hn3jMHtKLgiPB0FpWQIVsFLFlE9-Vz8UVMQRJFC3igHl1cQDEUB38qIbi~6wTghnDgtevFWYND23yLy~r44dHWV3t~YQQKHX8UWybnOxnPhgAa3diCskt6iDNwKoXv6ONgwgQE2k3wk7GNmXSSkqy0gDGgyUnYz7B0v1DMQg-BUxbeUjrhFGMvusPwxVEfARsEqThykIlIqh92Mp7DG8oA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)