Abstract
Infantile malignant osteopetrosis (IMO) is a fatal disease caused by lack of functional osteoclasts, and the only available treatment is hematopoietic stem cell (HSC) transplantation. In the majority of patients, the TCIRG1 gene, coding for a subunit of a proton pump essential for bone resorption, is mutated. Oc/oc mice have a deletion in the homologue gene (tcirg1) and die at 3 to 4 weeks, but can be rescued by neonatal transplantation of HSCs. Here, HSC-targeted gene therapy of osteopetrosis in the oc/oc mouse model was developed. Oc/oc fetal liver cells depleted of Ter119-expressing erythroid cells were transduced with a retroviral vector expressing tcirg1 and GFP, and subsequently transplanted intraperitoneally to irradiated neonatal oc/oc mice. Eight of 15 mice survived past the normal life span of oc/oc mice. In vitro osteoclastogenesis revealed formation of GFP-positive osteoclasts and bone resorption, albeit at a lower level than from wild-type cells. The skeletal phenotype was analyzed by X-ray and histopathology and showed partial correction at 8 weeks and almost normalization after 18 weeks. In summary, osteopetrosis in oc/oc mice can be reversed by neonatal transplantation of gene-modified HSCs leading to long-term survival. This represents a significant step toward the development of gene therapy for osteopetrosis.
Introduction
The osteoclast and the osteoblast are important mediators in the tightly regulated process of bone remodeling. The bone-forming osteoblasts are of mesenchymal origin, while the multinucleated osteoclasts that are responsible for bone resorption are derived from hematopoietic stem cells (HSCs). The balance between production and destruction of bone can be disturbed, and this will result in either reduction (osteoporosis) or increase (osteopetrosis) of bone mass.
Osteopetrosis comprises a heterogeneous group of diseases with varying degree of osteoclast dysfunction and the most severe form is called infantile malignant osteopetrosis (IMO).1 Children with this rare disorder have normal or elevated numbers of osteoclasts, which are unable to resorb bone due to defects in the acidification of the subcellular space between the osteoclast and the bone surface.2–5 Other cellular processes, such as cytoskeletal organization and ruffled border membrane formation, have also been suggested to be disturbed.6,7
The gene mutated in the majority of patients with IMO is called TCIRG1 (or ATP6I or OC116).8 The 2.7-kbp transcript codes for the α3 subunit of a proton pump used by the osteoclast to acidify the resorption area.9 As a consequence of the lack of resorption, remodeling of bone is severely hampered, which results in dense and fragile bone.10 This, in turn, causes bone marrow (BM) failure followed by anemia and hepatosplenomegaly.1,11,12
The only curative treatment for IMO is HSC transplantation, but this form of therapy is associated with high mortality, especially when HLA-identical donors are not available.13 IMO is thus a candidate disease for development of gene therapy because of its fatal outcome early in life if treatment with HSC transplantation is not possible. Since progression of the disease is rapid, it is of importance to initiate the treatment as early as possible. Experience from gene therapy studies of other diseases with hematopoietic involvement, such as severe combined immunodeficiency (SCID) and chronic granulomatous disease,14,15 shows promising results. Gene therapy of IMO has so far not been tested in animal models or in patients. We have previously demonstrated that a mouse model of IMO, the oc/oc mouse, which has a 1500-bp deletion in the tcirg1 gene, can be treated with neonatal BM transplantation.16 Here we show, both in vitro and in vivo, that the osteopetrotic phenotype in oc/oc mice can be reversed by transplantation of fetal liver–derived oc/oc HSCs retrovirally transduced to express a nonmutated form of tcirg1. This represents the first report of osteoclast-directed gene therapy and a first but significant step toward the development of gene therapy for osteopetrosis.
Materials and methods
Mice
Two pairs of (C57BL/6J × C3HheB/FeJ) F1 oc/+ mice (Ly5.2) were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained in the conventional animal facility at the Biomedical Centre, University of Lund. The mice had a mixed genotype in their HLA-locus (b/k), and were bred to obtain homozygotes (b/b). oc/oc mice that underwent transplantation were fed mashed food due to lack of teeth eruption or due to dental abnormalities in mice in which teeth had erupted. Because of abnormal wearing, teeth had to be kept short by cutting. All experiments were performed according to protocols approved by the local ethics committee. Oc/+ mice appeared normal and were phenotypically indistinguishable from wild-type (WT) littermates.
Genotyping of mice
Mice were genotyped on the day of birth using DNA extracted from the tip of the tail. Polymerase chain reaction (PCR) was performed using 2 forward primers, F1 (ATCGTAAGGCTGGGTTGTCCACT) and F3 (GATCATGGGCTCTATGGTTCCG), and one reverse primer, R1 (GCTCATTCCATGGGATGTGAATC). Forward primer F3 binds to the sequence deleted in tcirg1 in oc/oc mice. For identification of the genotypes, the PCR products were loaded onto a 1% agarose gel. Oc/oc mice were identified by a single band of 306 bp; oc/+ mice, by one band of 306 bp and one band of 482 bp; and WT mice, by a single band of 482 bp (Figure 1D).
Vector design and experimental setup. (A) Design of retroviral vectors. The backbone in both vectors contains SFFV LTRs and a wPRE. The upper vector contains murine tcirg1 cDNA followed by an IRES, and GFP and is denoted the tcirg1 vector. The lower vector control vector contains an IRES sequence followed by GFP. (B) Northern blot showing mRNA expression of tcirg1 in the transduced cell line 3T3. (C) FL harvest, Ter119 depletion, in vitro transduction, and analysis. (D) Genotyping, irradiation, and transplantation of oc/oc mice.
Vector design and experimental setup. (A) Design of retroviral vectors. The backbone in both vectors contains SFFV LTRs and a wPRE. The upper vector contains murine tcirg1 cDNA followed by an IRES, and GFP and is denoted the tcirg1 vector. The lower vector control vector contains an IRES sequence followed by GFP. (B) Northern blot showing mRNA expression of tcirg1 in the transduced cell line 3T3. (C) FL harvest, Ter119 depletion, in vitro transduction, and analysis. (D) Genotyping, irradiation, and transplantation of oc/oc mice.
Retroviral vectors and producer cell lines
cDNA for the murine tcirg1 gene was amplified from a murine BM cDNA library (generated from a C57/BL6 mouse) using the forward primer TTTCGAAGCTGGAGTGAGCTGCACT and reverse primer CCTGGGCCAGCCAGCTCTTTAT in a first round and forward primer AGCCGAAGTCGACCGAAGAGTCCGCCGGCCACCATGGGCTCTATGTTCCGG (for insertions of new restriction sites) and reverse primer TCCCACTTCGGAGAATTCGGGAT in a second round. The amplified cDNA was checked by sequencing. The tcirg1 cDNA followed by an internal ribosomal entry site (IRES), GFP cDNA, and a woodchuck posttranscriptional regulatory element (wPRE) was cloned into pSF91P,17 a retroviral vector backbone with a spleen focus-forming virus (SFFV) long terminal repeat (LTR) driving the expression of the transcripts. This vector will be referred to as the tcirg1 vector (Figure 1A). A pSF91P vector expressing GFP preceded by IRES was used as a control vector (Figure 1A). Both vectors were transfected into Phoenix Ampho cells (Nolan lab; Stanford University, Palo Alto, CA), and supernatants were harvested for transduction of the stable producer cell line GP+E86 (ecotrophic envelope) followed by selection of high-titer clones. The titers were approximately 5 × 105 for the tcirg1 vector and 1 × 107 for the control vector.
Northern blot on 3T3 cells transduced with the tcirg1 vector
3T3 cells were transduced with vector-containing medium (VCM) from the tcirg1 stable vector-producing cell line and total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). Untransduced 3T3 cells were used as control cells. Briefly, RNA was separated on an agarose gel and blotted to a membrane. For the hybridization, a probe produced by digestion of the tcirg1 vector with FspI (1995-bp band) was used.
Harvest and enrichment of fetal liver hematopoietic cells
On embryonic day 14.5, pregnant mice were killed by CO2 poisoning, and embryos were removed. Fetal livers (FLs) were dissected out and put into PBS (Invitrogen) supplemented with 2% FCS (Invitrogen). Single-cell suspensions were prepared by drawing liver cells through a 23-gauge needle followed by filtering through a 50-μm cell strainer. Individual FLs were genotyped by lysing a cell sample and running the PCR reaction described above in genotyping of mice. To enrich for stem and progenitor cells, Ter119-positive erythroid cells were depleted by incubation with a Ter119 antibody (Becton Dickinson, Franklin Lakes, NJ) and subsequent incubation with sheep antirat antibody–conjugated beads (Dynal Biotech, Oslo, Norway) followed by magnetic separation. Ter119-depleted oc/oc cells were used for gene therapy experiments. For initial transplantation experiments, Ter119-depleted WT and +/oc cells were used.
Transduction of fetal liver cells
Suspension culture plates (24-well) were coated with Retronectin CH-296 (Takara Shuzo, Otsu, Japan) and blocked with 2% BSA in PBS followed by preloading of 125 μL VCM. Ter119-depleted FL cells were suspended in VCM supplemented with murine stem cell factor, Flt3-ligand, and thrombopoietin, all at 50 ng/mL (Peprotek, Rocky Hill, NJ), and seeded into the wells at a concentration of 6 × 105 cells/mL. Transduction was repeated after approximately 18 hours and cells were frozen after a total culture period of 24 hours. Aliquots were kept in culture for an additional 48 hours and analyzed by flow cytometry to determine the fraction of GFP-positive cells.
Transplantations
One-day-old oc/oc mice were irradiated with 400 cGy administered from a 137Cs source. Four hours later mice received an intraperitoneal transplant of freshly thawed transduced FL cells in 30 μL PBS. Oc/oc mice that received a transplant of oc/oc cells transduced with the tcirg1 vector will be denoted oc/oc+tcirg1 mice.
Engraftment and lineage distribution analysis of peripheral blood
Peripheral blood (PB) was collected in heparin (LEO Pharma, Thornhill, ON) after tail clipping of mice, put in a 96-well U-bottom plate, and mixed with equal volumes of PBS + 2% FCS. Following centrifugation, the supernatant was poured off, erythrocytes were lysed with NH4Cl, and the cells were washed twice with PBS + 2% FCS. Subsequently, cells were incubated on ice for 20 to 30 minutes with APC-conjugated antibodies directed against B220, CD3, Gr-1, and Mac-1 (multilineage analysis) (Becton Dickinson). GFP+ cells were detected in the FL-1 channel. The cells were suspended in 300 μL PBS + 2% FCS followed by addition of 1 μg/mL 7-amino-actinomycin D (7-AAD, for detection of nonviable cells; Sigma, St Louis, MO) before analysis using a fluorescence-activated cell sorting (FACS) Calibur Instrument (Becton Dickinson). For engraftment analysis, the fraction of GFP+ cells was measured.
Isolation and sorting of bone marrow cells from oc/oc+tcirg1 and WT mice
BM from 13-week-old oc/oc+tcirg1 and age- and sex-matched WT mice was isolated as previously described.18 Mice were killed with a peritoneal injection of a lethal dose of euthesate (8 mg sodium pentobarbital per mouse; Sanofi Santé Animale Benelux, Maassluis, the Netherlands). Femurs and tibiae were removed, cleaned of soft tissue, and ground in a mortar with culture medium α-minimal essential medium (Gibco, Paisley, Scotland) supplemented with 5% FCS (HyClone, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL amphotericin B (antibiotic antimyotic solution; Sigma) and heparin (170 IE/mL; Leo Pharmaceutical Products, Weesp, the Netherlands). The cell suspension was aspirated through a 21-gauge needle and filtered over a 100-μm pore size Cell Strainer filter (Falcon; Becton Dickinson). Aliquots of cells from oc/oc+tcirg1 mice were sieved through 50-μm filters (filcons; Becton Dickinson) before sorting into GFP+ and GFP− populations. Depending on the availability of sorters, cells were sorted either on a BD FacsAria (Becton Dickinson) or a MoFlo (Dako, Glostrup, Denmark). Until plating, all cell suspensions were kept on ice.
In vitro osteoclastogenesis
BM isolates and sorted cells were plated in 96-well flat-bottom tissue culture–treated plates (Costar, Cambridge, MA) at a density of 1 × 105 or 4 × 105 cells per well in 150 μL culture medium containing 30 ng/mL recombinant murine M-CSF (R&D systems, Minneapolis, MN) with 20 ng/mL recombinant murine RANKL (RANKL-TEC; R&D systems). In addition, cells were seeded on 650-μm–thick bovine cortical bone slices. Culture media were replaced every 3 days. At the end of the culture period, cells were fixed in PBS-buffered 4% formaldehyde and stained for tartrate-resistant acid phosphatase (TRACP) activity using the leukocyte acid phosphatase kit (Sigma). Nuclei were stained with diamidino-2phenylindole dihydrochloride (DAPI).
In vitro bone resorption
For bone resorption assays, the bone slices were cleaned from the cells by incubating the slices in 0.25 M NH4OH. The slices were washed in distilled water, incubated in a water saturated alum (KAl(SO4)2 · 12H2O) solution, washed in distilled water, and stained with Coomassie Brilliant blue. The areas of individual resorption pits were measured using Image-Pro Plus software (MediaCybernetics, Silver Spring, MD).
Quantitative PCR
RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The RNA concentration was measured with the NanoDrop (Nanodrop technologies, Wilmington, MA). RNA (100-150 ng) was used in the reverse-transcriptase (RT) reaction that was performed according to the MBI Fermentas cDNA synthesis kit (Vilnius, Lithuania), using both the Oligo(dT)18 and the D(N)6 primers. Quantitative real-time PCR was performed using SybrGreen I on a LightCycler (Roche Diagnostics, Mannheim, Germany) for measurement of tcirg1 and hypoxanthine phosphoribosyltransferase (HPRT) as a reference. cDNA (2 μL, 0.5 ng/μL) was used in a 10-μL final volume reaction containing 1U Platinum Taq DNA polymerase (Invitrogen), 1 × buffer (provided with the enzyme) 0.8 mM dNTP, 3 mM MgCl2, 0.5 mg/mL BSA (Sigma Aldrich), 5% DMSO (Sigma Aldrich), 0.5 μM forward primer, 0.5 μM reverse primer, and 1:20 000 dilution of SybrGreen I. Tcirg1 forward primer was GATCATGGGCTCTATGTTCCG; tcirg1 reverse primer, ACCTGCCCGCTGCACTTCTT; hprt forward primer, CACAGGACTAGAACACCTGC; and hprt reverse primer, GCTGGTGAAAA GGACCTC.
For provirus copy number determination, DNA was isolated from transduced oc/oc fetal liver cells and quantitative PCR was performed as described in previous paragraph using the following primers: Tcirg1 forward primer GATCATGGGCTCTATGTTCCG and reverse primer GTCTCTGAACTCCACGAG and actin forward primer CAC CACAGCTGAGAGGGAAA and reverse primer GTCAGGCAGCTCATA GCTCTT.
Analysis was done with LightCycler Software version 5.32 (Roche Diagnostics). Tcirg1 PCR values were normalized to the corresponding hprt and actin values.
X-ray analysis
Prior to X-ray analysis, mice that underwent transplantation or control mice were killed with CO2. The X-ray examination was performed at the University Hospital in Lund.
Histology
Femurs were dissected from the mice and following fixation in PBS containing 4% formaldehyde, the bone was put in Parengy decalcification solution (0.15% chromethrioxid, 4.3% nitric acid, and 30% ethanol) (Bie & Berntsen, Copenhagen, Denmark) for 48 hours. After paraffin embedding, the femurs were subsequently sectioned and stained with Erlish eosin for microscopic examination.
Statistical analysis
Mean values and standard deviations (SDs) were computed in Excel (Microsoft, Redmond, WA). Student t test was used for comparisons. Levels of significant difference shown as * (P < .05) and ** (P < .005).
Results
Ter119-depleted fetal liver HSCs can be efficiently transduced with retroviral vectors
We have previously shown that oc/oc mice can be cured by neonatal transplantation of normal BM cells.16 For gene therapy experiments, another source of HSCs had to be used because of the severe reduction of cells in the BM of oc/oc mice. During late embryogenesis, the FL serves as the major site for hematopoiesis, and as there are no differences in FL cell number between oc/oc and WT mice (data not shown), these cells were targeted.
FL cells were enriched for HSCs by depletion of erythroid cells using a Ter119 antibody. The average yield of cells after this procedure was 1.9 × 106 cells per FL (range, 0.75-2.7 × 106). Subsequently, cells were transduced in a short 24-hour transduction protocol with either the bicistronic retroviral vector expressing tcirg1 and GFP or the GFP control vector. In vitro transduction efficiency was higher than 90% with the GFP vector (Table 1) as determined by FACS and somewhat lower with the tcirg1 vector, 42% to 85% (Table 2). The provirus copy number in transduced cells was determined by quantitative PCR and found to be in the range of 3.5 to 5.5 copies per GFP-positive cell when the transduction efficiency was 81% (SD 3%, n = 6).
Transplantation of normal fetal liver cells transduced with a GFP vector cures oc/oc mice from osteopetrosis and gives rise to long-term engraftment
To determine if FL HSCs have the same ability to cure oc/oc mice as BM cells, Ter119-depleted WT FL cells transduced with the GFP control vector were transplanted into sublethally irradiated 1-day-old oc/oc mice (n = 3, Table 1). In accordance with our previous study, transplantation of WT hematopoietic cells, although this time from a different source, cured oc/oc mice from osteopetrosis. All mice survived past the expected life span and exhibited teeth eruption and weight gain in the same manner as seen after the BM transplantation.16 The percentage of GFP+ cells in PB was analyzed 3, 8, and 18 weeks after transplantation and transduced cells of all hematopoietic lineages were identified (data not shown), indicating the presence of transduced HSCs in the mice.
Neonatal transplantation of oc/oc FL cells transduced with the tcirg1 vector results in long-term survival of oc/oc mice
Next we determined if oc/oc FL cells, genetically modified to express a nonmutated form of tcirg1, and subsequently transplanted to neonatal oc/oc mice, could rescue these mice from fatal progression of osteopetrosis. Ter119-depleted oc/oc FL cells were transduced with the tcirg1 vector (Figure 1). Transduced cells were transplanted intraperitoneally into 1-day-old mice irradiated with 400 cGy (Figure 1). Eight of 15 mice that underwent transplantation survived past the normal short life span of oc/oc mice and these are shown in Table 2, as well as the number of cells injected, in vitro transduction efficiency, and the level of GFP-positive cells in the peripheral blood at various time points. Oc/oc mice that underwent transplantation that did not survive died early, 12 to 22 days after birth. The cell dose and in vitro transduction efficiency of cells transplanted in these mice was in the same range as for surviving mice. Only 3 of the animals that died early lived long enough (3 weeks) to be subjected to analysis of frequency of GFP+ cells in PB, which was less than 15% in all cases. Mice that underwent transplantation increased in weight but remained smaller than both normal mice and irradiated normal mice (data not shown). In contrast to mice that received a transplant of normal BM or FL cells, teeth eruption in the mice that received a transplant of gene-modified cells was almost absent or very modest. Oc/oc mice that received a transplant of oc/oc FL cells transduced with the GFP-expressing control vector (n = 4, 3 × 106 cells/mouse, > 90% transduction efficiency in vitro) all died around after 13 to 15 days, showing that the transplantation procedure, per se, did not prolong the life span of treated oc/oc mice.
Multilineage engraftment of gene-modified cells in oc/oc mice that underwent transplantation and reversal of peripheral blood B-cell deficiency
Reconstitution in oc/oc+tcirg1 mice was analyzed by flow cytometry of PB at different time points after transplantation. All surviving mice had sustained reconstitution of GFP+ cells as shown in Table 2, and also exhibited multilineage GFP marking (Figure 2A), suggesting transduction of hematopoietic stem or early progenitor cells. It has previously been shown that oc/oc mice have a block in B lymphopoiesis, probably due to reduced production of IL-7 in the BM microenvironment, and as a result a reduced number of B cells in PB.19,20 In order to investigate if the peripheral B-cell deficiency was corrected, multilineage analysis of PB was performed at 8 and 12 to 18 weeks after transplantation (Figure 2A). We previously showed that PB lineage distribution was normalized in oc/oc mice that received a transplant of normal BM cells.16 In the present study, we can see a significant rise in the mature B220+ B-cell population in oc/oc+tcirg1 mice 12 to 18 weeks after transplantation compared to untreated oc/oc mice, and the level is not significantly different from WT controls (Figure 2B).
Multilineage reconstitution of transduced cells in oc/oc+tcirg1 mice and reversal of peripheral blood B-cell deficiency in oc/oc+tcirg1 mice. (A) The peripheral blood of an 18-week-old oc/oc+tcirg1 mice was analyzed for expression of GFP and lineage markers. Percentage positive cells as indicated in the graph. (B) The percentage of B220-positive cells in the peripheral blood of oc/oc, oc/oc+tcirg1, and WT mice was analyzed at the time points indicated. The mean value and SD is shown (n = 4-6 mice per group).
Multilineage reconstitution of transduced cells in oc/oc+tcirg1 mice and reversal of peripheral blood B-cell deficiency in oc/oc+tcirg1 mice. (A) The peripheral blood of an 18-week-old oc/oc+tcirg1 mice was analyzed for expression of GFP and lineage markers. Percentage positive cells as indicated in the graph. (B) The percentage of B220-positive cells in the peripheral blood of oc/oc, oc/oc+tcirg1, and WT mice was analyzed at the time points indicated. The mean value and SD is shown (n = 4-6 mice per group).
Bone marrow cells harvested from oc/oc+tcirg1 mice that underwent transplantation form bone-resorbing osteoclasts in vitro
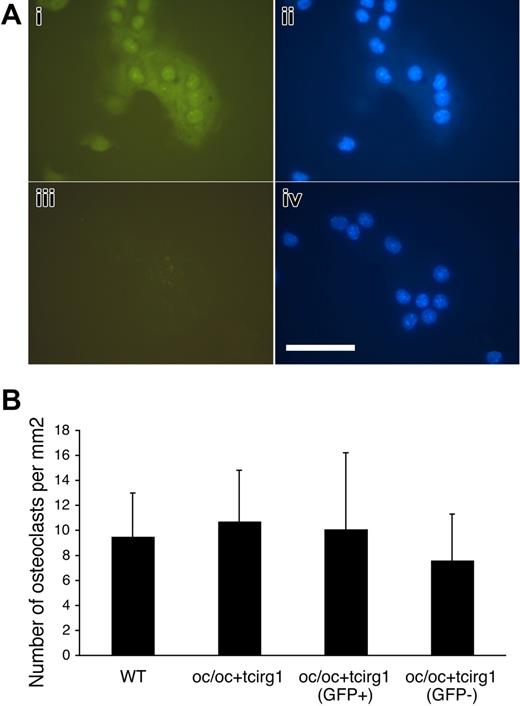
To investigate if transduced oc/oc FL cells can differentiate into bone-resorbing osteoclasts, we harvested BM cells from oc/oc+tcirg1 mice and cultured these in vitro with M-CSF and RANKL. Both green fluorescent and nonfluorescent multinucleated cells formed from oc/oc+tcirg1 BM after 6 days of culture, whereas only nonfluorescent cells formed from WT BM (Figure 3A). BM from oc/oc+tcirg1 mice was also sorted into populations of GFP+ and GFP− cells before in vitro culture. The number of osteoclasts (TRACP-positive cells with ≥ 3 nuclei per cell) that formed was similar between genotypes (WT and oc/oc+tcirg1) and the 2 sorted cell populations from the oc/oc+tcirg1 mice (Figure 3B). The distribution of the various categories of multinucleated cells (3-5, 6-10, and more than 10 nuclei per cell) was similar between these 4 groups (data not shown).
Cells harvested from oc/oc+tcirg1 mice can be in vitro–differentiated to osteoclast-like cells in normal numbers. BM cells from oc/oc+tcirg1 mice were cultured for 6 days with M-CSF and RANK-L after which generation of osteoclast-like cells was analyzed. (A) Both GFP+ (i-ii) and GFP− (iii-iv) osteoclast-like cells were formed. (i, iii) Green fluorescent; (ii, iv) blue fluorescent (DAPI) image. Bar represents 100 μm. Microscope: Leica DM IL PLAN 40× magnification, 0.5 lens; camera: Leica DFC320; and acquisition software: Leica IM500. (B) Osteoclasts were cultured on bone slices from BM cells obtained from WT or oc/oc+tcirg1 mice. Oc/oc+tcirg1 cells were also FACS sorted into GFP+ and GFP− populations. After culture with M-CSF and RANKL for 6 days, the number of multinucleated (≥ 3 nuclei) cells was enumerated. Mean ± SD is shown.
Cells harvested from oc/oc+tcirg1 mice can be in vitro–differentiated to osteoclast-like cells in normal numbers. BM cells from oc/oc+tcirg1 mice were cultured for 6 days with M-CSF and RANK-L after which generation of osteoclast-like cells was analyzed. (A) Both GFP+ (i-ii) and GFP− (iii-iv) osteoclast-like cells were formed. (i, iii) Green fluorescent; (ii, iv) blue fluorescent (DAPI) image. Bar represents 100 μm. Microscope: Leica DM IL PLAN 40× magnification, 0.5 lens; camera: Leica DFC320; and acquisition software: Leica IM500. (B) Osteoclasts were cultured on bone slices from BM cells obtained from WT or oc/oc+tcirg1 mice. Oc/oc+tcirg1 cells were also FACS sorted into GFP+ and GFP− populations. After culture with M-CSF and RANKL for 6 days, the number of multinucleated (≥ 3 nuclei) cells was enumerated. Mean ± SD is shown.
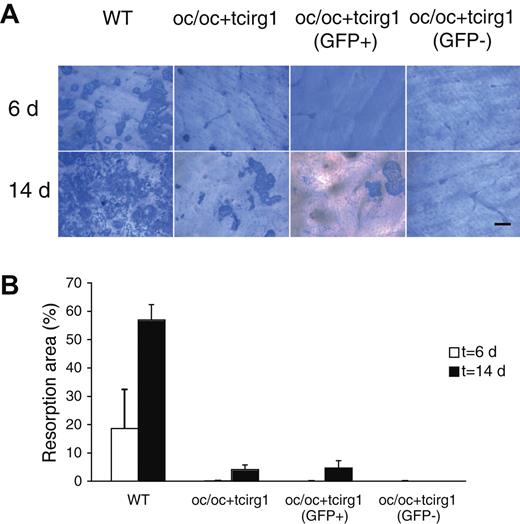
We further assessed the in vitro bone-resorbing activity of osteoclasts derived from BM cells from WT mice and oc/oc+tcirg1 mice after 6 and 14 days of culture on bovine bone slices. After 6 days, only WT cells exhibited signs of bone resorption (Figure 4), whereas after 14 days of culture also the oc/oc+tcirg1 cells were able to resorb bone, albeit at a lower level than WT cells. When oc/oc+tcirg1 BM was sorted into GFP+ and GFP− cell populations and cultured on bone slices, only GFP+ cells exhibited bone-resorbing activity but not to any greater degree than nonsorted oc/oc+tcirg1 cells. GFP− cells from oc/oc+tcirg1 BM exhibited no signs of bone resorption even after 14 days of culture on bone slices, while osteoclasts derived from oc/oc mice that received a transplant of normal FL cells transduced with the GFP vector exhibited the same level of resorption as WT cells (data not shown). This indicates that the stem cell source, transduction, and transplantation procedure as such did not reduce the resorption capacity of osteoclast-like cells formed.
In vitro–differentiated cells from oc/oc+tcirg1 mice are capable of bone resorption. Osteoclasts were cultured from BM cells harvested from WT or oc/oc+tcirg1 mice. In addition, oc/oc+tcirg1 cells were FACS sorted into GFP+ and GFP− populations. After culture with M-CSF and RANKL for 6 or 14 days on cortical bone slices, cells were removed and resorption pits were visualized. (A) Examples of bone resorption pits formed by WT, oc/oc+tcirg1–, oc/oc+tcirg1 (GFP+)–, and oc/oc tcirg1 (GFP−)–derived osteoclasts after 6 (upper panel) and 14 (lower panel) days. Bar represents 100 μm. For microscope and camera model, see Figure 3B legend. (B) Percentage of bone surface resorbed after 6 (empty bars) or 14 (solid bars) days of culture. Mean ± SD of a triplicate plating is shown.
In vitro–differentiated cells from oc/oc+tcirg1 mice are capable of bone resorption. Osteoclasts were cultured from BM cells harvested from WT or oc/oc+tcirg1 mice. In addition, oc/oc+tcirg1 cells were FACS sorted into GFP+ and GFP− populations. After culture with M-CSF and RANKL for 6 or 14 days on cortical bone slices, cells were removed and resorption pits were visualized. (A) Examples of bone resorption pits formed by WT, oc/oc+tcirg1–, oc/oc+tcirg1 (GFP+)–, and oc/oc tcirg1 (GFP−)–derived osteoclasts after 6 (upper panel) and 14 (lower panel) days. Bar represents 100 μm. For microscope and camera model, see Figure 3B legend. (B) Percentage of bone surface resorbed after 6 (empty bars) or 14 (solid bars) days of culture. Mean ± SD of a triplicate plating is shown.
The level of expression of tcirg1 in oc/oc+tcirg1 osteoclasts is reduced compared to WT cells
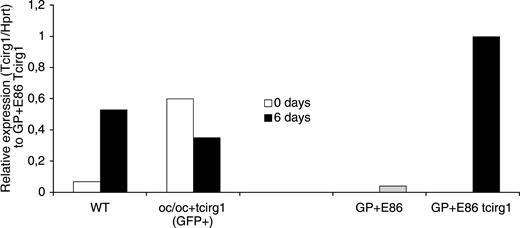
Quantitative RT-PCR was performed on cDNA from freshly harvested BM cells (0 days) and in vitro–differentiated osteoclasts (6 days) from WT and oc/oc+tcirg1 mice to determine the level of tcirg1 expression (Figure 5). In addition, the vector-producing cell lines GP+E86 (control vector) and GP+E86 tcirg1 (tcirg1 vector) were used as a comparison of vector expression.
Expression of WT tcirg1 is up-regulated during osteoclast differentiation, while vector-mediated expression is down-regulated. Quantitative RT-PCR was performed on cDNA from freshly isolated BM cells (0 days) harvested from WT and oc/oc+tcirg1 mice (GFP+ cells) and after 6 days of culture with M-CSF and RANKL to determine the level of tcirg1 expression. Tcirg1 PCR values were normalized against the corresponding HPRT value giving a relative intensity value for comparison of different samples. Expression levels are compared to the tcirg1 vector GP+E86 producer cell line and the GFP vector GP+E86 producer cell line.
Expression of WT tcirg1 is up-regulated during osteoclast differentiation, while vector-mediated expression is down-regulated. Quantitative RT-PCR was performed on cDNA from freshly isolated BM cells (0 days) harvested from WT and oc/oc+tcirg1 mice (GFP+ cells) and after 6 days of culture with M-CSF and RANKL to determine the level of tcirg1 expression. Tcirg1 PCR values were normalized against the corresponding HPRT value giving a relative intensity value for comparison of different samples. Expression levels are compared to the tcirg1 vector GP+E86 producer cell line and the GFP vector GP+E86 producer cell line.
In WT cells, tcirg1 was up-regulated as the cells differentiated toward osteoclast-like cells, which is in line with previous observations.21 In freshly harvested BM cells from oc/oc+tcirg1 mice, expression from the LTR-driven vector insert was high. However, during the in vitro differentiation toward the osteoclast lineage, the expression of tcirg1 from the vector decreased. Tcirg1 expression could not be detected in the GFP-negative cells sorted from oc/oc+tcirg1 mice (data not shown).
X-ray examination and histology show formation of bone marrow space
In order to examine the skeletal phenotype mice, were analyzed with X-ray and histology. The first analysis was performed 8 weeks after transplantation. At this time, partial remodeling of the bone was observed as detected by X-ray analysis and histology (Figure 6). Analysis 18 weeks after transplantation showed that the bone structure and bone marrow were almost normalized (Figure 6). Transduction of oc/oc HSCs with the tcirg1 vector can thus restore the bone-resorbing activity in vivo to a sufficient degree to reverse the osteopetrotic process in oc/oc mice, even though bone resorption in vitro was limited.
Reversal of osteopetrotic phenotype in oc/oc+tcirg1 mice as demonstrated by X-ray and histology. The left column (A-D) shows X-ray images and the right column (E-H) shows the corresponding histology images (femur). Three-week-old untreated control mouse (A,E), a 3-week-old untreated oc/oc mouse (B,F), 8-week-old oc/oc+tcirg1 mouse (C,G), and 18-week-old oc/oc+tcirg1 mouse (D,H). Note that bone marrow volume increases at the expense of bone volume in oc/oc+tcirg1 compared to untreated oc/oc mouse. Olympus BX45 microscope, (PLAN 4× magnification, 0.1 aperture for [E], [F], and [H]; 1.25× magnification and 0.04 aperture for [G]) and Nikon Prixera PRO 150 ES were used for the histology images.
Reversal of osteopetrotic phenotype in oc/oc+tcirg1 mice as demonstrated by X-ray and histology. The left column (A-D) shows X-ray images and the right column (E-H) shows the corresponding histology images (femur). Three-week-old untreated control mouse (A,E), a 3-week-old untreated oc/oc mouse (B,F), 8-week-old oc/oc+tcirg1 mouse (C,G), and 18-week-old oc/oc+tcirg1 mouse (D,H). Note that bone marrow volume increases at the expense of bone volume in oc/oc+tcirg1 compared to untreated oc/oc mouse. Olympus BX45 microscope, (PLAN 4× magnification, 0.1 aperture for [E], [F], and [H]; 1.25× magnification and 0.04 aperture for [G]) and Nikon Prixera PRO 150 ES were used for the histology images.
Discussion
The field of osteoclast biology has developed rapidly during recent years, in part due to knowledge gained from genetic and molecular examination of several mouse models of osteopetrosis, both spontaneous and induced.22 This condition involves an increase in bone mass, which leads to a number of symptoms of variable severity depending on the mechanism underlying the disease. Osteopetrosis may be caused by defects in osteoclast development or by a reduction in osteoclast function. In terms of correlation between the osteopetrotic mouse models and human forms of these diseases, only mutations affecting osteoclast function have so far been identified as having human counterparts.22,23 The most severe form of osteopetrosis in humans is IMO. Mutations in 3 genes, giving rise to 3 separate single gene disorders all recessive in trait, have so far been shown to cause IMO.2–4,6 The most common gene involved is called TCIRG1, the product of which functions as a subunit of a proton pump used by the osteoclast to acidify the space between the cell and underlying bone. The homologue tcirg1 is spontaneously mutated in the oc/oc mouse leading to progressive osteopetrosis and death 3 to 4 weeks after birth. A previous report concluded that the oc/oc mouse was resistant to treatment with BM transplantation.24 This observation was somewhat puzzling considering that osteoclasts develop from HSCs and that the human form of disease can be treated with HSC transplantation. However, recently, we were able to show that the oc/oc mouse is indeed curable by neonatal transplantation of BM cells if these, in contrast to the previous study, are enriched for H2-matched stem cells.16
HSCs have been one of the primary targets for gene therapy since these are a readily accessible cell source that can be harvested from patients and reinfused after ex vivo manipulation. However, despite the fact that the osteoclast is a part of the hematopoietic system, no gene therapy attempts targeting osteoclasts or their precursors have, to our knowledge, been performed previously.
Here we demonstrate for the first time that osteopetrosis can be reversed by transplantation of gene-modified HSCs leading to long-time survival of oc/oc mice suffering from an otherwise rapidly and lethally progressive form of this disease. Eight of 15 treated oc/oc animals survived past the normal life span of oc/oc mice and exhibited signs of osteoclast activity both in vivo and in vitro. In our previously published transplantation study, we concluded that all mice with at least 15% to 20% of WT donor cells in the PB survived long term, while lower levels of engraftment gave variable results. The results in the present study seem to follow the same pattern, as all mice with 15% of GFP-positive cells or more in the blood lived past the expected 3- to 4-week life span of untreated mice. The level of GFP-positive cells in peripheral blood was maintained or increased slightly during the life span of the mice and there was also evidence of multilineage engraftment indicating stem cell transduction. However, secondary transplantations would have to be performed to formally prove stem cell gene transfer.
The overall response rate in the present study (8/15) is approximately the same as seen in a similar murine ex vivo gene therapy study of ADA-SCID.25 In the present study, we chose to deliver the cells by intraperitoneal injection as this resulted in a high survival rate in our previous transplantation study.16 It is possible, though, that intravenous injection of gene-modified cells would improve the results shown here and maybe also lower the cell dose required.
When osteoclasts were generated and cultured in vitro from BM of oc/oc+tcirg1 mice, there was no sign of bone resorption during a standard culture period of 6 days. Increasing the culture period from 6 to 14 days resulted in bone resorption, however to a significantly lower level than mediated by WT cells (about 10% of normal). Thus partial restoration of osteoclast activity, as determined in vitro, was achieved but not normalization. Theoretically, reasons for this could be impaired development of osteoclasts from transduced cells, a low fraction of corrected cells, or a low level of expression of the transgene in the transduced cells. Previously, it has been shown that the resorption capacity of osteoclasts is correlated to the number of nuclei in the cells.26 The in vitro cultures in the present study demonstrate that multinucleated cells are formed to the same extent from oc/oc+tcirg1 cells as from WT cells. Also the number of nuclei per osteoclast did not differ between oc/oc+tcirg1 and WT cells, indicating that the differentiation of transduced HSCs into osteoclasts was not impaired in this respect. Even when BM cells from oc/oc+tcirg1 mice were sorted for GFP-positive cells to enrich for cells expressing tcirg1 and subsequently cultured on bone slices, the level of in vitro bone resorption remained low. This indicates that the low level of resorption cannot be explained by a low fraction of gene-corrected cells either. Quantitative PCR, on the other hand, revealed that the expression of tcirg1 in osteoclasts derived after in vitro culture of GFP+ cells from oc/oc+tcirg1 mice was lower than in osteoclasts that differentiated from WT cells. It is possible that the level of the transgene in the individual osteoclasts is one factor that limits the bone-resorbing capacity of the gene-corrected cells. Down-regulation of expression from retroviral vectors has previously been described as osteoclast progenitor cells fuse and differentiate into osteoclast-like cells27 and also when human CD34+ cells differentiate to dendritic cells.28 In the latter case down-regulation was observed with a number of different vector constructs, indicating a general mechanism affecting retroviral enhancer/promoter sequences. For this first study of gene correction of osteopetrosis, we selected a vector where the transgene is driven by the strong SFFV promoter, which generally leads to high expression in most cell types.17 However, in the future there will be a need for the development of vectors with osteoclast-specific promoters that selectively can lead to a high level of expression in mature osteoclasts and hopefully a higher degree of functional correction.
It is interesting and encouraging that despite the fairly low level of correction of osteoclast function observed in vitro there was an almost complete normalization of the skeletal phenotype as judged by histology and X-ray in mice that survived long term. This indicates that only a small portion of the bone-resorbing capacity of osteoclasts is needed to restore the balance between production and destruction of bone in oc/oc mice over time. In comparison to our previous study in which neonatal mice received a transplant of WT BM, reversal of the phenotype in the current study was slower, and was not observed until the age of 18 weeks compared with 8 weeks for normal BM cells. Other signs of slow bone resorption were also present. Tooth eruption was almost absent in the mice in the present study, in contrast to mice that received a transplant of WT cells in which teeth erupted, albeit not in a normal manner. Even though transplantation of gene-modified cells to oc/oc mice may lead to correction of osteopetrosis and long-term survival, the animals are not cured from all manifestations of the disease even if they undergo transplantation early after birth. We believe that the signs of slow bone resorption and the fact that not all treated mice survive point to the need to enhance osteoclast function early after transplantation. Ways of achieving this could involve, in addition to improving vectors as already mentioned, differentiating part of the transduced cells toward the osteoclastic lineage before transplantation or treating mice that have undergone transplantation with an osteoclast-stimulating cytokine, for example RANK-ligand.29
The only existing curative treatment for patients with IMO is HSC transplantation, which is associated with high mortality, especially if an HLA-identical sibling donor is not available.13 This, in combination with the rapid progression of the disease, makes gene therapy an attractive option. A potential problem for gene therapy is obtaining a sufficient amount of BM cells for in vitro transduction, since affected children have reduced BM cavities due to reduction of the marrow space. However, as IMO patients have increased levels of stem and progenitor cells circulating in the PB, one option would be to harvest CD34+ cells for in vitro transduction from the PB, possibly without need for any prior mobilization regimen to be given.30 However, it should be stated that a fairly high number of gene-corrected cells was transplanted to each recipient in the current study, and the amount of cells needed to correct the disease in humans remains to be investigated.
As seen in mice that received a transplant of normal BM,16 oc/oc+tcirg1 mice remain growth retarded. Growth retardation is likely to be sustained also in patients after gene therapy since the experience from HSC transplantation show sustained growth impairment even in patients who underwent transplantation early (before 3 months of age).13 Only transplantation in utero seems to be able to result in normal size of oc/oc mice.31 However, in the clinical setting, this form of therapy will be an option only in families with a known history of the disease when a prenatal diagnosis is made, and it is also technically more complicated to perform.
In conclusion, we have demonstrated that the osteopetrosis characteristic for oc/oc mice can be reversed by neonatal transplantation of gene-modified hematopoietic stem and progenitor cells. This can lead to long-term survival of treated mice even though only a portion of transplanted cells expresses the transgene and to a level that is lower than in WT osteoclasts. Our findings represent a first but significant step toward the development of gene therapy for osteopetrosis. However, further development of osteoclast-specific vectors and optimized transplantation procedures is needed in order to move these findings closer to the clinic.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swedish Children's Cancer Foundation, Lund University Hospital Foundations, and Clinical Research Support (ALF) from Lund University Hospital.
We thank Lena Persson-Feld and her staff in the animal facility for taking care of the mice and Marie-Louise Olsson and Per Danielsson at Lund University Hospital for help with X-ray analysis. We thank Christopher Baum for the pSF91P vector and Elena Comandasu, Tom o'Toole, and Angele Kelder for expert assistance in FACS analysis and sorting of cells from mice that underwent transplantation.
Authorship
M.K.J. designed and performed research, collected, analyzed and interpreted data, and wrote first draft of paper; T.J.V., T.S., and V.E. designed and performed research, collected, analyzed, and interpreted data, and wrote parts of the paper; A.C.M.B. helped in designing, performing, and analyzing the qPCR; M.E. performed histology analysis; A.F. and S.K. helped initiate the study and contributed to writing the paper; J.R. initiated and designed the study, analyzed and interpreted data, and wrote final version of paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johan Richter, Molecular Medicine and Gene Therapy, Lund University, 221 84 Lund, Sweden; e-mail: johan.richter@med.lu.se.



![Figure 6. Reversal of osteopetrotic phenotype in oc/oc+tcirg1 mice as demonstrated by X-ray and histology. The left column (A-D) shows X-ray images and the right column (E-H) shows the corresponding histology images (femur). Three-week-old untreated control mouse (A,E), a 3-week-old untreated oc/oc mouse (B,F), 8-week-old oc/oc+tcirg1 mouse (C,G), and 18-week-old oc/oc+tcirg1 mouse (D,H). Note that bone marrow volume increases at the expense of bone volume in oc/oc+tcirg1 compared to untreated oc/oc mouse. Olympus BX45 microscope, (PLAN 4× magnification, 0.1 aperture for [E], [F], and [H]; 1.25× magnification and 0.04 aperture for [G]) and Nikon Prixera PRO 150 ES were used for the histology images.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-12-061382/4/m_zh80120701340006.jpeg?Expires=1767708156&Signature=sItO5B7hWzhgXHvTmpz~uru76PqCiF-C8yRUxV3-EXUCq6ZM23LcY53bJ0lYuzzQoTaqmVEIZyUo-D1jaatJW8dhseMHZSBiTF9YNLVe6mpLJ5E76~NtgxBsg3njVvKAXBYn5BKFBWtLJvbALf5YeNRoDk1tP9bVquuh0Sc9RSCa6-VHpZAcCQF0KKEmcbJFGcDPQGtjPuEaZt2B55bccM-xFxcDa90Yvh--PjOtCSP38~1hmUuRAYSd9bU36A7RNDfdi9bQCqlGHAtQin61MoUf92zN4sGp3wpr-z2lvtBBJGG4iQlP3FBvxrUoEV1ETFpVHJuAqHnpSl0CtvWQYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
